Published online Dec 28, 2007. doi: 10.3748/wjg.v13.i48.6506
Revised: May 12, 2007
Accepted: May 26, 2007
Published online: December 28, 2007
AIM: To investigate the inhibitory effect and possible mechanism of action of schisandrin B in SC-B on gastric cancer cells in vitro.
METHODS: SC-B consisted of schisandrin B, aloe-emodin, and Astragalus polysaccharides. Exponentially growing human gastric cancer SGC-7901 cells were divided into six treatment groups: (1) control group (RPMI 1640 medium); (2) negative control group (2% DMSO); (3) positive control group (50 mg/L 5-Fluorouracil, 5-FU); (4) low-dose group (LSC, final concentration of schisandrin B, 25 mg/L); (5) moderate-dose group (MSC, final concentration of schisandrin B, 50 mg/L); (6) high-dose group (HSC, final concentration of schisandrin B, 100 mg/L). Follow-up was done at 12-48 h. An MTT (Methylthiazolyldiphenyl-tetrazolium bromide) assay was used to examine the inhibitory effect of SC-B on gastric cancer cells. The mitosis index was assessed using an inverted microscope. Flow cytometry was used to visualize the cell cycle. An RT-PCR (Reverse transcription-Polymerase chain reaction) -based assay was used to detect mRNA expression for cyclin D1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
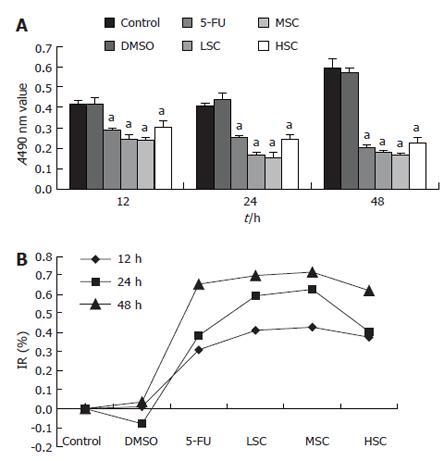
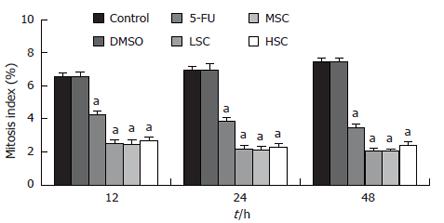
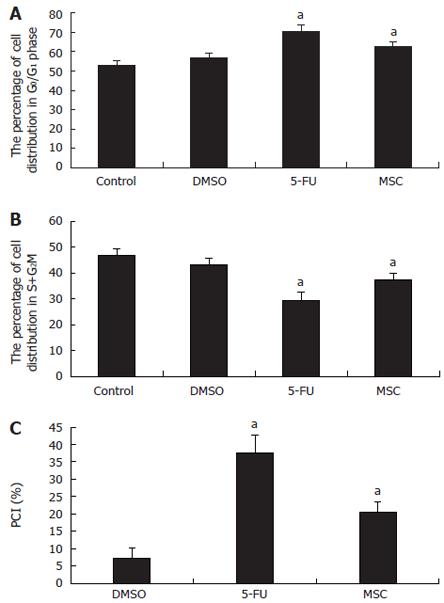
RESULTS: The MTT assay showed that the number of living cells in the LSC, MSC and HSC groups was significantly smaller than that in the DMSO-treated group (P < 0.05) at 12-48 h. The inhibitory rate (IR) of the LSC group was 41.15% ± 3.86%, 59.24% ± 5.34% and 69.93% ± 7.81% at 12, 24 and 48 h, respectively. The IR of the MSC group was 42.82% ± 4.94%, 62.68% ± 7.58% and 71.79% ± 8.12% at 12, 24 and 48 h, respectively. The IR of the HSC group was 37.50% ± 3.21%, 40.34% ± 2.98% and 61.99% ± 4.88% at 12, 24 and 48 h, respectively. These results suggested that a moderate dosage had the most obvious inhibitory efficacy at 48 h. Compared to the DMSO group, the mitosis index of the LSC, MSC, HSC groups was greatly decreased (P < 0.05) at all time points. Any dose of SC-B suppressed mitosis within 12-48 h. Compared to the DMSO group, the percentage of cells in the G0/G1 phase of the MSC group was greatly increased, and that of the S + G2M phase was greatly decreased, while the percentage of cell inhibition (PCI) in the MSC group was greatly increased (P < 0.05). This suggested that SC-B could exclusively arrest cells in the G0/G1 phase. Cyclin D1 mRNA expression was lower in the MSC group than that in the DMSO group (P < 0.05).
CONCLUSION: SC-B can inhibit the proliferation and aberrant mitosis of human gastric cancer SCG-7901 cells in vitro. This inhibitory effect may be due to the down-regulation of cyclin D1 mRNA expression, which causes cell cycle arrest of gastric cancer cells.
-
Citation: Liu XN, Zhang CY, Jin XD, Li YZ, Zheng XZ, Li L. Inhibitory effect of schisandrin B on gastric cancer cells
in vitro . World J Gastroenterol 2007; 13(48): 6506-6511 - URL: https://www.wjgnet.com/1007-9327/full/v13/i48/6506.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i48.6506
Gastric cancer is one of the most prevalent tumors. In China, its incidence and death rate are above those for all other tumors[1]. Therefore, the development of drugs for the treatment of gastric cancer is of great importance. Traditional Chinese medicines and their effective components have certain antitumor effects. This study investigated the inhibitory effect and mechanism of action of SC-B (consisting of schisandrin B, aloe-emodin and Astragalus polysaccharides) on the SGC-7901 cell line in vitro.
Schisandrin B and aloe-emodin were purchased from the National Institute for the Control of Pharmaceutical and Biological Products. Astragalus polysaccharides (UV > 70%) were from East Plant Health Protection. MTT, trypsin (1:250), fetal bovine serum and DMSO were from Sigma (St. Louis, MO, USA). RPMI 1640 medium and Trizol reagent were from Gibco/BRL (Gaithersburg, MD, USA). Oligo-dT primers, reverse transcriptase (AMV) and Taq polymerase were all from Promega (Madison, WI, USA).
Human gastric cancer cells (SGC-7901) were purchased from the Department of Cellular and Molecular Biology, Shanghai Institute of Biochemistry and Cell Biology, Academia Sinica. Cells were cultured in MEM containing 10% heated-inactivated fetal calf serum and PNS (100 U/mL penicillin and 10 mL/L streptomycin), at 37°C, in a humidified atmosphere of 95% O2/5% CO2. Cells were sub-cultured every 2 or 3 d.
Cells were classified into the following groups: control group (treated with RPMI 1640 medium); negative control group (treated with DMSO; final concentration, 2%); positive control group (treated with 5-FU; final concentration, 50 mg/L), low-dose group (LSC, containing 25 mg/L schisandrin B); moderate-dose group (MSC, containing 50 mg/L schisandrin B); and high-dose group (HSC, containing 100 mg/L schisandrin B). Note that SC-B consisted of schisandrin B, aloe-emolin and Astragalus polysaccharides at a ratio of 2:1:1.
Exponentially growing SGC-7901 cells were digested by 0.25% trypsin for 1-2 min, and washed three times with PBS. RPMI 1640 medium containing 10% newborn bovine serum was added to obtain a cell density of 5 × 108 cells/L. Final cell suspensions (100 μL) were placed in 96-well plates in an incubator containing 5% CO2, and incubated at 37°C for 24 h. Then, 100 μL RPMI 1640 medium containing different concentrations of schisandrin B was added to each plate. The effects of schisandrin B were assessed using six replicates for each concentration of schisandrin B. Cells were cultured for 12, 24 and 48 h. The culture medium was changed every 24 h. Four hours before the end of the culture, 20 μL 5 g/L MTT was added to the wells. Optical density (OD) values for each well were measured at 490 nm using an enzyme-linked immunosorbent assay meter. Inhibitory rate (IR) was calculated according to the formula: IR = [1 - (mean of treated group)/(mean of control group)] × 100%.
Cellular mitosis was observed using an inverted microscope, and the proportion of mitotic cells in 1000 cells was calculated after SC-B was added to the plates. The drug concentrations and incubation times were the same as those used in the MTT experiment.
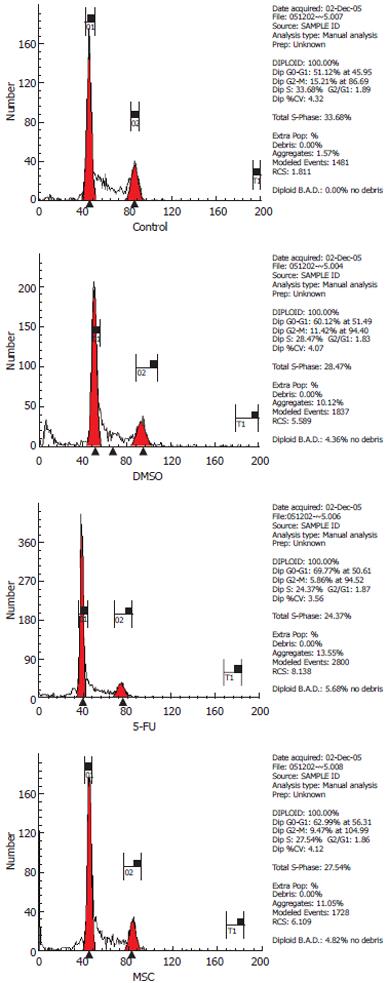
Exponentially growing SGC-7901 cells were used for this experiment, and the cell density was adjusted to 3 × 108 cells/L by adding RPMI 1640 medium containing 10% newborn bovine serum, to obtain a 2 mL final volume in each six-well plate. Six-well plates were placed at 37°C in an incubator containing 5% CO2 for 24 h. The culture medium was changed 24 h later. Once the 2 mL final cell suspensions (containing SC-B with a final concentration of schisandrin B of 50 mg/L) were added, the cells were left to incubate for 48 h. Finally, samples were processed to obtain simple cell suspensions (target cell number was > 5 × 109 cells/L), which were fixed with 70% cold ethanol for 24 h, after centrifugation for 5 min at 200 ×g. The cells were further centrifuged for 15 min at 200 ×g, and then resuspended in 0.5 mL PBS. Then, they were digested by Rnase-A (final concentration, 50 mg/L) for 30 min at 37°C, and stained with propidium iodide (final concentration, 65 mg/L) for 30 min. Finally, cell suspensions were analyzed for the cell cycle using a nylon monofilament mesh screen with 44-μm openings and flow cytometry. The percentage of cell inhibition (PCI) was calculated according to the formula: PCI = [(Percentage of S + G2M in DMSO group - Percentage of S + G2M in treatment group) / Percentage of S+G2M in DMSO group] × 100%.
The methods for cell culture, including drug concentrations and incubation times were the same as those for the cell cycle analysis.
Cells in each six-well plate were homogenized in 1 mL Trizol and 0.2 mL chloroform, and the mixture was placed on ice for 5 min. After sedimentation by centrifugation at 12 000 ×g at 4°C, RNA was precipitated by combining the aqueous phase with an equal volume of isopropanol. The precipitate was recovered by centrifugation (12 000 ×g at 4°C), washed once with 75% ethanol, and solubilized in diethylpyrocarbonate (DEPC)-treated water. RNA concentrations were measured with a UV spectrometer. RNA/DNA (A260/A280) ratio had to be > 1.8.
Each RT reaction consisted of 1 μg extracted RNA, 1 μL 10 × RT buffer, 4 μL dNTPs (10 mmol/L), 0.5 μL oligo-dT primers, 1 μL AMV, and 0.25 μL RNase inhibitor, and DEPC-treated water was added to obtain a final reaction volume of 10 μL. RT was initially performed at 95°C for 3 min, and placed on ice. Other reactants were added to the reaction system, and the mixture was further incubated at 42°C for 70 min, and held at 95°C for 3 min. Samples were frozen for later use.
Each PCR reaction included 10 μL 5 × PCR Buffer, 1.6 μL dNTPs, 0.5 μL of a 25 μmol/L solution of each primer, 0.25 μL Taq DNA polymerase, 10 μL RT products, and DEPC-treated water. The total reaction volume was 40 μL. PCR reaction conditions are shown in Table 1. The amplified products were visualized by electrophoresis on a 1.5% agarose gel, stained with 1 mg/L ethidium bromide, and illuminated and analyzed with a UVP gel imaging system (BIO-PRO, Carlsbad, CA, USA).
| mRNA | Primer (5’-3’) | Primer size (bp) | PCR conditions |
| CyclinD1 | F: TGGATGCTGGAGGTCTGCGAGGAA | 573 | 95°C/3 min; |
| R: GGCTTCGATCTGCTCCTGGCAGGC | |||
| GAPDH | F: GAAGGTGAAGGTCGGAGT | 320 | (94°C/30 s; 55°C/30 s; 72°C/60 s) 35 cycles |
| R: GA AGATGGTGATGGGATTTA |
Data were processed using SPSS10.0 software. One-way ANOVA analysis was used to examine the differences between the groups.
Our results showed that the living cell numbers in the LSC, MSC, HSC groups were significantly smaller than that in the DMSO group (P < 0.05), as measured by the MTT assay, at 12, 24 and 48 h. The IR of the LSC group was 41.15% ± 3.86%, 59.24% ± 5.34%, and 69.93% ± 7.81% at 12, 24 and 48 h, respectively. The IR of the MSC group was 42.82% ± 4.94%, 62.68% ± 7.58% and 71.79% ± 8.12% at 12, 24 and 48 h, respectively. The IR of the HSC group was 37.50% ± 3.21%, 40.34% ± 2.98% and 61.99% ± 4.88% at 12, 24 and 48 h, respectively. These results suggested that a moderate dosage of SC-B had the most obvious inhibitory effect at 48 h (Figure 1).
Compared to the DMSO group, the mitosis index of the LSC, MSC, HSC groups was greatly decreased (P < 0.05) at different time points. This suggested that SC-B could suppress mitosis of SGC-7901 cells treated with the three doses within 12 to 48 h (Figure 2).
Compared to the DMSO group, in the MSC group, the number of cells in the G0/G1 phase was greatly increased (62.59% ± 2.42% vs 56.76% ± 2.56%), but the number in the S + G2M phase was greatly decreased (37.42% ± 2.42% vs 43.25% ± 2.56%). The PCI of the MSC group was greatly increased compared to the DMSO group (20.42% ± 3.11% vs 7.29% ± 2.83%, P < 0.05). This suggested that SC-B could exclusively arrest cells in the G0/G1 phase (Figures 3 and 4).
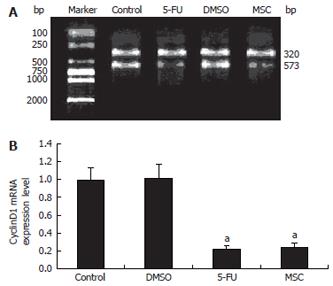
GAPDH mRNA was used as the standard for PCR (320 bp). The ratio of cyclin D1 mRNA to GAPDH mRNA was used to quantify the expression level of cyclin D1 mRNA (573 bp). The results showed that cyclin D1 mRNA expression in the MSC group was lower than that in the DMSO group (P < 0.05) (Figure 5).
Schisandrin B, extracted from the fruit of Schisandra chinensis Baill, has been reported to have antitumor effects. For example, schisandrin B can inhibit the proliferation of human hepatoma SMMC-7721 cells and induce apoptosis, involving the caspase-3-dependent and caspase-9-independent pathways, accompanied by down-regulation of Hsp70 protein expression at an early stage[2]. Schisandrin B enhances doxorubicin-induced apoptosis of SMMC7721 cells and MCF-7, a human breast cancer cell line, but not normal cells. This enhancement is associated with the activation of caspase 9[3]. Schisandrin B inhibits DNA synthesis in ascitic hepatoma cells[4]. Aloe-emodin is purified from Aloe vera leaves. Aloe-emodin modulates protein kinase C isozymes, inhibits proliferation, and induces apoptosis in U-373MG glioma cells[5]. The aloe-emodin-induced increase in the amount of proforms and fragments of nucleophosmin in the cytoplasm may be one of the important events for aloe-emodin-induced H460 cell apoptosis[6]. All this information supports the hypothesis that aloe-emodin represents a novel antitumor chemotherapeutic drug. Astragalus mongholicus polysaccharides have been isolated from one of the Chinese herbs, and are known to have various immunomodulatory activities[7]. We have previously shown that schisandrin B and aloe-emodin clearly inhibit the multiplication of human SGC-7901 cells within 12 to 48 h, and we have found that the most effective concentration ratio of schisandrin, aloe-emodin and Astragalus polysaccha-rides in SC-B is 2:1:1. The inhibitory effect of SC-B is significantly higher than that of schisandrin, aloe-emodin and Astragalus polysaccharides on their own[8].
This study investigated the inhibitory effect and mechanism of SC-B on the SGC-7901 gastric cancer cell line in vitro. We showed that SC-B (25-100 mg/L) clearly inhibited the multiplication and mitosis of human gastric cancer SGC-7901 cells within 12-48 h, but a dose of 50 mg/L SC-B had the strongest inhibitory effect at 48 h. Therefore, when we further investigated the possible mechanisms of SC-B, we decided to use the moderate concentration (50 mg/L) and the 48-h time point.
The cell cycle consists of the G1 phase (growth and preparation of the chromosomes for replication), S phase (synthesis of DNA), G2 phase (preparation for mitosis), and M phase (mitosis). A eukaryotic cell cannot divide into two, and the resulting two cells cannot divide into four, unless two processes are alternated. These include the doubling of the DNA genome in the S phase (synthesis phase), and the halving of this genome during mitosis (M phase). The period between M and S is called the G1 phase, and the period between S and M is the G2 phase. Sometimes, a cell leaves the cell cycle temporarily or permanently. It exits the cycle at G1, and enters a stage designated G0. G0 represents the absence of signals for mitosis, and an active repression of the genes needed for mitosis. Cancer cells cannot enter G0, and are destined to repeat the cell cycle indefinitely[9]. If some mechanism can prevent S phase cancer cells from entering the M phase, then the infinite growth of cancer cells could be controlled. In our study, exponentially growing cells were used to analyze the changes in the cell cycle. We showed that SC-B (50 mg/L) exclusively arrested cells in the G0/G1 phase compared to the DMSO group. The number of cells in the G0/G1 phase in the MSC group was greatly increased (P < 0.05 vs DMSO group), which suggests that SC-B (50 mg/L) can prevent G1 phase cancer cells from entering the M phase.
Quality controls of the cell cycle are cell-cycle checkpo-ints. Some key checkpoints are: G1 checkpoint (to assess DNA damage before the cells enter S phase); S checkpoint (to monitor the presence of the Okazaki fragments on the lagging strand during DNA replication); G2 checkpoint (during S phase, and after DNA replication); and M checkpoint (to detect any failure of spindle fibers to attach to the kinetochores, and arrest the cell in metaphase)[10-14]. Checkpoints can become activated due to DNA damage, exogenous stress signals, defects during DNA replication, or failure of the chromosomes to attach to the mitotic spindle[15]. Whether the cell cycle starts to allow the cells to proliferate depends on the G1 checkpoint, which determines if the cells enter the S phase. The cell cycle is controlled by proteins in the cytoplasm. One of the main players in animal cells is cyclin D1. This is one of the important regulative factors that can induce cells from G1 to enter the S phase. Increased amounts of cyclin D1 bind to cyclin-dependent kinases 4/6, which is the signal to prepare the chromosomes for replication[16,17]. RT-PCR was used to measure cyclin D1 mRNA expression in this study. The results showed that cyclin D1 mRNA expression was significantly reduced in the MSC group (50 mg/L) compared to the DMSO group (P < 0.05). This suggested that SC-B suppressed the multiplication and aberrant mitosis of human gastric cancer SGC-7901 cells by reducing cyclin D1 mRNA expression, which caused the cell cycle to arrest by inhibiting the G1 checkpoint (i.e. by preventing gastric cancer cells from G1 entering the S phase).
Gastric cancer is one of the most prevalent malignant tumors, and is classified as one of the first and most severe form of cancer harming people’s health. Therefore, the exploitation of drugs to treat gastric cancer is of great significance. Traditional Chinese medicines and their effective components have certain antitumor effects.
Schisandrin B, a dibenzocyclooctadiene derivative was isolated from the fruit of Schisandra chinensis. Many studies showed that schisandrin B has anticancer effects. Now, studies on the application of Schisandrin B in the preparation of anticancer medications, particularly for the treatment of multidrug resistant (MDR) cancer are hot research topics. Schisandrin B effectively reverses MDR cancer in combination with other anticancer chemotherapeutic agents.
Many other researches have revealed that Schisandrin B has anticancer effects, but Schisandrin B is cytotoxic to human cancer cells, which restricts its application in cancer chemotherapy. In this study, we successfully prepared SC-B for human use by mixing schisandrin B with aloe-emodin and Astragalus polysaccharides. The anticancer effect of SC-B was greatly increased compared with that of Schisandrin B on its own. In this study, we observed the influence of SC-B on living cell numbers, mitosis index, cell cycle, and CyclinD1 expression in SGC-7901 cells. The results suggested that SC-B could suppress the multiplication and aberrant mitosis of human gastric cancer SGC-7901 cells by reducing CyclinD1 mRNA expression, causing cell-cycle arrest by inhibiting the G1 checkpoint.
SC-B could be applied in the treatment of gastric cancer, and might provide a potential therapeutic anticancer drug.
Schisandrin B: Schisandrin B is a dibenzocyclooctadiene derivative isolated from the fruit of Schisandra chinensis and Schisandra chinensis, a woody vine which bears numerous clusters of tiny, bright red berries; Cell cycle: The cell cycle or cell-division cycle refers to the series of events that take place in a eukaryotic cell from its formation and the moment it replicates itself; Cyclins: Cyclins are a family of proteins involved in the progression of cells through the cell cycle. The main players in animal cells are: G1 cyclin (cyclin D), S-phase cyclin (cyclins E and A), and mitotic cyclins (cyclins B and A).
This is a good descriptive report on the potential chemotherapeutic effects of schisandrin B in gastric cancer, in vitro.
| 1. | Xu B, Wang JM. Epidemiologic research on gastric cancer. Aizheng Jinzhan. 2006;13:1-7. |
| 2. | Wu YF, Cao MF, Gao YP, Chen F, Wang T, Zumbika EP, Qian KX. Down-modulation of heat shock protein 70 and up-modulation of Caspase-3 during schisandrin B-induced apoptosis in human hepatoma SMMC-7721 cells. World J Gastroenterol. 2004;10:2944-2948. [PubMed] |
| 3. | Li L, Lu Q, Shen Y, Hu X. Schisandrin B enhances doxorubicin-induced apoptosis of cancer cells but not normal cells. Biochem Pharmacol. 2006;71:584-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Liu LS, Wang Q, Zheng RL, Sun XF, Zhang LD. Effects of γ-Schisandrin on immunity, DNA, synthesis and survival in mice bearing ascitic hepatoma. Lanzhou Daxue Xuebao. 1990;26:55-59. |
| 5. | Acevedo-Duncan M, Russell C, Patel S, Patel R. Aloe-emodin modulates PKC isozymes, inhibits proliferation, and induces apoptosis in U-373MG glioma cells. Int Immunopharmacol. 2004;4:1775-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Lee HZ, Wu CH, Chang SP. Release of nucleophosmin from the nucleus: Involvement in aloe-emodin-induced human lung non small carcinoma cell apoptosis. Int J Cancer. 2005;113:971-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Shao P, Zhao LH, Zhi-Chen JP. Regulation on maturation and function of dendritic cells by Astragalus mongholicus polysaccharides. Int Immunopharmacol. 2006;6:1161-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Fu ZL, Liu XN, Zhang CY, Jin XD, Zheng XZh. Inhibition Effect of Aloeemodin on Human Gastric Cancer Cell Line SGC-7901. Mudanjiang Yixueyuan Xuebao. 2006;27:8-11. |
| 9. | Bruce Alberts, Alexander Johnson, Julian Lewis, Martin Raff, Keith Roberts, Peter Walter. Molecular Biology of the Cell. 4th ed. London: Garland Science 2002; 968. |
| 10. | Andreassen PR, Ho GP, D'Andrea AD. DNA damage responses and their many interactions with the replication fork. Carcinogenesis. 2006;27:883-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Baker DJ, Chen J, van Deursen JM. The mitotic checkpoint in cancer and aging: what have mice taught us. Curr Opin Cell Biol. 2005;17:583-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol. 2006;24:1770-1783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 794] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 13. | Deep G, Singh RP, Agarwal C, Kroll DJ, Agarwal R. Silymarin and silibinin cause G1 and G2-M cell cycle arrest via distinct circuitries in human prostate cancer PC3 cells: a comparison of flavanone silibinin with flavanolignan mixture silymarin. Oncogene. 2006;25:1053-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 126] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Vigneron A, Roninson IB, Gamelin E, Coqueret O. Src inhibits adriamycin-induced senescence and G2 checkpoint arrest by blocking the induction of p21waf1. Cancer Res. 2005;65:8927-8935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Giono LE, Manfredi JJ. The p53 tumor suppressor participates in multiple cell cycle checkpoints. J Cell Physiol. 2006;209:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 222] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 16. | Saab R, Bills JL, Miceli AP, Anderson CM, Khoury JD, Fry DW, Navid F, Houghton PJ, Skapek SX. Pharmacologic inhibition of cyclin-dependent kinase 4/6 activity arrests proliferation in myoblasts and rhabdomyosarcoma-derived cells. Mol Cancer Ther. 2006;5:1299-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Marzec M, Kasprzycka M, Lai R, Gladden AB, Wlodarski P, Tomczak E, Nowell P, Deprimo SE, Sadis S, Eck S. Mantle cell lymphoma cells express predominantly cyclin D1a isoform and are highly sensitive to selective inhibition of CDK4 kinase activity. Blood. 2006;108:1744-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
S- Editor Ma N L- Editor Kerr C E- Editor Ma WH