Published online Dec 28, 2007. doi: 10.3748/wjg.v13.i48.6558
Revised: September 29, 2007
Accepted: October 6, 2007
Published online: December 28, 2007
AIM: To explore the expression of PlexinA1 in gastric carcinoma and its relationship with tumor angiogenesis and proliferation.
METHODS: PlexinA1 mRNA and protein expressions of Semaphorin6D were measured using semi-quantity reverse transcription PCR and Western blotting in 20 cases of gastric carcinoma and corresponding normal gastric mucosa. PlexinA1, Ki-67 expression and microvessel density (MVD) were detected by immunohistochemistry in 50 cases of gastric carcinoma and 20 cases of normal gastric mucosa.
RESULTS: The mRNA and protein expressions of PlexinA1 in gastric carcinoma were significantly higher than that in normal gastric mucosa (0.71 ± 0.37 vs 0.60 ± 0.25, P = 0.0299 < 0.05, and 0.47 ± 0.16 vs 0.21 ± 0.08, P = 0.0000 < 0.01), and MVD within tumor tissues increased significantly with PlexinA1 mRNA expression (r =0.8736, P < 0.01) and PlexinA1 protein expression (r = 0.7286, P < 0.01), and MVD of the PlexinA1 positive staining group (25.25 ± 3.93) was significantly higher than that of the negative group (19.56 ± 1.75), (P < 0.01). Proliferation index of tumor cells within tumor tissues were positively correlated with PlexinA1 mRNA expression (r = 0.5420, P = 0.014 < 0.01) and PlexinA1 protein expression (r = 0.5024, P = 0.024 < 0.05). The proliferation index of the PlexinA1 positive staining group (567.69 ± 125.61) was significantly higher than that of the negative group (369.58 ± 116.88), (P < 0.01).
CONCLUSION: PlexinA1 may play an important role in the occurrence and development of gastric carcinoma, and be related to tumor angiogenesis and proliferation.
- Citation: Zhao XY, Chen L, Li YH, Xu Q. PlexinA1 expression in gastric carcinoma and its relationship with tumor angiogenesis and proliferation. World J Gastroenterol 2007; 13(48): 6558-6561
- URL: https://www.wjgnet.com/1007-9327/full/v13/i48/6558.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i48.6558
Primary gastric carcinoma is one of the most common malignant tumors in China. Invasion and metastasis are the main causes for the death of cancer patients. On the other hand, invasion and metastasis of malignant tumors are closely related to the proliferation of tumor cells and angiogenesis of tumor tissues. PlexinA1 is a large transmembrane protein that is a major receptor for multiple classes of Somaphorins, either alone or in combination with neuropilins. Although PlexinA1 has pleiotropic function in formation of nervous systems, embryogenesis, angiogenesis and immunoreaction[1-4], the function of Plexin A1 in carcinogenesis remain unrevealed. Therefore, we detected the protein and mRNA expression of PlexinA1 in gastric carcinoma and normal gastric mucosa by the semi-quantitative RT-PCR, Western blotting and immunohistochemistry in order to explore the expression of PlexinA1 in gastric carcinoma and its relationship with tumor angiogenesis and proliferation.
Twenty fresh gastric carcinoma specimens and corresponding normal gastric mucosa specimens were analyzed by RT-PCR and Western blotting. All specimens were obtained from patients (8 women and 12 man; mean age 54 years) who underwent surgery for gastric carcinoma between April 2006 and August 2006 in the General Hospital of PLA. Of the 20 patients, 9 showed high-moderate differentiation, 11 poor differentiation and 10 lymph node metastasis. Fifty paraffin-embedded gastric carcinoma specimens and twenty normal gastric mucosa specimens were collected respectively for immunohistochemistry. The patients with gastric carcinoma consisted of 21 women and 29 men with a mean age of 56 years. Of the 50 gastric carcinoma specimens, 23 were highly-moderately differentiated and 27 poorly differented, and 28 had lymph node metastasis. The diagnosis was confirmed by pathological examination.
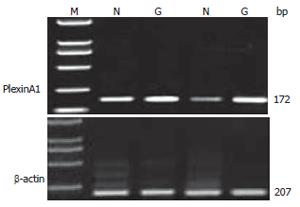
Total mRNA was isolated by Trizol reagent according to the procedure of the supplier (BioDev-tech, Beijing, China), and the concentration was determined by measuring the absorbance at 260 nm and using the following data: 1 optical density unit = 40 mg of RNA/mL. A 1.5 μg aliquot of total RNA from each specimen was reverse-transcribed into single-strand cDNA using oligo (dT)16 primer for 2 h at 37°C, each single-strand cDNA was used for subsequent PCR amplification of PlexinA1 and β-actin with the latter used as a quantitative control. The PCR was carried out in a reaction volume of 50 μL for 5 min at 95°C for initial denaturing, followed by 37 cycles of 94°C for 50 s, 55°C for 50 s, and 72°C for 1 min, then extend at 72°C for 10 min on the Authorized Thermal Cycler for PCR. The primer sequences used for amplification were 5’-TGTGGACGACCCCAAATTCTA-3’ and 5’-CTGGGCAAACACGGTGAAC-3’ for PlexinA1, 5’-ACACCTACCAGGGAACGGAG-3’ and 3’-GCCTCTGCACATACCTGCT-5’ for β-actin. The primer sequences were synthesized by Beijing Genomics Institute (China). PCR products were resolved in 2% agarose gels and visualized by staining with ethidium bromide. To quantify PCR products, the bands representing amplified products were analyzed by Quantity One Analysis Software (BIO-RAD Co. America).
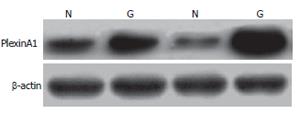
Expression of the PlexinA1 protein was detected using the Western blot method. After washing in ice-cold PBS, the samples were finely minced and suspended in ice-cold homogenization buffer (2 mL/g tissue), which contained protease inhibitors to minimize protein degradation. The suspension was firstly homogenized, then centrifuged at 12 000 ×g for 30 min at 4°C to remove the nuclei and cell debris. The supernatant (total protein extract) was collected. Equal amount (50 μg) of proteins was run on a 10% SDS-PAGE gel and electrotransferred onto Hybond-polyvinylidene difluoride membranes (Amersham, Arlington Heights, USA). The membranes were blocked for 2 h at room temperature, followed by incubation with the primary anti-PlexinA1 antibody 1:50 (Santa Cruz Co, USA) at 4°C overnight. The primary antibody was diluted in TBST containing fat-free milk. After three 10-min washes in TBST, the membrane was incubated in peroxidase-conjugated secondary antibody (Sigma, St. Louis, USA) diluted 1:800 at room temperature for 1 h. Immunoreactive proteins were visualized by autoradiogram using ECL Western blotting detection reagents (Amershan Pharmacia, Uppsala, Sweden) and exposing to X-Omat BT film (Kodak, New York, USA). Bands were analyzed by Quantity One Analysis Software.
PlexinA1 multiclonal antibody 1:50 was purchased from American Santa Cruz Co. Monoclonal antibody of factorVIII and monoclonal antibody of Ki-67 (ready to use) were supplied by the Chinese Boster Co. All operations were performed according to the instructions of the manufacturers. Positive specimens were used as positive controls and PBS in substitution of the first antibody was used as a negative control at the same time.
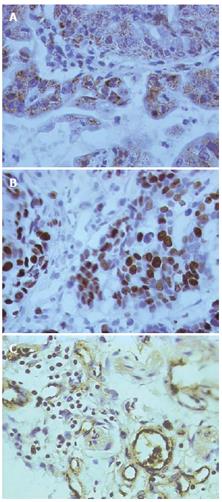
The cytoplasm and membrane of positive cells were stained brown by PlexinA1. The result of immunostaining was recorded as negative or positive based on the expression of protein detected. At least 10 surface areas were scored, and the percentage of positive cells was calculated for each specimen. Specimens were classified as positive if more than 30% of the cells were stained at ×400 magnification.
The nuclei of positive cells were stained deep brown by Ki-67. The number of positive cells among 1000 tumor cells was counted per slide and taken as the tumor cell proliferation index.
Gastric carcinoma vascular endothelial cells were stained brown. The isolated brown and yellow blood vessel endothelial cells or cell clusters in gastric carcinoma tissues were regarded as a single microvessel (Figure 1A). Areas with the highest microvessel densities were selected under × 100 microscopic magnification. Then the number of microvessels stained by facto VIII antibody in 5 vision fields was counted under × 400 magnification, respectively. The average values were taken as MVD[5]. Indistinguishable or indistinct cells were excluded.
All data were analyzed by SPSS12.0 statistical software. t test, analysis of Chi-square test, and linear correlation analysis were used. P < 0.05 was taken as significant.
Two percent of Agarose gel electrophoresis showed a 172 bp PlexinA1 fragment by RT-PCR amplification from gastric caner specimens and normal gastric mucosa (Figure 2). The PlexinA1 mRNA amplification was successful in all tissues. The expression level was higher in tumor (0.71 ± 0.37) than in normal gastric mucosa (0.60 ± 0.25, P = 0.0299 < 0.05).
The affinity-purified anti-plexinA1 antibody detected a major band at 90 kD in protein extracts from all samples tested (Figure 3). The expression level was much higher in tumor (0.47 ± 0.16) than that in normal gastric mucosa (0.21 ± 0.08, P < 0.01). This result is matched with that of RT-PCR.
In immunohistochemical staining, PlexinA1 located at the membrane and cytoplasm of gastric carcinoma cells appeared as brown particles. The positive expression rate of PlexinA1 in gastric carcinoma was 52%, significantly higher than that in normal gastric mucosa (25%), (P < 0.05. However, the PlexinA1 expression level had no correlation with the age of patients, tumor size, invasion depth, differentiation degree and lymph node metastasis (Table 1).
| Characteristics | PlexinA1 | Total | P value | |
| Negative | Positive | |||
| Age (yr) | ||||
| ≥ 45 | 12 | 14 | 26 | > 0.05 |
| < 45 | 12 | 12 | 24 | |
| Size | ||||
| ≥ 5 cm | 8 | 11 | 19 | > 0.05 |
| < 5 cm | 16 | 15 | 31 | |
| Differentiation | ||||
| Well-moderate | 13 | 10 | 23 | > 0.05 |
| Poorly | 11 | 16 | 27 | |
| Invasion depth | ||||
| Lamina and muscularis propria | 5 | 6 | 11 | > 0.05 |
| Visceral peritoneum | 19 | 20 | 39 | |
| Lympth node metatasis | ||||
| Negative | 10 | 12 | 22 | > 0.05 |
| Positive | 14 | 14 | 28 | |
PlexinA1 mRNA and protein expression ratio of gastric carcinoma and the corresponding normal gastric mucosa (T/N) was significantly correlated with proliferation index of tumor cells in gastric carcinoma. Proliferation index of tumor cells within tumorous tissues increased significantly with PlexinA1 mRNA expression (r = 0.5420, P = 0.014 < 0.05) and PlexinA1 protein expression (r = 0.5024, P = 0.024 < 0.05). The proliferation index of the PlexinA1 positive staining group (567.69 ± 125.61) was significantly higher than that of the negative group (369.58±116.88), (P < 0.01).
PlexinA1 mRNA and protein expression ratio of gastric carcinoma and the corresponding normal gastric mucosa (T/N) was significantly correlated with the MVD of gastric carcinoma. MVD was elevated with the increase of PlexinA1 mRNA and protein expression. MVD within tumorous tissues increased significantly with PlexinA1 mRNA expression (r = 0.8736, P < 0.01) and PlexinA1 protein expression (r = 0.7286, P < 0.01). Also, with increase of PlexinA1 expression of gastric carcinoma tissues by immunohistochemistry, MVD rose as well. The MVD of the PlexinA1 positive staining group (25.25 ± 3.93) was significantly higher than that of the negative group (19.56 ± 1.75), (P < 0.01).
PlexinA1 is a large transmembrane protein that is receptor of Semaphorins, either alone or in combination with neuropilin-1 or -2[1,6]. Despite the fact that PlexinA1 plays a crucial role in formation of the nervous system, increasing evidence attested to the significance of PlexinA1 in cardiogenesis. Some works reported that PlexinA1 has multiple functions in cardiogenesis as a receptor for the transmembrane Semaphorin, Sema6D, independent of neuropilins, and it plays a critical role in cardiac morphogenesis by regulating epithelial cell migration[2,7]. These findings suggest that PlexinA1 may regulate angiogenesis in vivo, and raise the intriguing possibility that PlexinA1 may play a role in tumor-induced angiogenesis.
As it is well known, the growth and metastasis of tumors require induction of angiogenesis[8,9]. Without the ability to induce angiogenesis, most neoplasms would fail to grow > 2 mm in diameter or metastasize, including gastric carcinoma. We detected the mRNA and protein expression of PlexinA1 in gastric carcinoma and normal gastric mucosa by the semi-quantity RT-PCR and Western blotting. The results showed that the expression of PlexinA1 in gastric carcinoma was significantly higher than those in normal gastric mucosa. We further detected the expression of PlexinA1 in gastric carcinoma and normal gastric mucosa by immunohistochemistry. The positive expression rate of PlexinA1 in gastric carcinoma was significantly higher than that in normal gastric mucosa. However, the PlexinA1 expression level was not correlated with the age of patients, tumor size, invasion depth, differentiation degree, and lymph node metastasis. These results suggest that PlexinA1 may play an important role in the occurrence and development of gastric carcinoma. MVD and Ki-67 are important index of judging tumor angiogenesis and tumor cell proliferation[10-12].
Under the effect of angiogenesis and proliferation factors, tumor and endothelial cells proliferate and migrate to form new blood vessel networks and induce tumor invasion and metastasis. This study showed that MVD within tumor tissues increased significantly with PlexinA1 mRNA and protein expression, and PlexinA1 expression level of cancer tissues was positively correlated with Ki-67. These results suggest that PlexinA1 may contribute to tumor angiogenesis and tumor cell proliferation through binding its ligands named Semaphorins.
In conclusion, this is the first investigation about the expression of PlexinA1 in gastric carcinoma and its clinicopathological significance. The results of our study shed some light on the pathogenesis of gastric carcinoma, and may represent a new therapeutic target for gastric carcinoma treatment.
Primary gastric carcinoma is one of the most common malignant tumors in China. Invasion and metastasis are the main causes for the death of cancer patients, and invasion and metastasis of malignant tumors are closely related with proliferation of tumor cells and angiogenesis of tumor tissues. PlexinA1 is a large transmembrane protein that is a major receptor for multiple classes of Somaphorins, and has pleiotropic function in formation of nervous systems, embryogenesis, angiogenesis and immunoreaction, yet the function of PlexinA1 in carcinogenesis has not been intensively studied, including in gastric carcinoma.
Experiments have been employed to study the expression of PlexinA1 in gastric carcinoma and its relationship with tumor angiogenesis and proliferation. These studies show that the expression level in gastric carcinoma is higher than that in normal gastric mucosa, and is positively related to tumor angiogenesis and proliferation.
This is the first investigation about the expression of PlexinA1 in gastric carcinoma and its clinicopathological significance. PlexinA1 is found positively related to tumor angiogenesis and proliferation, which shed some light on the pathogenesis of gastric carcinoma.
This study may represent a new therapeutic target for gastric carcinoma treatment.
The authors studied the expression of PlexinA1 in gastric carcinoma and its relationship with tumor angiogenesis and proliferation, and showed that the expression level in gastric carcinoma is higher than that in normal gastric mucosa, and is positively related to tumor angiogenesis and proliferation, which may be useful in basic research of gastric carcinoma, and provide a new thought about gastric carcinoma treatment.
| 1. | Pasterkamp RJ, Verhaagen J. Emerging roles for semaphorins in neural regeneration. Brain Res Brain Res Rev. 2001;35:36-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 100] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Suto F, Kamei J, Aoki K, Yabuki M, Hori M, Fujisawa H. Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev. 2004;18:435-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 238] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 3. | Perälä NM, Immonen T, Sariola H. The expression of plexins during mouse embryogenesis. Gene Expr Patterns. 2005;5:355-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Takegahara N, Takamatsu H, Toyofuku T, Tsujimura T, Okuno T, Yukawa K, Mizui M, Yamamoto M, Prasad DV, Suzuki K. Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat Cell Biol. 2006;8:615-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 210] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 5. | Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4014] [Cited by in RCA: 4111] [Article Influence: 117.5] [Reference Citation Analysis (0)] |
| 6. | Murakami Y, Suto F, Shimizu M, Shinoda T, Kameyama T, Fujisawa H. Differential expression of plexin-A subfamily members in the mouse nervous system. Dev Dyn. 2001;220:246-258. [PubMed] |
| 7. | Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Yabuki M, Harada K, Hori M, Kikutani H. Guidance of myocardial patterning in cardiac development by Sema6D reverse signalling. Nat Cell Biol. 2004;6:1204-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 175] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5621] [Cited by in RCA: 5546] [Article Influence: 178.9] [Reference Citation Analysis (0)] |
| 9. | Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2432] [Cited by in RCA: 2498] [Article Influence: 108.6] [Reference Citation Analysis (0)] |
| 10. | Salvesen HB, Iversen OE, Akslen LA. Identification of high-risk patients by assessment of nuclear Ki-67 expression in a prospective study of endometrial carcinomas. Clin Cancer Res. 1998;4:2779-2785. [PubMed] |
| 11. | Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3536] [Cited by in RCA: 3581] [Article Influence: 96.8] [Reference Citation Analysis (0)] |
| 12. | Kraizer Y, Mawasi N, Seagal J, Paizi M, Assy N, Spira G. Vascular endothelial growth factor and angiopoietin in liver regeneration. Biochem Biophys Res Commun. 2001;287:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
S- Editor Zhu LH L- Editor Ma JY E- Editor Lu W