Published online Dec 21, 2007. doi: 10.3748/wjg.v13.i47.6410
Revised: September 13, 2007
Accepted: November 10, 2007
Published online: December 21, 2007
AIM: Consecutive monitoring of intragastric pH using the Bravo® capsule.
METHODS: We put threads through a Bravo® capsule and then affixed it to the gastric wall by endoscopic hemoclipping in seven subjects. Study data were uploaded to a computer via Datalink every 48 h. In this way, repeated monitoring of intragastric pH was undertaken.
RESULTS: All subjects were able to monitor gastric pH over a 1-wk period, and five for > 2 wk. No complications were encountered during the monitoring. After pH monitoring, we safely retrieved the capsule endoscopically.
CONCLUSION: Clipping a Bravo® capsule onto the gastric wall enabled long-term intragastric pH monitoring. This is a methodological report of pH monitoring over a period of > 2 wk.
- Citation: Ono S, Kato M, Ono Y, Asaka M. New method for long-term monitoring of intragastric pH. World J Gastroenterol 2007; 13(47): 6410-6413
- URL: https://www.wjgnet.com/1007-9327/full/v13/i47/6410.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i47.6410
The Bravo® pH monitoring system is a catheter-less system. It has been reported to be useful clinically because it enables monitoring without pain while the subject undertakes daily activities[1-5]. The Bravo® capsule is attached to the esophageal mucosa by suction via the delivery system, and it is usually eliminated spontaneously within 1 wk[2]. According to manufacturer Medtronic, the average life of a capsule’s battery is 14 d, and consecutive pH monitoring is theoretically possible until the capsule battery goes flat. We used these characteristics of the Bravo® capsule to develop a new method for long-term monitoring of intragastric pH[6].
The subjects of this study were seven Helicobacter pylori-negative volunteers. The Bravo® pH monitoring system (Medtronic, Shoreview, MI, USA), nylon threads, flexible overtube® (Sumitomo Bakelite, Tokyo, Japan), and stainless steel hemoclips (HX-200L-135; Olympus Optical, Tokyo, Japan) were prepared.
First, we removed the Bravo® capsule from its delivery system and we tied two nylon threads to the capsule and made each thread into a 2-cm diameter ring. Next, we confirmed a connection between the capsule and the receiver. The capsule was then calibrated in buffer solutions.
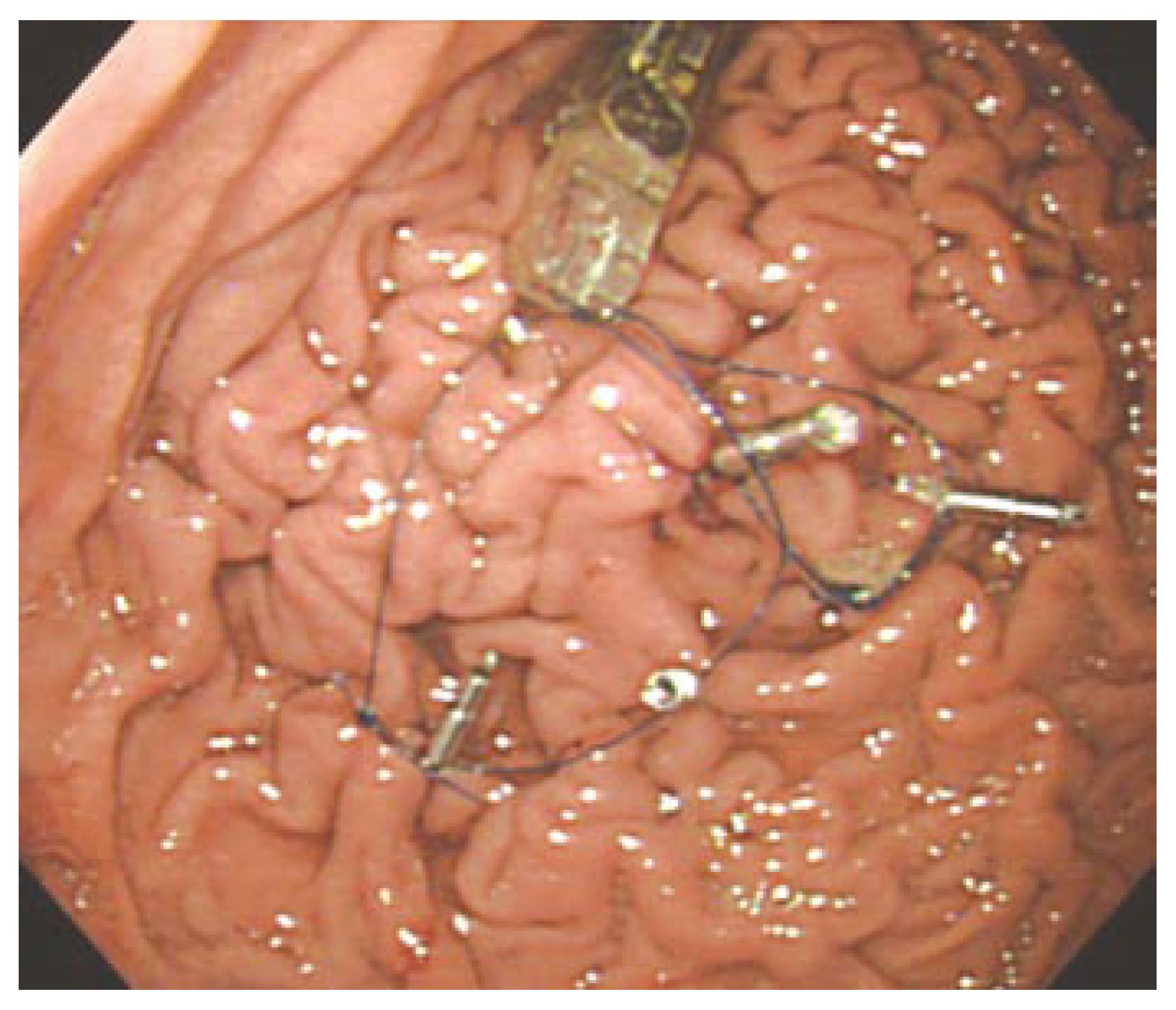
Endoscopic examinations with sedation were conducted on the subjects and results revealed the absence of any abnormal lesions. The flexible overtube was back-loaded onto the endoscope prior to the procedure. We carefully inserted the flexible overtube® with the tip of the endoscope inside the stomach. The Bravo® capsule was then passed through the tube into the stomach. Using hemoclips, the threads attached to the capsule were fixed on the greater curvature of the upper corpus (Figure 1).
We administered antacids to determine whether the Bravo® capsule precisely monitored changes in intragastric pH. The subject was administered 150 mg ranitidine hydrochloride orally twice daily at 8:00 and 20:00 h. No limitation of meals and activities was imposed but meal times were set at 7:00, 12:00 and 19:00 h. Alcohol and tobacco were prohibited.
Study data were uploaded to a computer via Datalink (Medtronic) every 48 h and analyzed using basic computer software (EXCEL; Microsoft, Redmond, WA, USA). After monitoring was completed, the fixed Bravo® capsules were removed endoscopically. We cut the threads tied to the capsule using scissors forceps (FS-3L-1; Olympus Optical), and put the capsule in the Roth Retrieval Net TM (United States Endoscopy Group, Mentor, OH, USA). Then, we retrieved the capsule by pulling it into an attachment mounted on the tip of the fiberscope.
This study was approved by our hospital ethics committee and informed consent for participation in the study was obtained from all subjects.
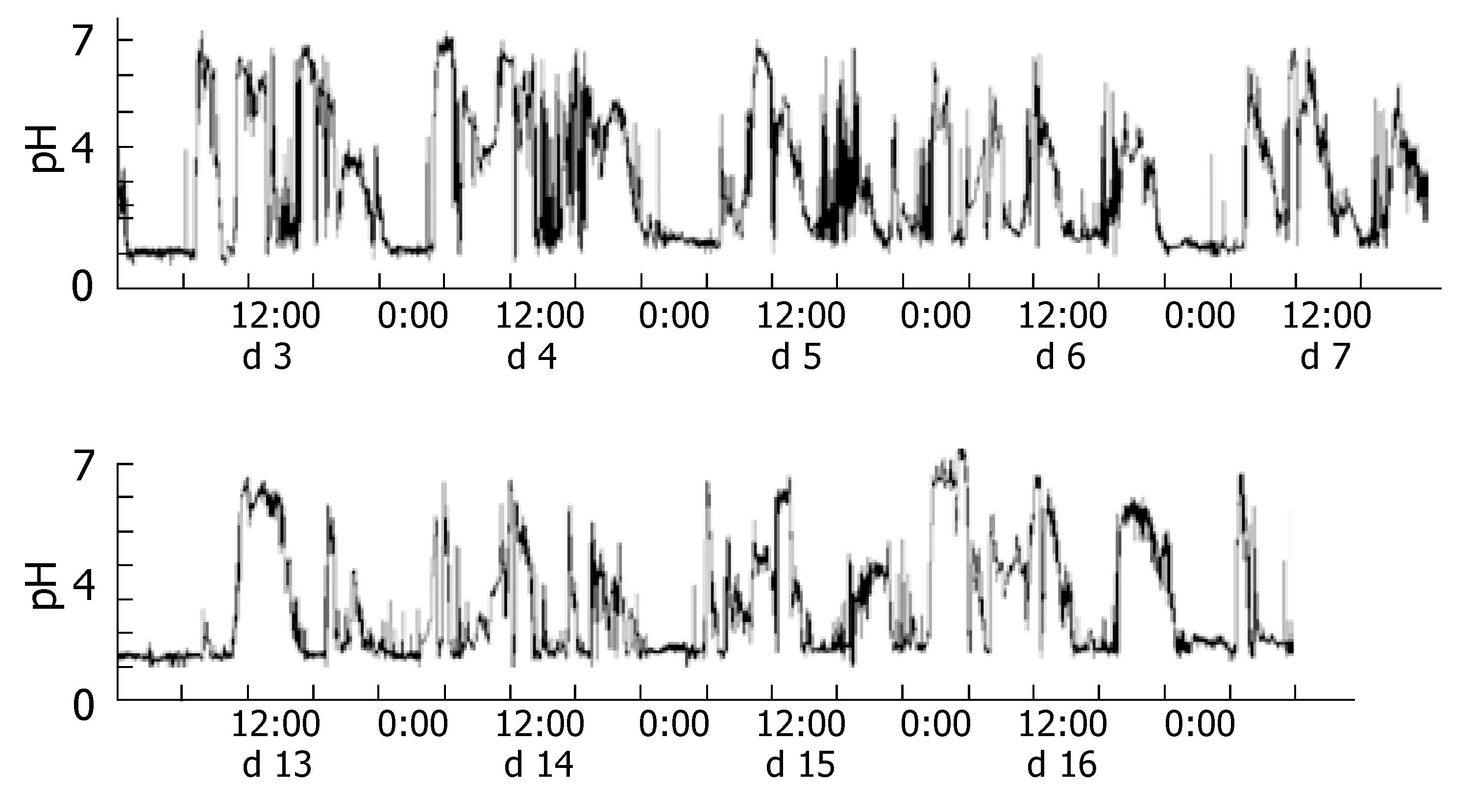
Placement of the Bravo® capsules took less than 5 min. No complications or side effects were observed during pH monitoring. All subjects were able to perform normal daily activities. Two of the seven subjects ended gastric pH monitoring because their capsule batteries went flat (at d 7 and 13). The other five succeeded in pH monitoring for 16 consecutive days. During this study, the average rate of captured data per day was 97.7%. A selection of the pH monitoring data is shown in Figure 2.
Gastric acid secretion was suppressed, beginning on the first day of ranitidine intake. However, during the medication period, the percentage time with pH < 4 increased and the median pH decreased. At the time of re-administration, the effect of the drug developed more slowly than at the first time it was given.
Retrieval of the Bravo® capsules took less than 5 min. No capsule suffered any damage.
Using the standard vacuum method, it is more difficult to attach the Bravo® capsule to a large lumen such as the stomach, than to the esophagus. It took us 15 min to place the capsule using the conventional method. The capsule was then eliminated on the fifth day. In previous studies of the Bravo® system, intragastric pH monitoring was carried out for just 48 h[7-9]. Since an endoscopic procedure and administration of premedication can influence gastric acid secretion[10], a longer period for pH monitoring would be helpful for diagnosis[11-13]. In our study, we were able to monitor gastric pH for at least 1 wk.
We had some difficulty in passing threads through the capsule beforehand, but we needed only about 5 min to affix the capsule under endoscopic guidance[7]. The Bravo® system has been reported to be safe[2,14,15], although there is a report of esophageal perforation during attachment of the capsule[16]. We used a flexible overtube® so that the capsule could pass safely through the cervical esophagus, a physiologic stricture. However, complications with the flexible overtube® have been reported[17], and so care must be taken when inserting it.
In addition, the precision of long-term pH monitoring using an antimony electrode fixed in stomach may create problems. However, in our study, pH rhythm was reflected by the effects of meals and medication, but the precision itself was not influenced. Some studies have suggested that the effects of histamine H2-receptor antagonists are attenuated during continuous treatment; an effect expressed as tolerance or tachyphylaxis[18-21]. Tachyphylaxis of ranitidine was observed in our study. The rates of captured data per day in the esophagus and stomach have been reported as 97.7% to 99.3%[7,14] and 98.3%[7], respectively. In our study, the rate was high and did not decrease over the 16-d period.
After monitoring for 16 d, we endoscopically retrieved the fixed capsules from the stomach. The Bravo® capsule attached to the esophagus by suction was eliminated spontaneously through the gastrointestinal tract. However, we confirmed that a capsule fixed to the stomach for a long period did not suffer any damage.
H2-receptor antagonists and proton-pump inhibitors rapidly and potently suppress gastric acid secretion and are widely used for treatment of acid-related diseases[22-27]. However, they have some clinical weaknesses, tachyphylaxis and nocturnal gastric acid breakthrough[28-30], which have not been resolved. One reason has been the availability of only intermittent pH monitoring, but not long-term continuous monitoring. Our method is useful clinically not only for the diagnosis of acid-related diseases, but also for the elucidation of their problems.
In conclusion, we present a newly developed method for easy and simple long-term monitoring of intragastric pH using the Bravo® pH monitoring system.
We thank Mrs. E Shouji (Department of Gastroenterology and Hematology, Hokkaido University Graduate School of Medicine) and Mr. T Iwasaki (Medical Care Support Division, Hokkaido University Hospital) for endoscopic support.
The Bravo® capsule is usually attached to esophageal mucosa by suction via the delivery system and eliminated spontaneously within 1 wk. However, consecutive pH monitoring is theoretically possible until the capsule battery goes flat.
It has been reported that an endoscopic procedure and administration of premedication can influence gastric acid secretion when using the Bravo® pH monitoring system. In previous studies of the Bravo® system using its conventional vacuum method of attachment, it was only possible to carry out intragastric pH monitoring for 48 h.
Clipping of a Bravo® capsule onto the gastric wall enabled intragastric pH monitoring for > 2 wk. The methodology is easy and simple.
Our method is clinically useful, not only for diagnosis of acid-related diseases, but also for the elucidation of tolerance of H2-receptor antagonists and nocturnal gastric acid breakthrough of proton-pump inhibitors.
Bravo® pH monitoring system is a catheter-less pH monitoring system.
The authors have reported a modified method for long-term monitoring of intragastric pH using the Bravo capsule fixed on the gastric wall by endoscopic hemoclipping. The contents of the manuscript are reasonable, and this may be a useful method for the elucidation of tolerance to H2-receptor antagonists and nocturnal gastric acid breakthrough of proton-pump inhibitors, as the authors state.
| 1. | Remes-Troche JM, Ibarra-Palomino J, Carmona-Sánchez RI, Valdovinos MA. Performance, tolerability, and symptoms related to prolonged pH monitoring using the Bravo system in Mexico. Am J Gastroenterol. 2005;100:2382-2386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Pandolfino JE, Richter JE, Ours T, Guardino JM, Chapman J, Kahrilas PJ. Ambulatory esophageal pH monitoring using a wireless system. Am J Gastroenterol. 2003;98:740-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 355] [Article Influence: 15.4] [Reference Citation Analysis (1)] |
| 3. | Gillies RS, Stratford JM, Booth MI, Dehn TC. Oesophageal pH monitoring using the Bravo catheter-free radio capsule. Eur J Gastroenterol Hepatol. 2007;19:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Pandolfino JE, Schreiner MA, Lee TJ, Zhang Q, Boniquit C, Kahrilas PJ. Comparison of the Bravo wireless and Digitrapper catheter-based pH monitoring systems for measuring esophageal acid exposure. Am J Gastroenterol. 2005;100:1466-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Ono S, Kato M, Ono Y, Nakagawa M, Shimizu Y, Takeda H, Asaka M. A new procedure for 16 consecutive days monitoring of intragastric pH. J Clin Gastroenterol. 2008;42:960. [PubMed] |
| 7. | Yamaguchi T, Seza A, Odaka T, Shishido T, Ai M, Gen S, Kouzu T, Saisho H. Placement of the Bravo wireless pH monitoring capsule onto the gastric wall under endoscopic guidance. Gastrointest Endosc. 2006;63:1046-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Pandolfino JE, Schreiner MA, Lee TJ, Zhang Q, Kahrilas PJ. Bravo capsule placement in the gastric cardia: a novel method for analysis of proximal stomach acid environment. Am J Gastroenterol. 2005;100:1721-1727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Mori M, Suzuki H, Masaoka T, Imaeda H, Nomoto Y, Hosoda H, Nishizawa T, Kangawa K, Hibi T. Intravenous ghrelin administration enhances gastric acid secretion - evaluation using wireless pH capsule. Aliment Pharmacol Ther. 2006;24 Suppl 4:96-103. |
| 10. | Ward EM, Devault KR, Bouras EP, Stark ME, Wolfsen HC, Davis DM, Nedrow SI, Achem SR. Successful oesophageal pH monitoring with a catheter-free system. Aliment Pharmacol Ther. 2004;19:449-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Bhat YM, McGrath KM, Bielefeldt K. Wireless esophageal pH monitoring: new technique means new questions. J Clin Gastroenterol. 2006;40:116-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Ahlawat SK, Novak DJ, Williams DC, Maher KA, Barton F, Benjamin SB. Day-to-day variability in acid reflux patterns using the BRAVO pH monitoring system. J Clin Gastroenterol. 2006;40:20-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Tseng D, Rizvi AZ, Fennerty MB, Jobe BA, Diggs BS, Sheppard BC, Gross SC, Swanstrom LL, White NB, Aye RW. Forty-eight-hour pH monitoring increases sensitivity in detecting abnormal esophageal acid exposure. J Gastrointest Surg. 2005;9:1043-1051; discussion 1051-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Wenner J, Johnsson F, Johansson J, Oberg S. Wireless oesophageal pH monitoring: feasibility, safety and normal values in healthy subjects. Scand J Gastroenterol. 2005;40:768-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Marchese M, Spada C, Iacopini F, Familiari P, Shah SG, Tringali A, Costamagna G. Nonendoscopic transnasal placement of a wireless capsule for esophageal pH monitoring: feasibility, safety, and efficacy of a manometry-guided procedure. Endoscopy. 2006;38:813-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Fajardo NR, Wise JL, Locke GR, Murray JA, Talley NJ. Esophageal perforation after placement of wireless Bravo pH probe. Gastrointest Endosc. 2006;63:184-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Holderman WH, Etzkorn KP, Patel SA, Harig JM, Watkins JL. Endoscopic findings and overtube-related complications associated with esophageal variceal ligation. J Clin Gastroenterol. 1995;21:91-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Wilder-Smith C, Halter F, Ernst T, Gennoni M, Zeyen B, Varga L, Roehmel JJ, Merki HS. Loss of acid suppression during dosing with H2-receptor antagonists. Aliment Pharmacol Ther. 1990;4 Suppl 1:15-27. [PubMed] |
| 19. | Nwokolo CU, Smith JT, Gavey C, Sawyerr A, Pounder RE. Tolerance during 29 days of conventional dosing with cimetidine, nizatidine, famotidine or ranitidine. Aliment Pharmacol Ther. 1990;4 Suppl 1:29-45. [PubMed] |
| 20. | Lachman L, Howden CW. Twenty-four-hour intragastric pH: tolerance within 5 days of continuous ranitidine administration. Am J Gastroenterol. 2000;95:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Komazawa Y, Adachi K, Mihara T, Ono M, Kawamura A, Fujishiro H, Kinoshita Y. Tolerance to famotidine and ranitidine treatment after 14 days of administration in healthy subjects without Helicobacter pylori infection. J Gastroenterol Hepatol. 2003;18:678-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Welage LS. Overview of pharmacologic agents for acid suppression in critically ill patients. Am J Health Syst Pharm. 2005;62:S4-S10. [PubMed] |
| 23. | Richardson P, Hawkey CJ, Stack WA. Proton pump inhibitors. Pharmacology and rationale for use in gastrointestinal disorders. Drugs. 1998;56:307-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 169] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Sanders SW. Pathogenesis and treatment of acid peptic disorders: comparison of proton pump inhibitors with other antiulcer agents. Clin Ther. 1996;18:2-34; discussion 1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Langtry HD, Markham A. Rabeprazole: a review of its use in acid-related gastrointestinal disorders. Drugs. 1999;58:725-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Adachi K, Komazawa Y, Mihara T, Azumi T, Fujisawa T, Katsube T, Furuta K, Kinoshita Y. Comparative study of the speed of acid-suppressing effects of oral administration of cimetidine and famotidine. J Gastroenterol Hepatol. 2005;20:1012-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Abe Y, Inamori M, Togawa J, Kikuchi T, Muramatsu K, Chiguchi G, Kawamura H, Kobayashi N, Kirikoshi H, Sakaguchi T. The comparative effects of single intravenous doses of omeprazole and famotidine on intragastric pH. J Gastroenterol. 2004;39:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Peghini PL, Katz PO, Bracy NA, Castell DO. Nocturnal recovery of gastric acid secretion with twice-daily dosing of proton pump inhibitors. Am J Gastroenterol. 1998;93:763-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 193] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 29. | Katsube T, Adachi K, Kawamura A, Amano K, Uchida Y, Watanabe M, Kinoshita Y. Helicobacter pylori infection influences nocturnal gastric acid breakthrough. Aliment Pharmacol Ther. 2000;14:1049-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Katz PO, Anderson C, Khoury R, Castell DO. Gastro-oesophageal reflux associated with nocturnal gastric acid breakthrough on proton pump inhibitors. Aliment Pharmacol Ther. 1998;12:1231-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 131] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
S- Editor Zhu LH L- Editor Kerr C E- Editor Lu W