Published online Dec 14, 2007. doi: 10.3748/wjg.v13.i46.6231
Revised: September 6, 2007
Accepted: October 28, 2007
Published online: December 14, 2007
AIM: To evaluate the combination of bevacizumab with infusional 5-fluorouracil (5-FU), leucovorin (LV) and irinotecan (FOLFIRI) in patients with advanced colorectal cancer (CRC) pretreated with combination regimens including irinotecan and oxaliplatin.
METHODS: Fourteen patients (median age 56 years) with advanced CRC, all having progressed after oxaliplatin- and irinotecan-based combination chemotherapy, were enrolled in this study. Patients were treated with 2 h infusion of irinotecan 150 mg/m2 on d 1, plus bevacizumab 5 mg/kg iv infusion for 90 min on d 2, and iv injection of LV 20 mg/m2 followed by a bolus of 5-FU 400 mg/m2 and then 22 h continuous infusion of 600 mg/m2 given on two consecutive days every 14 d.
RESULTS: The median number of cycles of chemotherapy was six (range 3-12). The response rate was 28.5%, one patient had a complete response, and three patients had a partial response. Eight patients had stable disease. The median time to progression was 3.9 mo (95% CI 2.0-8.7), and the median overall survival was 10.9 mo (95% CI 9.6-12.1). Grade 3/4 neutropenia occurred in five patients, and two of these developed neutropenic fever. Grade 3 hematuria and hematochezia occurred in one. Grade 2 proteinuria occurred in two patients. However, hypertension, bowel perforation or thromboembolic events did not occur in a total of 90 cycles.
CONCLUSION: Bevacizumab with FOLFIRI is well tolerated and a feasible treatment in patients with heavily treated advanced CRC.
- Citation: Kwon HC, Oh SY, Lee S, Kim SH, Kim HJ. Bevacizumab plus infusional 5-fluorouracil, leucovorin and irinotecan for advanced colorectal cancer that progressed after oxaliplatin and irinotecan chemotherapy: A pilot study. World J Gastroenterol 2007; 13(46): 6231-6235
- URL: https://www.wjgnet.com/1007-9327/full/v13/i46/6231.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i46.6231
Colorectal cancer (CRC) is the second most common cause of cancer deaths worldwide, with 945 000 new cases and 492 000 CRC-related deaths in 2000[1,2]. Up to 30% of patients present with metastatic disease, and approximately 50%-60% eventually develop metastatic or advanced disease[1]. The management of patients with metastatic CRC has changed dramatically over the last 5 years, with increased chances of prolonged survival. In particular, combination chemotherapy regimens including irinotecan and oxaliplatin have markedly improved response rates and prolonged median survival over fluorouracil (FU) with leucovorin (LV)[3,4]. However, there is a paucity of data about third-line chemotherapy in patients who are resistant to irinotecan- or oxaliplatin-based chemotherapy[5-7].
Angiogenesis is required for tumor growth and meta-stasis, which makes it an attractive target for biologically based cancer therapy[8-10]. Vascular endothelial growth factor (VEGF) is the most potent and specific target for cancer therapy, and has been identified as a crucial regulator of both normal and pathological angiogenesis, with increased expression being observed in many human tumor types[11-13]. In CRC, increased VEGF expression correlates with invasiveness, vascular density, metastasis, recurrence and prognosis[14,15]. In preclinical studies, a murine anti-human monoclonal antibody directed against VEGF has been shown to inhibit the growth of human tumor xenografts[16-18]. As well, the combination of anti-VEGF antibody and chemotherapy in nude mice injected with human cancer xenografts has demonstrated an increased antitumor effect compared with antibody or chemotherapy treatment alone[19].
Bevacizumab, a recombinant humanized monoclonal antibody targeting VEGF, has been evaluated in various solid tumors[20]. In phase I trials, bevacizumab was generally well tolerated and did not demonstrate dose-limiting toxicity or interactions with commonly used chemotherapy regimens[21]. In a phase 2 trial of treatment of CRC, the addition of bevacizumab to FU/LV increased the response rate, the median time to disease progression, and the median duration of survival[22]. Recently, it has been shown in randomized phase III trials that bevacizumab, when combined with irinotecan plus bolus FU/LV in the first-line treatment of metastatic CRC, and with oxaliplatin plus continuous FU/LV (FOLFOX) in second-line treatment leads to an increased median survival, progression-free survival (PFS), and response rate compared with cytotoxic chemotherapy alone[23,24].
The goal of this trial was to evaluate the safety and activity of bevacizumab plus LV, 5-FU and irinotecan (FOLFIRI) in patients with advanced CRC that had progressed after treatment with both irinotecan- and oxaliplatin-based chemotherapy regimens.
The eligibility criteria were as follows: histologically confirmed CRC (adenocarcinoma), bidimensionally measurable disease, no secondary malignancy, age > 18 years, Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0-2, and a life expectancy of > 3 mo. Adequate hematological, hepatic and renal function (including urinary excretion of no more than 500 mg protein/d) were also required. Patients should have had and failed both oxaliplatin- and irinotecan-based treatment prior to enrolment. Failure due to significant intolerance to either drug was allowed.
Exclusion criteria included thromboembolism that required therapeutic anticoagulation, central nervous system metastasis, and major surgery within 6 wk, non-healing wounds, uncontrolled hypertension, pregnant or lactating women, bleeding diathesis, active or recent cardiovascular disease or cerebrovascular accident, or prior bevacizumab therapy. The pretreatment characteristics of the patients are presented in Table 1. Written informed consent was required before chemotherapy.
| Characteristics | No. of patients |
| Median age (range) | 56 yr (29-69) |
| Sex | |
| Male | 9 |
| Female | 5 |
| Performance (ECOG) | |
| 0-1 | 12 |
| 2 | 2 |
| CEA (ng/mL) | |
| < 5 | 3 |
| ≥ 5 | 11 |
| Primary site | |
| Colon | 8 |
| Rectum | 6 |
| Sites of metastasis | |
| Liver | 7 |
| Lung | 8 |
| Lymph nodes | 7 |
| Peritoneum | 3 |
| Others | 2 |
| Number of metastasis | |
| 1 | 3 |
| ≥ 2 | 11 |
| Adjuvant chemotherapy | |
| Yes | 9 |
| No | 5 |
On d 1, irinotecan (150 mg/m2) was administered in 500 mL normal saline or dextrose as a 2-h iv infusion. On d 1 and 2, LV (20 mg/m2) was administered as a iv bolus, immediately followed by 5-FU (400 mg/m2) given as a 10-min iv bolus, followed by 5-FU (600 mg/m2) as a continuous 22-h infusion. Bevacizumab administration always followed chemotherapy. Bevacizumab was given at 5 mg/kg as an iv infusion every 2 wk. The first infusion was given over 90 min, the second over 60 min, and if both were well tolerated, subsequent infusions were given over 30 min. No premedication was given.
Dose modifications of irinotecan or 5-FU were made for hematological or non-hematological toxicity, on the basis of the most severe grade of toxicity that occurred during the previous cycle. Treatment was delayed until the absolute number of neutrophils was > 1500/μL, platelets were > 100 000/μL, and recovery occurred from mucositis, diarrhea, or skin toxicity to grade 1 or less. The 5-FU dose was reduced after the occurrence of National Cancer Institute Common Toxicity Criteria (NCI-CTC) grade 3 diarrhea, stomatitis or dermatitis. For toxicity of grade 3 or higher, a dose reduction of irinotecan by 20% was prescribed by the protocol. Bevacizumab was retained for uncontrolled hypertension or proteinuria of > 2 g in 24 h. Bevacizumab was discontinued for grade 3 or 4 hemorrhage, thromboembolic events that required full-dose anticoagulation, or any grade 4 toxicity.
Treatment was administered until the disease progressed, unacceptable toxic effects developed, or the patient refused further treatment.
Pretreatment evaluation included physical examination, complete blood cell counts, blood chemistry, tumor marker level, and radiological examination [chest posterior-anterior (PA) view radiography, computed tomography (CT) and other imaging techniques as clinically indicated] within 1 mo of starting chemotherapy. Tumor responses were determined by WHO criteria[25]. Complete blood cell counts, serum chemistry, including liver and renal function, and chest PA radiography were performed at least every 2 wk, and tumor assessment by CT was performed every three cycles.
Efficacy analysis was performed according to the intention-to-treat principle. Patients were considered assessable for response if they were eligible, had measurable disease, and had received at least one dose of study therapy. In the analysis of survival and subsequent treatment, all patients were followed until death, loss to follow-up, or termination of the study.
PFS and overall survival (OS) were calculated using the Kaplan-Meier method. PFS was calculated from the date therapy started to the date of disease progression, and OS was calculated from the date therapy started to the date of death. All data were analyzed using SPSS software (version 12.0, Chicago, IL, USA).
Between June 2005 and June 2006, a total of 14 patients were assigned for treatment at the Department of Internal Medicine, Dong-A University Medical Center, Busan, Korea. Demographic details of the patients included in the study are shown in Table 1. There were nine male and five female patients, median age 59 years (range 29-69). All patients had progressed after prior irinotecan- and oxaliplatin-based regimens. All patients used bevacizumab/FOLFIRI treatment within 6 mo after both irinotecan and oxaliplatin treatment failures. All 14 patients were assessable for response and for toxicity and survival.
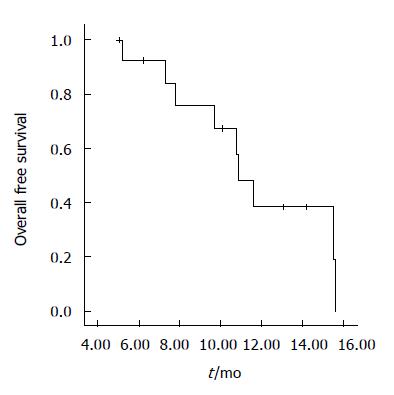
There were a median six cycles of chemotherapy (range 3-12). Chemotherapy was stopped due to disease progression in 12 patients, one discontinued because of toxicity and another because of unrelated events with no progression. Median follow-up duration was 10.1 mo. Response rate was 28.5% (95% confidence interval (CI) 15.0%-36.5%), one patient had a complete response, and three had a partial response. The median duration of response was 7.7 mo. Eight patients had stable disease, and two had disease progression. PFS was 3.9 mo (95% CI; 2.0-8.7), and median OS was 10.9 mo (95% CI; 9.6-12.1). Figures 1 and 2 show PFS and OS curves, respectively. Oral 5-FU was administered to the 12 patients with disease progression. No objective responses were documented after salvage therapy.
A total of 90 cycles of chemotherapy were administered to the patients. All patients who received at least one dose of bevacizumab and chemotherapy were assessable for adverse events. Dose modifications or interruptions were required in 30% of patients. The incidence of hematological and non-hematological toxicity is summarized in Table 2. The major grade 3/4 hematological toxicity included neutropenia (35.7%) and thrombocytopenia (7.1%). There were two cycles of neutropenic fever. Grade 1/2 nausea, vomiting, diarrhea and mucositis developed in 6 patients; however, this toxicity was mild and manageable. In one patient who had rectal cancer with bladder invasion, massive hematuria and hematochezia occurred; he was taken off the study because of toxicity. However, hypertension, bowel perforation or thromboembolic events did not occur. There was no treatment-related death. There have been nine reported deaths, of which eight were because of disease progression, and one was because of pneumonia in the present study.
| NCI-CTC grade | ||||
| 1 | 2 | 3 | 4 | |
| Hematological | ||||
| Anemia | 7 | 3 | ||
| Leukopenia | 2 | 2 | 2 | |
| Neutropenia | 3 | 4 | 1 | |
| Thrombocytopenia | 1 | 3 | 1 | |
| Non-hematological | ||||
| Nausea/vomiting | 2 | 1 | ||
| Mucositis | 1 | |||
| Diarrhea | 2 | |||
| Proteinuria | 1 | 2 | ||
| Hematuria | 1 | 1 | ||
| Asthenia | 4 | |||
Patients with advanced CRC treated with 5-FU, irinotecan and oxaliplatin in combination or sequentially may survive for 18-21 mo[3,4,26,27]. However, if these three standard drugs fail, there are no accepted treatment options. There have been few clinical trials in a third-line setting that can provide historical estimates of PFS and OS[5-7,28,29]. A recent study has shown that patients treated with cetuximab in combination with irinotecan achieved significant activity[6]. The response rate was 22.9% and time to progression and OS were 4.1 and 8.6 mo, respectively. Promising data from a small randomized phase II trial have recently shown that bevacizumab when added to cetuximab or to cetuximab plus irinotecan has a high activity in chemotherapy-refractory CRC[28]. Panitumumab, a human monoclonal antibody against epidermal growth factor receptor (EGFR), has also been shown to be active in irinotecan- and oxaliplatin-refractory metastatic CRC[23]. However, other reports have shown no clinical benefits[5,7].
The improvement in the clinical outcome afforded by the addition of bevacizumab to 5-FU suggests that blocking VEGF may be a broadly applicable approach to the treatment of CRC[22]. Adding bevacizumab to both first- and second-line combination chemotherapy improves response, time to progression, and OS, but not without toxicity[23,24]. The addition of bevacizumab 5 mg/kg
biweekly significantly improved the primary outcome of median survival from 15.6 mo with irinotecan/5-FU bolus infusion/LV (IFL) alone to 20.3 mo with IFL/bevacizumab. Bevacizumab also significantly increased response rate from 34.8% to 44.8%, and prolonged time to progression from 6.2 to 10.6 mo[23]. Compliance was also excellent in this study. As well, results from a phase III study in patients with previously treated metastatic colon cancer have revealed improved OS in patients who receive bevacizumab (10 mg/kg) with FOLFOX, as compared with those treated with FOLFOX alone, 12.5 versus 10.7 mo[24].
However, a recent large non-randomized study has shown that the combination of bevacizumab and a bolus regimen of 5-FU/LV is not sufficiently active in heavily pretreated, bevacizumab-naive patients to support the use of bevacizumab with bolus 5-FU/LV in chemotherapy-refractory metastatic CRC. The combination of bevacizumab and 5-FU/LV was associated with a low response rate: 4% based on investigator assessment and 1% based on independent review. Median PFS and OS were 3.7 and 9.1 mo, respectively[7]. This study demonstrated that for patients with advanced CRC that had progressed after treatment with both oxaliplatin- and irinotecan-based chemotherapy regimens, response rate was 28.5%, with approximately 58% of the patients showing stable disease. Median PFS was 3.9 mo and median OS was 10.9 mo. We used irinotecan instead of bolus 5-FU/LV; therefore, the response rate and survival were increased compared with those in the earlier study. Further studies will be needed to confirm these results.
Previous phase 1 and 2 clinical trials have suggested that treatment with bevacizumab alone or with chemo-therapy results in an increased incidence of thrombosis, bleeding, proteinuria and hypertension[21,22]. In two phase III investigations, the risk of venous thromboembolism was not increased by bevacizumab, but there was a small increased risk of both bleeding and bowel perforations, as well as a consistent increase in hypertension[23,24]. Hemorrhage has also been seen more frequently with bevacizumab treatment as compared with chemotherapy alone[22]. The majority of patients had minor hemorrhage, but 10% of patients had gastrointestinal hemorrhage, and 43% were grade 3/4. However, a larger phase III trial did not demonstrate an increased incidence of grade 3/4 bleeding[23]. We did not find any excess of such side effects, compared with previous studies, except for one case of massive bleeding. The reason why there was no thrombosis, hypertension and bowel perforation may have been due to the small number of patients and their relatively young age.
An analysis of predictive markers has shown indeed that bevacizumab increases the activity of irinotecan plus FU/LV, regardless of the level of VEGF expression, thrombospondin expression, and microvessel density[30]. In this study, we evaluated the correlation between expression of VEGF and microvascular density and clinical outcome, and we found no significant results (data not shown).
There is a paucity of data about third-line chemotherapy in patients who are resistant to irinotecan- or oxaliplatin-based chemotherapy. Bevacizumab, a recombinant humanized monoclonal antibody targeting vascular endothelial growth factor (VEGF), has been evaluated in various solid tumors.
The goal of this trial was to evaluate the safety and activity of bevacizumab plus irinotecan (FOLFIRI) in patients with advanced colorectal cancer (CRC) that had progressed after treatment with both irinotecan- and oxaliplatin-based chemotherapy regimens.
Bevacizumab with FOLFIRI is well tolerated and feasible in heavily treated patients with advanced CRC.
We will evaluate correlations between expression of VEGF and microvascular density and clinical outcomes.
Bevacizumab: monoclonal antibody against VEGF, which aids growth and metastasis of several cancers. Irinotecan, oxaliplatin: chemotherapeutic agents that are useful in CRC.
The manuscript evaluates the safety and activity of bevacizumab plus FOLFIRI in patients with advanced CRC that had progressed after treatment with both irinotecan- and oxaliplatin-based chemotherapy regimens. The method is simple and correct. The manuscript is written in correct English with minor language polishing.
| 1. | Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1727] [Cited by in RCA: 1714] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 2. | Bae JM, Park JG. Annual report of the Korea Central Cancer Registry Program 2000: based on registered data from 131 hospitals. Cancer Res Treat. 2002;34:77-83. |
| 3. | Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2273] [Cited by in RCA: 2228] [Article Influence: 85.7] [Reference Citation Analysis (0)] |
| 4. | Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1779] [Cited by in RCA: 1729] [Article Influence: 78.6] [Reference Citation Analysis (2)] |
| 5. | Lim DH, Park YS, Park BB, Ji SH, Lee J, Park KW, Kang JH, Lee SH, Park JO, Kim K. Mitomycin-C and capecitabine as third-line chemotherapy in patients with advanced colorectal cancer: a phase II study. Cancer Chemother Pharmacol. 2005;56:10-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3767] [Cited by in RCA: 3731] [Article Influence: 169.6] [Reference Citation Analysis (7)] |
| 7. | Chen HX, Mooney M, Boron M, Vena D, Mosby K, Grochow L, Jaffe C, Rubinstein L, Zwiebel J, Kaplan RS. Phase II multicenter trial of bevacizumab plus fluorouracil and leucovorin in patients with advanced refractory colorectal cancer: an NCI Treatment Referral Center Trial TRC-0301. J Clin Oncol. 2006;24:3354-3360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2986] [Cited by in RCA: 3069] [Article Influence: 133.4] [Reference Citation Analysis (8)] |
| 9. | Ko AH. Future strategies for targeted therapies and tailored patient management in pancreatic cancer. Semin Oncol. 2007;34:354-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Saclarides TJ. Angiogenesis in colorectal cancer. Surg Clin North Am. 1997;77:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2348] [Cited by in RCA: 2504] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 12. | Díaz-Rubio E. Vascular endothelial growth factor inhibitors in colon cancer. Adv Exp Med Biol. 2006;587:251-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Unnithan J, Rini BI. The role of targeted therapy in metastatic renal cell carcinoma. ScientificWorldJournal. 2007;7:800-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Takahashi Y, Tucker SL, Kitadai Y, Koura AN, Bucana CD, Cleary KR, Ellis LM. Vessel counts and expression of vascular endothelial growth factor as prognostic factors in node-negative colon cancer. Arch Surg. 1997;132:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 235] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 15. | Tokunaga T, Oshika Y, Abe Y, Ozeki Y, Sadahiro S, Kijima H, Tsuchida T, Yamazaki H, Ueyama Y, Tamaoki N. Vascular endothelial growth factor (VEGF) mRNA isoform expression pattern is correlated with liver metastasis and poor prognosis in colon cancer. Br J Cancer. 1998;77:998-1002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 218] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2567] [Cited by in RCA: 2544] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 17. | Nakamura M, Abe Y, Tokunaga T. Pathological significance of vascular endothelial growth factor A isoform expression in human cancer. Pathol Int. 2002;52:331-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3808] [Cited by in RCA: 4020] [Article Influence: 167.5] [Reference Citation Analysis (0)] |
| 19. | Borgström P, Gold DP, Hillan KJ, Ferrara N. Importance of VEGF for breast cancer angiogenesis in vivo: implications from intravital microscopy of combination treatments with an anti-VEGF neutralizing monoclonal antibody and doxorubicin. Anticancer Res. 1999;19:4203-4214. [PubMed] |
| 20. | Presta LG, Chen H, O'Connor SJ, Chisholm V, Meng YG, Krummen L, Winkler M, Ferrara N. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593-4599. [PubMed] |
| 21. | Margolin K, Gordon MS, Holmgren E, Gaudreault J, Novotny W, Fyfe G, Adelman D, Stalter S, Breed J. Phase Ib trial of intravenous recombinant humanized monoclonal antibody to vascular endothelial growth factor in combination with chemotherapy in patients with advanced cancer: pharmacologic and long-term safety data. J Clin Oncol. 2001;19:851-856. [PubMed] |
| 22. | Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, Griffing S, Bergsland E. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1247] [Cited by in RCA: 1209] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 23. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7832] [Cited by in RCA: 7794] [Article Influence: 354.3] [Reference Citation Analysis (8)] |
| 24. | Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB 3rd. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 1738] [Article Influence: 91.5] [Reference Citation Analysis (2)] |
| 25. | Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 26. | Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2282] [Cited by in RCA: 2215] [Article Influence: 100.7] [Reference Citation Analysis (1)] |
| 27. | Dy GK, Krook JE, Green EM, Sargent DJ, Delaunoit T, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP. Impact of complete response to chemotherapy on overall survival in advanced colorectal cancer: results from Intergroup N9741. J Clin Oncol. 2007;25:3469-3474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Saltz LB, Lenz HJ, Kindler HL, Hochster HS, Wadler S, Hoff PM, Kemeny NE, Hollywood EM, Gonen M, Quinones M. Randomized phase II trial of cetuximab, bevacizumab, and irinotecan compared with cetuximab and bevacizumab alone in irinotecan-refractory colorectal cancer: the BOND-2 study. J Clin Oncol. 2007;25:4557-4561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 306] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 29. | Malik I, Hecht J, Patnaik A. Safety and efficacy of panitumumab monotherapy in patients with metastatic colorectal cancer. J Clin Oncol. 2005;23:3520 (Abstract). |
| 30. | Jubb AM, Hurwitz HI, Bai W, Holmgren EB, Tobin P, Guerrero AS, Kabbinavar F, Holden SN, Novotny WF, Frantz GD. Impact of vascular endothelial growth factor-A expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J Clin Oncol. 2006;24:217-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 305] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
S- Editor Zhu LH L- Editor Kerr C E- Editor Ma WH