Published online Feb 7, 2007. doi: 10.3748/wjg.v13.i5.683
Revised: November 23, 2006
Accepted: December 5, 2006
Published online: February 7, 2007
AIM: To develop a conditionally replicative gene-viral vector system called CNHK500-p53, which contains dual promoters within the E1 region, and combines the advantages of oncolytic virus and gene therapies for hepatocellular carcinoma (HCC).
METHODS: CNHK500-p53 was constructed by using human telomerase reverse transcriptase (hTERT) promoter to drive adenovirus E1a gene and hypoxia response element (HRE) promoter to drive adenovirus E1b gene. p53 gene expressing cassette was inserted into the genome of replicative virus. Viral replication experiments, cytopathic effect (CPE) and methyl thiazolyl tetrazolium (MTT) assay were performed to test the selective replication and oncolytic efficacy of CNHK500-p53.
RESULTS: Immunohistochemistry verified that infection with CNHK500-p53 was associated with selective replication of adenovirus and production of p53 protein in telomerase-positive and hypoxia-inducible factor-dependent HCC cells. p53 protein secreted from HepG2, infected with CNHK500-p53 was significantly higher than that infected with nonreplicative adenovirus Ad-p53 in vitro (388 ± 34.6 μg/L vs 76.3 ± 13.17 μg/L). Viral replication experiments showed that replication of CNHK500-p53 and CNHK500 or WtAd5, was much stronger than that of Ad-p53 in tested HCC cell lines. CPE and MTT assay indicated that CNHK500-p53 selectively replicated in and killed HCC cells while leaving normal cells unaffected.
CONCLUSION: A more efficient gene-viral system is developed by combining selective oncolysis with exogenous expression of p53 against HCC cells.
-
Citation: Zhao HC, Zhang Q, Yang Y, Lu MQ, Li H, Xu C, Chen GH. p53-expressing conditionally replicative adenovirus CNHK500-p53 against hepatocellular carcinoma
in vitro . World J Gastroenterol 2007; 13(5): 683-691 - URL: https://www.wjgnet.com/1007-9327/full/v13/i5/683.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i5.683
Hepatocellular carcinoma (HCC) is the fifth most common solid tumor worldwide, accounting for 500 000 new cases annually. The majority of patients presenting with advanced disease are not candidates for liver transplantation, surgical resection, or regional therapy. In 60% to 80% of patients with HCC, underlying liver cirrhosis and hepatic dysfunction complicate its treatment. Systemic treatments have minimal effects with significant toxicity, and cannot improve patient survival[1]. The search for alternative treatment modalities has revived the concept of using oncolytic viruses to treat cancer[2,3]. In this respect, conditional replicative adenoviruses (CRAds) appear to be attractive anticancer agents that are currently evaluated in clinical trials[4,5]. CRAds exert intrinsic anticancer activity through selective replication and lysis in cancer cells. In addition, release of CRAd progeny by infected tumor cells provides a potential to amplify the oncolytic effect by lateral spread through solid tumors.
Recent studies have shown that telomerase activity may serve as a general marker of cancer cells. Its activity in normal cells is restricted to fetal tissue, whereas it is elevated in tumors[6]. Although some tumors could activate a yet unknown alternative mechanism of telomere extension, the majority (> 85%) of human HCC cells acquire immortality by expressing telomerase reverse transcriptase (hTERT)[7]. It has been shown that hTERT expression is regulated at the transcriptional level, thereby providing a promising tool for tumor-specific gene expression.
Hypoxia occurs in virtually allsolid tumors as they outgrow their blood supply. Hypoxia augments cellular levels of hypoxia-inducible factor (HIF), a transcription factor that regulates target genes through the binding of hypoxia response elements (HRE). Activation of the HIF pathway enables cancer cells to survive and proliferate in a hypoxic environment and contributes to a more aggressive phenotype[8,9]. Therefore, the HIF/HRE system of gene regulation, which is active under hypoxia or as a result of genetic alterations during cell transformation, is particularly attractive to specific target solid tumors.
Besides viral oncolysis, CRAds can be exploited as vectors of gene therapies by advanced virology and viral vector design to enhance their oncolysis. Many malignant neoplasms have lost the function of p53. Although many oncolytic adenoviruses use p53-dependent pathways to cause cell death, several studies have shown that replicating adenoviruses kill cells more rapidly when expressing p53[10,11].
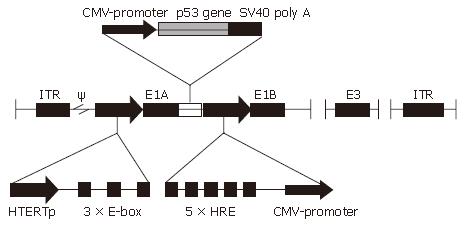
Here we have constructed a novel gene-viral vector system called CNHK500-p53, which uses hTERT promoter to drive adenovirus E1a gene and HRE promoter to drive adenovirus E1b gene. In addition, human p53 gene was cloned into the downstream of E1A of adenovirus. E1A gene is essential for adenoviral replication, and adenovirus can hardly propagate without it. Telomerase and hypoxia are two important features of human solid tumors. Making use of these two promoters, CNHK500-p53 will replicate only in telomerase positive cancer cells undergoing hypoxia in theory. We tested the replication ability and oncolytic activity of CNHK500-p53 in HCC cell lines in vitro.
pXC1 (wild-type adenovirus plasmid) and pBGHE3 (a plasmid-containing right arm of adenovirus type 5 with deletion of 188-1339 bp sequence.) were purchased from Microbix Biosystems Ltd (Toronto, Canada). pGEM-3ZF and pGEM-3ZF-p53 were purchased from Promega Ltd, USA. Human HCC cell lines HepG2, Hep3B, normal human liver cell line L02, normal human fibroblast cell lines MRC-5 and BJ were purchased from the American Type Culture Collection (Manassas, VA). Human HCC cell lines SMMC-7721, Bel-7402 and wild-type adenovirus 5 (WtAd5) were obtained from Second Military Medical University (Shanghai, China). Human embryonic kidney 293 cell line was obtained from Microbix Biosystems (Toronto, Canada). Hep3B, HepG2, SMMC-7721, Bel-7402 and human embryonic kidney 293 cells were cultured in DMEM (Life Technologies, Rockville, MD). L02 was cultured in RPMI 1640 medium. BJ was cultured in modified Eagle’s medium (MEM). All the media were supplemented with 10% heat-inactivated fetal bovine serum (Life Technologies), 4mmol/L L-glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin and cultured under a 5% CO2 atmosphere at 37°C.
Complete cDNA sequence of p53 gene was amplified by PCR from plasmid pGEM-3ZF-p53 by using the upstream primer VT182 (5’CCG GAA TTC (EcoRI) GCC ATG GAG GAG CCG CAG TCA GA3’) and downstream primer VT183(5’CGC GGA TCC (BamHI) TTA TCA GTC TGA GTC AGG CCC TTC TG3’). Synthetic DNA sequence was released with endonucleases EcoRI and BamHI (New England Biolabs, Beverley, MA) and ligated into plasmid pClon15 (made by ourselves, which contains the sequence of mouse cytomegalovirus promoter + multiple clone site + SV40 poly A) to generate pClon15-p53. pClon15-p53 was digested with endonucleases AgeI and NotI (New England Biolabs), a 1917-bp fragment containing mouse cytomegalovirus promoter + p53 gene + SV40 polyA was excised and inserted into AgeI and NotI sites of pSG500, which was constructed in our previous study and contained hTERT promoter core sequence with three extra E-boxes downstream and HRE promoter[12]. The plasmid resulting from the insertion of p53 gene cassette into the pSG500 in orthograde orientation was designated as pSG500-p53. pSG500-p53 and pSG500 were transfected by Lipofectamine 2000 (Life Technologies) into 293 cells together with pBHGE3. Viral plaques appeared 9-14 d after cotransfection and were sublimated three times. Recombinant adenoviruses, extracted using QIAamp DNA blood mini kit (Qiagen, Valencia, CA), were verified by PCR and named CNHK500-p53 and CNHK500. Ad-p53 was used as a control, which is a nonreplicative adenovirus vector carrying a SV40 early promoter-driven human p53 expression cassette[13]. A similar procedure was used by replacing the p53 gene with a 1538-bp fragment of the green fluorescent protein (GFP) expression cassette, obtained from plasmid pCA13-GFP (Takara Ltd, Japan), to derive conditionally replicative adenovirus CNHK500-GFP and nonreplicative adenovirus Ad-GFP. The nonreplicative adenovirus Ad-blank was used as a control.
Viruses were purified by CsCl density purification and propagated in 293 cells. After 72 h, the detached cells were harvested by centrifugation at 1000 ×g for 5 min at 4°C, resuspended in 10 mL cold PBS (free Ca2+ and Mg2+), and then lysed with three cycles of freeze and thaw. Lysate was collected by centrifugation at 1500 ×g for 10 min at 4°C, and the supernatant was placed on a gradient prepared with equal parts of CsCl in PBS and then centrifuged at 15 000 ×g for 2 h at 12°C. The virus band was removed and placed in a preformed CsCl gradient by ultracentrifugation for 18 h and dialyzed into 10 mmol/L Tris-HCl (pH 7.4) containing 10 mmol/L MgCl2 and 10% glycerol. Titers of the purified adenovirus were determined by plaque assays of the tissue culture infectious dose 50 methods and shown as plaque forming unit per milliliter (pfu/mL). All viral preparations were free of endotoxin.
Monolayer cells, including logarithmically growing Hep3B, HepG2, SMMC-7721 (105 cells/well), and contact-inhibition BJ, L02 (106 cells/well) were cultured in six-well dishes overnight and infected with CNHK500-p53, WtAd5, Ad-p53 at a multiplicity of infection (MOI) of 5.0 pfu/cell. Virus inocula were removed after 2 h. The cells then were washed twice with PBS and incubated at 37°C for 0, 12, 24, 48, or 96 h. Lysates of cells were prepared with three cycles of freeze and thaw. Serial dilutions of the lysates were titered on human embryonic kidney 293 cells with the tissue culture infectious dose 50 methods, normalized with that at the beginning of infection, and reported as multiples.
HepG2, Hep3B and BJ were seeded in 6-well plates at a density of 5 × 105 cells/well and infected with CNHK500-p53 or wtAd5 at a MOI of 1 after 24 h of incubation. Two days after viral infection, cells were harvested and lysed with M-PER mammalian protein extraction reagent (PI-ERCE, Rockford, IC). Concentration of the extracted protein was measured with a biophotometer (Eppendorf AG, Hamburg, Germany). Total proteins (20 μg) were separated on 10% SDS-polyacrylamide gel, electroblotted onto PROTRAN nitrocellulose transfer membrane (Schleicher & Schuell Inc, Dassel. Germany) and blocked with 5% fat-free milk in Tris-buffered saline (TBS: 10 mMTris, pH 7.5, 0.9% NaCl) containing 0.1% Tween-20 (TBST) at room temperature for 1 h. The membrane was incubated with either rabbit polyclonal antibody against Ad-E1A protein (Santa Cruz Biotechnology) or rat anti-Ad5 E1B 55k monoclonal antibody overnight at 4°C and repeatedly washed in TBST. After incubation for 1 h with appropriate secondary horseradish peroxidase-conjugated anti-bodies and extensive washing with TBST, immunocomplexes on the membrane were detected with LumiGLOTM reagent and visualized with Kodak BiomaxMR film. To detect the expression of E1A and E1B under hypoxic condition, CNHK500-p53 infected cells were exposed to 0.1% hypoxia for 16 h before harvest.
HepG2 cells were seeded in 24-well plates at a density of 5 × 104 cells/well and cultured for 24 h, followed by infection with CNHK500-p53 and Ad-p53 at a MOI of 0.1. On days 3, 5, 7, and 10 post-infection, the supernatants of cell cultures were collected and assayed for p53 gene expression levels using the ELISA kit of p53 (Chemicon International, Temecula, CA) and the manipulation was done according to the manufacturer’s instructions.
Cytopathic effect (CPE): Hep3B, HepG2, Bel-7402 (2 ×104 Cells/well) and BJ (6 × 104 cells/well) were dispensed in 24-well plates. The culture solution was removed on the second day, and 1 mL serum free DMEM and virus were added to each well. The multiplicity of infection (MOI) of each well was 0.01, 0.1, 1, 10 and 100, respectively. The culture plate was then incubated for 90 min in a 37°C incubator under the condition of 5% CO2 in DMEM containing 5% serum.
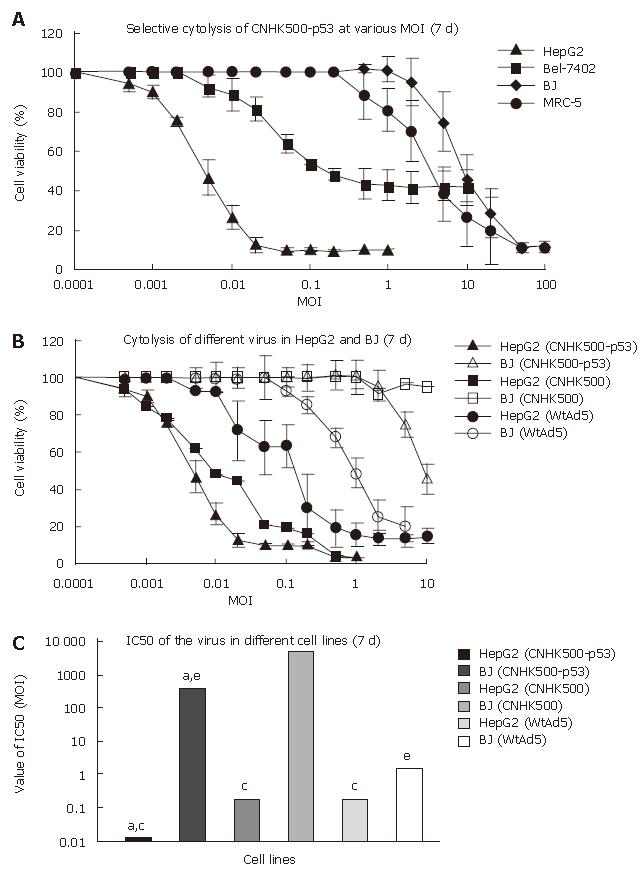
Methyl thiazolyl tetrazolium (MTT) assay: MTT assay was performed to determine cell viability at various viral MOIs. HepG2, Hep3B and BJ cells were plated at a density of 1 × 104 cells/well in 96-well plates (Falcon) and 24 h later, the cells were infected with CNHK500-p53 at serial MOIs from 0.001 to 100. After 7 d of incubation, cell viability was measured by MTT assay using a non-radioactive cell proliferation kit (Roche Molecular Biochemicals) according to its protocol, and the spectrophotometrical absorbance of samples was measured with a microplate reader model 550 (BIO-RAD Laborato-ries, Tokyo, Japan) at 570 nm with a reference of 655 nm. Percentage of cell survival was calculated using the formula: % cell survival = (OD value of infected cells/OD value of uninfected control cells) × 100%. Eight replicate samples were taken at each MOI and each experiment was repeated at least 3 times. IC50 of CNHK500-p53, CNHK500, and WtAd5 was calculated in HepG2 and BJ 7 d after infection. Statistical analysis was performed using Student’s t test for differences among groups. P < 0.05 was considered statistically significant.
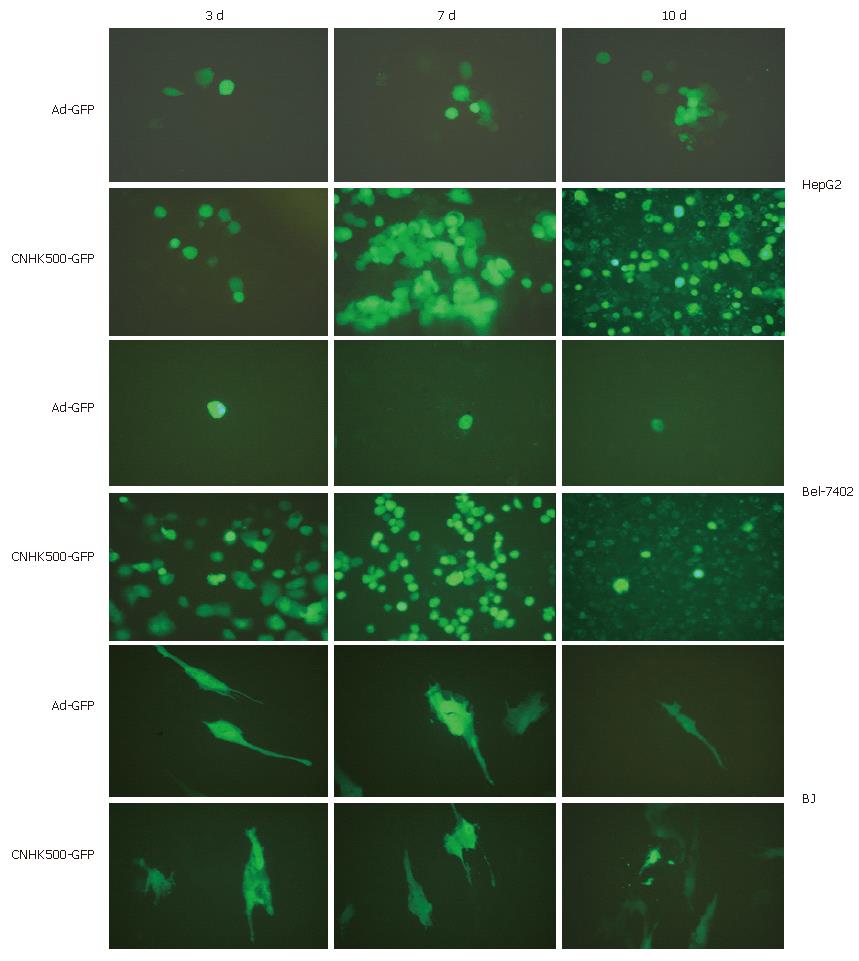
HepG2, Bel-7402 (1 × 105 cells/well), and BJ (1 × 106 cells/well) were inoculated into six-well plates, respectively. When the cells were confluent, CNHK500-EGFP or Ad-EGFP was added to each well at the MOI of 1 and then washed with PBS 2 h later. Cells were then coated with 1.25% agarose. On days 3, 7 and 10, the cells were observed under a fluorescence microscope and significant changes were photographed. Fluorescence microscopy was performed with routine methods with fluorescence in isothiocyanate (FITC), with the excitement and emission wavelength being 475 nm and 490 nm, respectively.
To make a conditional replicative gene-viral vector, we adopted a design as shown in Figure 1, in which the adenovirus E1a gene was placed under the control of hTERT promoter plus three extra E-boxes, and E1b gene was controlled by HRE promoter, p53 gene-expressing cassette was inserted between E1A and HRE promoter. CNHK500-p53 was successfully made and verified by PCR. We were able to produce the adenovirus with high titers (2 × 1010 pfu/mL).
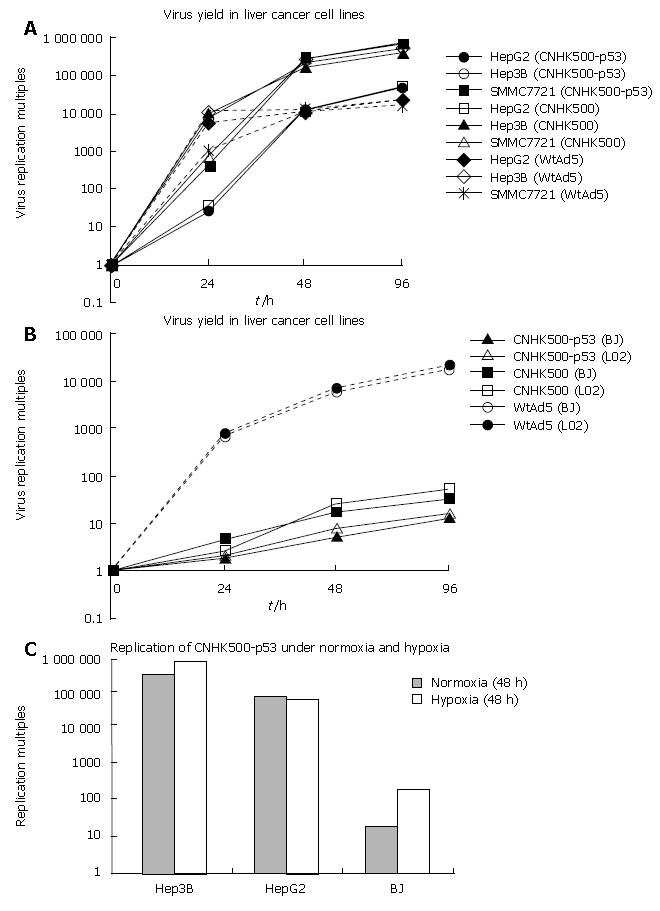
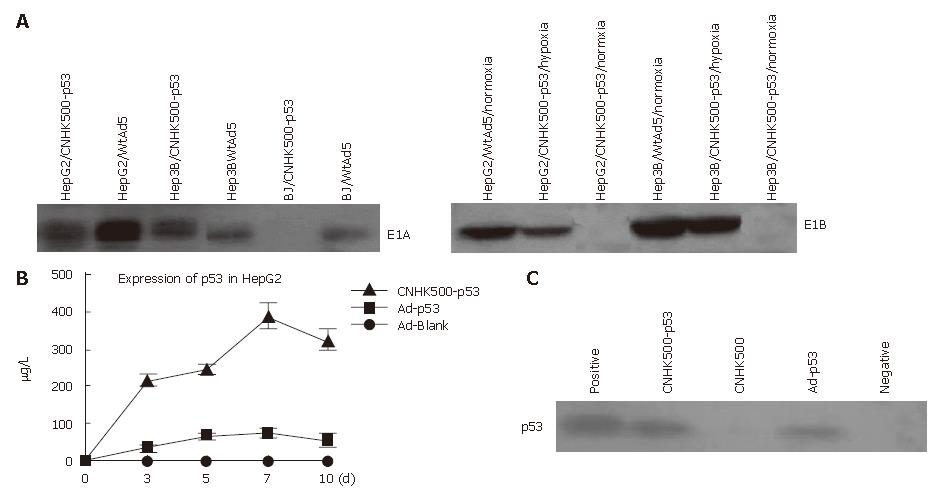
Selective replication of the new recombinant adenovirus CNHK500-p53 was evaluated using telomerase-positive HCC cell lines Hep3B, HepG2, SMMC-7721 and telomerase-negative normal cell lines BJ, L02. In HepG2, Hep3B and SMMC-7721, the replicative multiples increased to 47 230-, 459 837- and 669 251- fold respectively after 96 h of CNHK500-p53 replication, similar to those of CNHK500 and WtAd5 (Figure 2A). However, in normal cell lines BJ, L02, the replicative multiples of CNHK500-p53 and CNHK500 were only 12.8-, 16.3- and 31-, 53- fold at 96 h, and attenuated as much as 1354-, 1325- and 559-, 407.5- fold when compared with WtAd5 (Figure 2B). CNHK500-p53 showed enhanced replication ability both in HepG2, Hep3B and in BJ under hypoxia condition, but was higher in HepG2 and Hep3B cells than in normal BJ cells (Figure 2C). As hTERT promoter regulates E1a gene, E1A protein could be detected in telomerase positive hepatocellular cells HepG2 and Hep3B, but not in telomerase negative normal cells BJ. Under normoxic condition, E1B protein could hardly be detected due to poor activity of HRE promoter. When HCC cells were exposed to hypoxia, E1B protein was induced as a result of increased activity of HRE promoter (Figure 3A).
To verify that the p53 expressed by CNHK500-p53 could secrete efficiently into the media, the conditioned media from 5 × 104 HepG2 cells infected with CNHK500-p53 or Ad-p53 at a MOI of 0.1 were collected and analyzed for the presence of p53 protein by ELISA. The quantity of p53 expressed by CNHK500-p53 and Ad-p53 on d 3, 5, 7, and 10 post-infection is shown in Figure 3B, indicating that p53 expression in CNHK500-p53 was 5.1 times more than that in Ad-p53 in HepG2 on d 7. Western blot analysis revealed a clear band of Mr 53 000 in the conditioned media after HepG2 cells were infected with CNHK500-p53 or Ad-p53 at a MOI of 1 on d 3, suggesting that the protein in the media was p53 protein (Figure 3C).
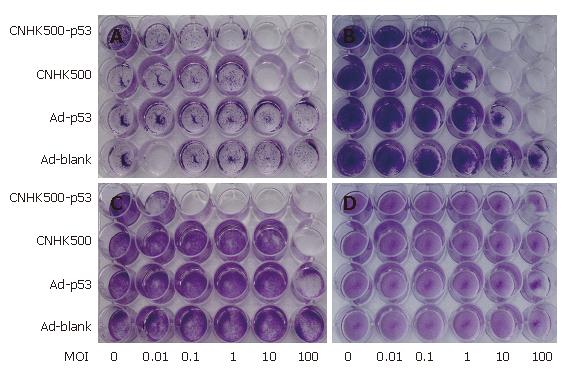
CPE was used to determine whether CNHK500-p53 infection induces selective cell lysis. HCC cell lines (HepG2, Hep3B, and Bel-7402) and the normal cell line (BJ) were infected with CNHK500-p53, CNHK500, Ad-p53, and Ad-Blank at various MOIs, fixed in methanol and stained with crystal violet 7 d after infection to visualize viable cells. CNHK500-p53 showed the strongest selective cytolysis effect among the viruses and killed all cancer cell lines in a dose-dependent fashion (Figure 4). Infection with CNHK500-p53 at a MOI of 0.1-1 was sufficient to induce its lytic effects, although the sensitivity varied among the cell types. In contrast, no apparent CPE was observed in BJ cells 7 d after CNHK500-p53 infection. Cytotoxicity of CNHK500-p53 was also assessed by MTT assay. HepG2, Hep3B and MRC-5, BJ cells were infected with CNHK500-p53 at various viral MOIs. As shown in Figure 5A, CNHK500-p53 induced more rapid cell death in HCC cells than in normal BJ cells 7 d after infection. Furthermore CNHK500-p53 showed a different ability to kill HepG2 and Bel-7402 with the IC50 being 0.012 and 0.28 of MOI. On the contrary, the IC50 in normal BJ cells was as high as 352.1 of MOI, suggesting that more than 29 341- or 1257-fold of CNHK500-p53 was needed to kill half BJ compared with HepG2 and Bel-7402. Comparison with CNHK500 and WAd5 infection, CNHK500-p53 not only demonstrated more apparent cytolysis against HepG2 than CNHK500 and WtAd5 but less profound cytotoxicity than WAd5 against BJ (Figure 5B and C).
To determine the selective replication of CNHK500-GFP, HCC cell lines including HepG2, Bel-7402 and normal cell line BJ were observed under fluorescent microscope at different time points after being infected with CNHK500-GFP and Ad-GFP. By conducting GFP expression, CNHK500-GFP demonstrated a greater replicative ability than Ad-GFP in HCC cell lines, with no significant difference in normal cell line. After 3, 7 and 10 d of infection with CNHK500-GFP and Ad-GFP, only a few scattered cells emitted fluorescence in normal cell line. However, in HCC cell lines, CPE such as deformation and aggregation appeared 3 d after infection, and the fluorescence emission spread from a single cell to many cells within a large area 7 d after infection. Bel-7402 cells were particularly sensitive to CNHK500-GFP, many cells died with GFP degradation and fluorescence extinction 10 d after infection (Figure 6), showing selective replication of CNHK500-derivative and correct insertion of GFP gene.
Besides conventional approaches, CRAds specifically killing tumor cells while sparing normal cells have been introduced as new agents for cancer therapy in the past decade[14,15]. The efficacy of CRAds against cancer, including HCC, is however, limited by several factors, mainly including tumor specificity and oncolysis[16].
With the advancement in molecular biology and understanding of the function of viral genes, it has become possible to genetically re-engineer viruses to make them selectively kill tumor cells over normal tissue. At present, tumor specificity has been achieved in oncolytic adenoviruses mainly (1) by altering viral genes that attenuate replication in normal tissue but not in tumor cells such as ONYX015 with E1B 55KD deleted[15], (2) by placing viral genes that initiate viral replication under the control of promoter sequences that are active in tumor cells such as using AFP promoter to restrict viral replication in AFP-producing HCC[17,18], and (3) by modifing viral coat proteins that function in host cell infection.
Studies showed that adenovirus-induced oncolysis can benefit from combined gene therapy[11,19]. Cancer gene therapy typically involves delivery of tumor suppressor, enzyme/pro-drug gene, cytotoxic/pro-apoptotic gene, immunogene, anti-angiogenic gene directly into tumor cells[20]. After more than two decades of study, the tumor suppressor p53 gene is widely regarded as the “genome guardian.” It has been estimated that at least half of all human malignancies, including HCC, are related to a mutation of the p53 gene[21].
In our previous study, we constructed a CRAd containing dual promoters within the E1 region, designated as CNHK500[12], in which the viral E1a gene is regulated by hTERT promoter and E1b gene by HRE promoter. Since telomerase is highly activated in most malignant tumors but inactive in normal somatic cells[22], CNHK500 can selectively replicate in telomerase-positive cancer cells. At the same time, its replication ability is further attenuated in normal cells as hypoxia microenvironment seldom exists among normal tissues. However, it may propagate very well in solid tumors because HRE promoter is transcriptally activated due to hypoxia, a unique feature of human solid tumors[23]. A further study suggested that CNHK500 is tumor-selective in vitro and vivo when compared with CNHK300 and WtAd5[24]. To enhance the oncolytic potency of CNHK500, we constructed a new CRAd CNHK500-p53 by combining oncolytic virotherapy with gene therapy, which expresses functional p53 during viral replication in HCC cells as verified by PCR, Western blot, and ELISA assay. We evaluated the efficacy of CNHK500 and CNHK500-p53 against human HCC cell lines in vitro.
We found that exogenous expression of p53 by CNHK500-p53 could lead to enhanced oncolytic potency compared with its parent CNHK500 on most HCC cell lines, while the ability of selective replication was not significantly different between them. The superior efficacy of CNHK500-p53 was independent of the cellular p53 genetic background. It was reported that the expressed p53 gene appears to exert its anticancer activities by one or more of the following mechanisms: (1) simultaneously triggering apoptotic pathways in tumor cells by a transcription-dependent mechanism in cell nuclei[25,26] and by a transcription-independent mechanism in mitochondria[27] and Golgi apparatus[28]; (2) activating immune response factors such as natural killer cells[29] to exert “bystander effects”; (3) inhibiting DNA repair and antiapoptosis functions in tumor cells[30]; (4) down-regulating the expression of multidrug resistance genes[31] to revert the resistance of tumor cells against radio- and chemotherapies as well as the vascular endothelial growth factor gene[32] to block the blood supply to tumor tissue and matrix metalloproteinase[33] to suppress tumor cell adhesion, infiltration, and metastasis; (5) blocking the transcription of survival signals in tumor cells[34,35], thus inhibiting the growth of tumor cells in any stage of the cell cycle. Hence, dysfunctional p53 of HCC cells might delay conditionally replicative adenovirus-induced cell death, thus limiting conditionally replicative adenovirus efficacy.
In conclusion, CNHK500-p53 has the selective replicative ability in HCC cell lines and a higher oncolytic efficacy than its parent CNHK500 in vitro. The enhanced oncolytic efficacy may be related to the expression of p53 gene carried by CNHK500-p53. Further experiments are needed to warrant its potential therapeutic effect against HCC.
We are grateful to Laboratory of Viral and Gene Therapy, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, Shanghai, China, for its help in constructing recombinant adenoviruses.
Hepatocellular carcinoma (HCC) is one of the most frequent and lethal malignancies worldwide especially in China. According to the reports of American cancer society (ACS) in 2005, the 5-year survival rate of HCC is only about 8.3%. Therefore, development of effective alternative approaches is needed. Replication-selective virus-mediated gene therapy holds great promise for the treatment of cancer, including HCC.
So far, 656 cancer gene therapy clinical protocols are in different phase of evaluation worldwide. Unfortunately, successful delivery and targeted expression of therapeutic gene into cancer cells still are very difficult to achieve. The main problem is due to the very low in vivo transduction and expression efficacy of available vectors. In this respect, conditionally replicative adenoviruses appear as attractive vectors. Combination of oncolytic virotherapy and gene therapy may be an effective alternative approach against cancer.
In order to construct tumor-specific conditionally replicative adenoviruses, adenovirus E1A gene expression was driven by the hTERT promoter and E1B gene by the hypoxia response (HRE) promoter through genetic engineering, which assures adenovirus replication only in telomerase-positive cells exposed to hypoxia. Besides, human p53 gene was cloned into the downstream of E1A of adenovirus to enhance oncolysis. It was different from other study in adenovirus reconstruction methods. Meanwhile, the recombinant adenovirus was verified by PCR assay, and showed tumor-specific replication and enhanced oncolysis against HCC cell lines in vitro.
The results of our present study demonstrate that p53-expressing conditionally replicative adenovirus has the selectively replictive ability in HCC cell lines and a higher oncolytic efficacy than non-p53-expressing conditionally replicative adenovirus in vitro. The enhanced oncolytic efficacy may be related to the expression of p53 gene. Further experiments are needed to warrant its potential therapeutic effect against HCC.
CRAds: conditional replicative adenoviruses, recombinant adenoviruses modified to selectively replicate in cancer cells; Oncolytic virotherapy: one of the cancer therapies by obtaining a virus that replicates and preferentially kills cancer cells, leaving the surrounding normal tissues relatively intact; Cancer gene therapy: Cancer gene therapy can be defined as transfer of nucleic acids into tumor or normal cells to eradicate or reduce tumor mass by direct killing of cells, immunomodulation or correction of genetic errors, and reversion of malignant status. Initially started with lots of optimism and enthusiasm, cancer gene therapy has shown limited success in treatment of patients.
This is an interesting manuscript. In this manuscript, Hing-Chuan Zhao et al take advantage of the selective expression of hTERT and HIF in tumoral cells to express under their respective promoters E1A and E1B assuring adenovirus replication only in telomerase cancerous cells exposed to hypoxia. The data are clearly presented. They show the selective replication and expression of adenoviral proteins as well as the selective cytopathic effect of their adenovirus.
| 1. | Thomas MB, Abbruzzese JL. Opportunities for targeted therapies in hepatocellular carcinoma. J Clin Oncol. 2005;23:8093-8108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | Nemunaitis J, Edelman J. Selectively replicating viral vectors. Cancer Gene Ther. 2002;9:987-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Ring CJ. Cytolytic viruses as potential anti-cancer agents. J Gen Virol. 2002;83:491-502. [PubMed] |
| 4. | Alemany R, Balagué C, Curiel DT. Replicative adenoviruses for cancer therapy. Nat Biotechnol. 2000;18:723-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 316] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 5. | Heise C, Kirn DH. Replication-selective adenoviruses as oncolytic agents. J Clin Invest. 2000;105:847-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 121] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Huang TG, Savontaus MJ, Shinozaki K, Sauter BV, Woo SL. Telomerase-dependent oncolytic adenovirus for cancer treatment. Gene Ther. 2003;10:1241-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 98] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Tahara H, Nakanishi T, Kitamoto M, Nakashio R, Shay JW, Tahara E, Kajiyama G, Ide T. Telomerase activity in human liver tissues: comparison between chronic liver disease and hepatocellular carcinomas. Cancer Res. 1995;55:2734-2736. [PubMed] |
| 8. | Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4764] [Cited by in RCA: 5116] [Article Influence: 222.4] [Reference Citation Analysis (0)] |
| 9. | Safran M, Kaelin WG. HIF hydroxylation and the mammalian oxygen-sensing pathway. J Clin Invest. 2003;111:779-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 247] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 10. | Dix BR, O'Carroll SJ, Myers CJ, Edwards SJ, Braithwaite AW. Efficient induction of cell death by adenoviruses requires binding of E1B55k and p53. Cancer Res. 2000;60:2666-2672. [PubMed] |
| 11. | van Beusechem VW, van den Doel PB, Grill J, Pinedo HM, Gerritsen WR. Conditionally replicative adenovirus expressing p53 exhibits enhanced oncolytic potency. Cancer Res. 2002;62:6165-6171. [PubMed] |
| 12. | Qi Z, Linhui P, Hongping W. Construction of CNHK500, a conditionally replicating adenovirus driven by the human telomerase reverse transcriptase promoter and hypoxia response promoter. Zhonghua Shiyan Waike Zazhi. 2004;21:1366-1368. |
| 13. | Ameyar M, Shatrov V, Bouquet C, Capoulade C, Cai Z, Stancou R, Badie C, Haddada H, Chouaib S. Adenovirus-mediated transfer of wild-type p53 gene sensitizes TNF resistant MCF7 derivatives to the cytotoxic effect of this cytokine: relationship with c-myc and Rb. Oncogene. 1999;18:5464-5472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Post DE, Khuri FR, Simons JW, Van Meir EG. Replicative oncolytic adenoviruses in multimodal cancer regimens. Hum Gene Ther. 2003;14:933-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1264] [Cited by in RCA: 1216] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 16. | Curiel DT. The development of conditionally replicative adenoviruses for cancer therapy. Clin Cancer Res. 2000;6:3395-3399. [PubMed] |
| 17. | Sangro B, Herraiz M, Prieto J. Gene therapy of neoplastic liver diseases. Int J Biochem Cell Biol. 2003;35:135-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Ohashi M, Kanai F, Tateishi K, Taniguchi H, Marignani PA, Yoshida Y, Shiratori Y, Hamada H, Omata M. Target gene therapy for alpha-fetoprotein-producing hepatocellular carcinoma by E1B55k-attenuated adenovirus. Biochem Biophys Res Commun. 2001;282:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Zhang Q, Nie M, Sham J, Su C, Xue H, Chua D, Wang W, Cui Z, Liu Y, Liu C. Effective gene-viral therapy for telomerase-positive cancers by selective replicative-competent adenovirus combining with endostatin gene. Cancer Res. 2004;64:5390-5397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Hernandez-Alcoceba R, Sangro B, Prieto J. Gene therapy of liver cancer. World J Gastroenterol. 2006;12:6085-6097. [PubMed] |
| 21. | Shiraishi K, Kato S, Han SY, Liu W, Otsuka K, Sakayori M, Ishida T, Takeda M, Kanamaru R, Ohuchi N. Isolation of temperature-sensitive p53 mutations from a comprehensive missense mutation library. J Biol Chem. 2004;279:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Shay JW, Wright WE. Telomeres and telomerase: implications for cancer and aging. Radiat Res. 2001;155:188-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Binley K, Askham Z, Martin L, Spearman H, Day D, Kingsman S, Naylor S. Hypoxia-mediated tumour targeting. Gene Ther. 2003;10:540-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Su CQ, Sham J, Xue HB, Wang XH, Chua D, Cui ZF, Peng LH, Li LF, Jiang LH, Wu MC. Potent antitumoral efficacy of a novel replicative adenovirus CNHK300 targeting telomerase-positive cancer cells. J Cancer Res Clin Oncol. 2004;130:591-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Matsuda K, Yoshida K, Taya Y, Nakamura K, Nakamura Y, Arakawa H. p53AIP1 regulates the mitochondrial apoptotic pathway. Cancer Res. 2002;62:2883-2889. [PubMed] |
| 26. | Taha TA, Osta W, Kozhaya L, Bielawski J, Johnson KR, Gillanders WE, Dbaibo GS, Hannun YA, Obeid LM. Down-regulation of sphingosine kinase-1 by DNA damage: dependence on proteases and p53. J Biol Chem. 2004;279:20546-20554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1484] [Cited by in RCA: 1601] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 28. | Bennett M, Macdonald K, Chan SW, Luzio JP, Simari R, Weissberg P. Cell surface trafficking of Fas: a rapid mechanism of p53-mediated apoptosis. Science. 1998;282:290-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 522] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 29. | Rosenblum MD, Olasz E, Woodliff JE, Johnson BD, Konkol MC, Gerber KA, Orentas RJ, Sandford G, Truitt RL. CD200 is a novel p53-target gene involved in apoptosis-associated immune tolerance. Blood. 2004;103:2691-2698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Sah NK, Munshi A, Nishikawa T, Mukhopadhyay T, Roth JA, Meyn RE. Adenovirus-mediated wild-type p53 radiosensitizes human tumor cells by suppressing DNA repair capacity. Mol Cancer Ther. 2003;2:1223-1231. [PubMed] |
| 31. | Krishna R, Mayer LD. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur J Pharm Sci. 2000;11:265-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 749] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 32. | Pal S, Datta K, Mukhopadhyay D. Central role of p53 on regulation of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) expression in mammary carcinoma. Cancer Res. 2001;61:6952-6957. [PubMed] |
| 33. | Ala-aho R, Grénman R, Seth P, Kähäri VM. Adenoviral delivery of p53 gene suppresses expression of collagenase-3 (MMP-13) in squamous carcinoma cells. Oncogene. 2002;21:1187-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Yin Y, Liu YX, Jin YJ, Hall EJ, Barrett JC. PAC1 phosphatase is a transcription target of p53 in signalling apoptosis and growth suppression. Nature. 2003;422:527-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 111] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Rother K, Johne C, Spiesbach K, Haugwitz U, Tschöp K, Wasner M, Klein-Hitpass L, Möröy T, Mössner J, Engeland K. Identification of Tcf-4 as a transcriptional target of p53 signalling. Oncogene. 2004;23:3376-3384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
S- Editor Liu Y L- Editor Wang XL E- Editor Liu WF