Published online Dec 14, 2007. doi: 10.3748/wjg.v13.i46.6203
Revised: September 16, 2007
Accepted: October 28, 2007
Published online: December 14, 2007
AIM: To investigate the individual and combined effects of allopurinol and hyperbaric oxygen (HBO) therapy on biochemical and histopathological changes, oxidative stress, and bacterial translocation (BT) in the experimental rat acute pancreatitis (AP).
METHODS: Eighty-five Sprague-Dawley rats were included in the study. Fifteen of the eighty-five rats were used as controls (sham, GroupI). AP was induced via intraductal taurocholate infusion in the remaining seventy rats. Rats that survived to induction of acute necrotizing pancreatitis were randomized into four groups. Group II received saline, Group III allopurinol, Group IV allopurinol plus HBO and Group V HBO alone. Serum amylase levels, oxidative stress parameters, BT and histopathologic scores were determined.
RESULTS: Serum amylase levels were lower in Groups III, IV and V compared to Group II (974 ± 110, 384 ± 40, 851 ± 56, and 1664 ± 234 U/L, respectively, P < 0.05, for all). Combining the two treatment options revealed significantly lower median [25-75 percentiles] histopathological scores when compared to individual administrations (13 [12.5-15] in allopurinol group, 9.5 [7-11.75] in HBO group, and 6 [4.5-7.5] in combined group, P < 0.01). Oxidative stress markers were significantly better in all treatment groups compared to the controls. Bacterial translocation into the pancreas and mesenteric lymph nodes was lower in Groups III, IV and V compared to Group II (54%, 23%, 50% vs 100% for translocation to pancreas, and 62%, 46%, 58% vs 100% for translocation to mesenteric lymph nodes, respectively, P < 0.05 for all).
CONCLUSION: The present study confirms the benefit of HBO and allopurinol treatment when administered separately in experimental rat AP. Combination of these treatment options appears to prevent progression of pancreatic injury parameters more effectively.
- Citation: Comert B, Isik AT, Aydin S, Bozoglu E, Unal B, Deveci S, Mas N, Cinar E, Mas MR. Combination of allopurinol and hyperbaric oxygen therapy: A new treatment in experimental acute necrotizing pancreatitis? World J Gastroenterol 2007; 13(46): 6203-6207
- URL: https://www.wjgnet.com/1007-9327/full/v13/i46/6203.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i46.6203
Acute pancreatitis (AP) is an untreatable condition with a wide clinical spectrum ranging from a mild, self-limited disease to severe organ failure[1]. Translocation of enteric bacteria is the most important cause of infection in the pancreatic tissue, and subsequent events such as sepsis and related complications[2,3]. Currently, several treatment options have been proposed for the septic complications of AP.
Hyperbaric oxygen (HBO) therapy has been investigated in several experimental and clinical conditions which cannot be treated with currently available medical or surgical options[4,5]. HBO has been shown to have bactericidal activity against anaerobic bacteria[6]. In addition, HBO reduces the incidence of bacterial translocation[7]. It also lowers nitric oxide production and enhances several activities including bactericidal action of neutrophils, angiogenesis and wound healing[8]. Lin et al showed that repeated HBO therapy in endotoxic rats reduced inflammatory mediators and free radicals, as well as mortality[9]. In a rat model of tourniquet-induced ischemia-reperfusion skeletal muscle injury, HBO attenuated the reperfusion-induced increase in catalase activity and malondialdehyde (MDA)[10]. These studies demonstrated that HBO treatment, especially when administered in repeated doses has antioxidant rather than oxidative effect.
Xanthine oxidase plays an important role in the migration of microorganisms from the intestinal lumen to intra-abdominal spaces in pathological conditions, an event termed bacterial translocation (BT)[11,12]. Allopurinol (ALPL) has antioxidant properties, and previous studies have shown that antioxidant therapy reduces tissue injury and bacterial translocation in experimental pancreatitis[13,14].
The present study was carried out to investigate the individual and combined effects of ALPL and HBO on biochemical and histopathological changes, oxidative stress, and BT during the course of experimental rat pancreatitis.
The study was approved by the Institutional Animal Use and Care Committee of the Gulhane Medical Academy and performed in accordance with the National Institutes of Health guidelines for the care and handling of animals.
Eighty-five male Sprague-Dawley rats weighing from 280 to 350 g were obtained from Gulhane School of Medicine Research Center (Ankara, Turkey). Before the experiment, the animals were fed standard rat chow and water ad libitum and housed in metabolic cages with controlled temperature and 12 h light/dark cycles for at least 1 wk.
Anesthesia was induced with Sevoflurane (Sevorane® Liquid 250 mL, Abbott, Istanbul, Turkey) inhalation. Laparotomy was performed through a midline incision. The common biliopancreatic duct was cannulated with a 28 gauge 1/2 inch, micro-fine catheter. One microaneurysm clip was placed on the bile duct below the liver and another around the common biliopancreatic duct at its entry into the duodenum to avoid reflux of enteric contents into the duct. One mL/kg of 3% sodium taurocholate (Sigma, St. Louis, MO, USA) was slowly infused into the common biliopancreatic duct, with the infusion pressure maintained below 30 mmHg, as measured with a mercury manometer[15]. When the infusion was finished, the microclips were removed, and the abdomen was closed in two layers. All procedures were performed using sterile techniques.
Fifteen rats had a sham operation and served as the controls (GroupI). AP was induced via intraductal taurocholate infusion in the remaining seventy rats. Five of seventy rats died during the 6 h induction period. All surviving animals were randomization into four groups, six hours after the induction of pancreatitis. Group II (n = 17) received saline (1 mL/kg, bid, sc), Group III (n = 16) ALPL (200 mg/kg per day, bid, sc)[16], Group IV (n = 16) ALPL plus HBO (2.8 atmospheric pressure, bid, 90 min each, total 4 sessions)[17] and Group V (n = 16) HBO alone. Five rats in Group II, three rats each in Groups III and Group IV, and four rats in Group V died during the treatment period. Fifty-four hours after induction, all the surviving animals were killed with an intracardiac injection of pentobarbital (200 mg/kg). Data was collected on serum amylase levels, oxidative stress parameters [MDA, superoxide dismutase (SOD) and glutathione peroxidase (GSHPx)], bacterial translocation and histopathologic scores.
Blood samples were taken from the heart before the animals were sacrificed for serum amylase levels. A Hitachi 917 auto analyzer (Roche Diagnostics, Germany) was used for the amylase assay. Amylase level was expressed as U/L.
A portion of the pancreatic tissue from each rat was fixed in 10% neutral buffered formalin and embedded in paraffin. One paraffin section, stained with hematoxylin and eosin, was examined from each animal. Two pathologists who were blinded to the treatment protocol scored the tissues for edema, acinar necrosis, inflammatory infiltrate, hemorrhage, fat necrosis, and perivascular inflammation, in 20 different fields. The scores for each of the histologic abnormalities were added up, with a maximum score of 24, as defined by Schmidt et al[18].
The areas of the pancreas showing macroscopic necrosis and visible mesenteric lymph nodes were excised, weighed, and homogenized. The homogenates were diluted serially, quantitatively plated in duplicate on phenylethyl alcohol and MacConkey II agar, and incubated aerobically at 37°C for 24 h. The bacterial counts were expressed as colony-forming units (cfu/g tissue), and counts of 1000 cfu/g or higher were considered to be indicative of a positive culture. Gram-negative bacteria were identified with the API-20E system (BioMerieux Vitek, Hazelwood, MO, USA). Gram-positive bacteria were identified to the genus level by means of standard microbiologic methods[19,20].
Pancreatic tissue samples were homogenized in cold KCl solution (1.5%) in a glass homogenizer on ice. The samples were centrifuged and the supernatant was used for the assays described below.
Tissue MDA concentration was estimated by the method of Ohkawa et al[21]. The supernatant was resuspended in 4 mL water, 0.5 mL glacial acetic acid and 0.5 mL 0.33% aqueous thiobarbituric acid solution. The mixture was heated for 60 min in a boiling water bath. After cooling, the complex formed by thiobarbituric acid reactant substances was extracted into an n-butanol phase, and the formed chromogen was measured at 532 nm by spectrophotometer. A standard absorption curve for MDA was prepared using tetramethoxy propane solution. MDA levels were expressed as nmol/g tissue.
For the measurement of SOD activity, the supernatant was diluted 1:400 with 10 mmol/L phosphate buffer, pH 7.00. Twenty five μL of diluted supernatant was mixed with 850 μL of substrate solution containing 0.05 mmol/L xanthine sodium and 0.025 mmol/L 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride (INT) in a buffer solution containing 50mmol/L 3-cyclohexylaminol-1-propanesulfonic acid (CAPS) and 0.94 mmol/L ethylenediamine tetraacetic acid (EDTA) at pH 10.2. At this stage, 125 μL of xanthine oxidase (80 U/L) was added to the mixture and the absorbance increase was followed at 505 nm for 3 min against air. Twenty five microlitres of phosphate buffer or 25 μL of various standard concentrations were used as blank or standard determinations. SOD activity was expressed as U/g tissue[22].
For GSHPx measurement, the reaction mixture consisted of 50 mmol/L tris buffer, pH 7.6 containing 1mmol/L of Na2EDTA, 2 mmol/L of reduced glutathione (GSH), 0.2 mmol/L of reduced nicotinamide adenine dinucleotide (NADPH), 4 mmol/L of sodium azide and 1000 U of glutathione reductase (GR), Fifty microlitres of supernatant and 950 μL of reaction mixture, or 20 μL of supernatant and 980 μL of reaction mixture were mixed and incubated for 5 min at 37°C. The reaction was initiated with 8 mmol/L H2O2, and the decrease in NADPH absorbance was followed at 340 nm for 3 min. The enzyme activity was expressed as U/g tissue[23].
The results of parametric tests were expressed as mean ± SE. Nonparametric values were expressed as median (25-75 percentiles). The significance of differences in the histopathologic scores and serum amylase levels was assessed by the Kruskal-Wallis test. Subgroup analyses were performed by the Mann-Whitney U test or t-test as appropriate. The significance of differences in oxidative stress parameters was determined by Oneway ANOVA test and Tukey HSD procedure as post hoc test. Probabilities less than 0.05 were considered significant. All statistical measurements were made using SPSS PC ver. 11.05 (SPSS Inc. USA).
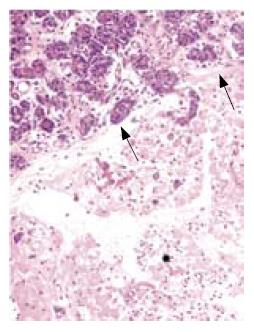
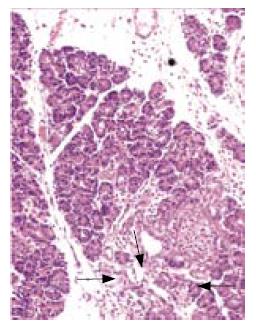
All rats except those in GroupIdeveloped acute pancreatitis, demonstrated by macroscopic parenchymal necrosis, and abundant turbid peritoneal fluid (Figure 1). Histopathological scores were significantly lower in all treatment groups (Group III, Group IV and Group V)compared to Group II (13 [12.5-15], 6 [4.5-7.5], 9.5 [7-11.75], 18 [14.5-19]; P < 0.01, P < 0.001, and P < 0.001, respectively). The most favorable results were seen in the combination treatment group (Figure 2, Table 1).
| Group I(n = 15) | Group II(n = 12) | Group III(n = 13) | Group IV(n = 13) | Group V(n = 12) | |
| Amylase (U/L) | 278 ± 44 | 1664 ± 234 | 974 ± 110 | 384 ± 40 | 851 ± 56 |
| Oxidative stress | |||||
| MDA (nmol/g) | 12.3 ± 0.4 | 28.3 ± 0.7 | 18.2 ± 0.4 | 16.4 ± 0.4 | 20.1 ± 0.5 |
| SOD (U/g) | 395 ± 7 | 254 ± 6 | 345 ± 16 | 282 ± 8 | 300 ± 9 |
| GSHPx (U/g) | 51.6 ± 2.0 | 30.8 ± 0.9 | 35.8 ± 1.5 | 48.4 ± 0.7 | 45.6 ± 1.4 |
| Bacterial translocation | |||||
| MLNs | 2 (13%) | 12 (100%) | 8 (62%) | 6 (46%) | 7 (58%) |
| Pancreas | 2 (13%) | 12 (100%) | 7 (54%) | 3 (23%) | 6 (50%) |
| Histopathologic score | 2 (1-3) | 18 (14.5-19) | 13 (12.5-15) | 6 (4.5-7.5) | 9.5(7-11.75) |
| Mortality | 0/15 (0%) | 5/17 (29%) | 3/16 (19%) | 3/16 (19%) | 4/16 (25%) |
Serum amylase levels were lower in Groups III, IV and V compared to Group II (974 ± 110, 384 ± 40, 851 ± 56, 1664 ± 234 U/L; P < 0.05, P < 0.001, and P < 0.02, respectively, Table 1). Oxidative stress markers showed significantly lower levels in all treatment groups compared to the controls. Tissue MDA levels in Groups III, IV, and V were significantly lower than in Group II (18.2 ± 0.4, 16.4 ± 0.4, 20.1 ± 0.5 nmol/g, vs 28.3 ± 0.7 nmol/g, respectively, P < 0.01 for all, Table 1). Tissue SOD activity in Groups III, IV, and V was significantly higher compared to Group II (345 ± 16 U/g, 282 ± 8 U/g, 300 ± 9 U/g, vs 254 ± 6 U/g; respectively, P < 0.01 for all, Table 1). In addition, GSHPx activity in Groups III, IV and V was significantly higher than in Group II (35.8 ± 1.5 U/g, 48.4 ± 0.7 U/g, 45.6 ± 1.4 U/g, vs 30.8 ± 0.9 U/g; respectively, P < 0.01 for all, Table 1). BT to pancreas and mesenteric lymph nodes was reduced significantly in the three treatment groups (Group III, IV and V) compared to the control group (pancreatic tissue: 54%, 23%, 50%, vs 100%; P < 0.02, P < 0.001, and P < 0.002, respectively; MLNs: 62%, 46%, 58%, vs 100%; P < 0.05, P < 0.01, and P < 0.05, respectively, Table 1). Bacterial growth was seen in all tissue specimens obtained from the pancreas and MLNs in the control groups. Of the three treatment groups, combination treatment (Group IV) was most effective in preventing BT (3/13 [23%] to pancreatic tissue, and 6/13 [46%] in MLNs). The best results in terms of amylase levels, histopathological score, oxidative stress markers and BT were seen in rats receiving the combination treatment, compared to animals receiving a single treatment and the control group. Five rats in Group II, three rats each in Groups III and IV, and four rats in Group V died before the 54th h of induction of pancreatitis. Mortality rates between groups, except the sham group, were statistically not significant.
Pancreatic infection is a serious complication of acute necrotizing pancreatitis. The failure of gut barrier results in bacterial translocation and subsequently septic complication of pancreatitis[2,3,24]. For this reason, prevention of contamination of the necrotic pancreatic tissue is very important, and the new generation antibiotics are of significant advantage in this respect. Although in experimental and clinical studies, the use of antibiotic has been shown to be beneficial[2]; in a randomized, controlled study prophylactic antibiotic therapy was found to have no effect on the mortality[25].
Although the potential role of xanthine oxidase in the presence of barrier failure and translocation of bacteria across the gut lumen has been shown in a previous study[14], the degree to which such a mechanism is involved in the pathogenesis of pancreatic infection is not known, and whether an inhibitor of this enzyme has a preventive effect is not clear[11,12]. The role of HBO therapy in the prevention of infectious complications, mainly through the reduction of oxidative stress and bacterial translocation in experimental acute pancreatitis has been reported previously[7,26]. Our group had previously investigated the efficacy of individual administration of allopurinol and HBO in preventing bacterial contamination of pancreatic tissue. In the present study, we examined the impact of combining allopurinol and HBO therapy[7,14].
We observed that both allopurinol and HBO had beneficial effects on the biochemical and histological abnormalities, oxidative stress and bacterial translocation. The present report represents the first study examining the effects of a xanthine oxidase inhibitor plus HBO therapy in acute pancreatitis. The individual effects of the two treatments on amylase levels were nearly the same. However, HBO treatment resulted in greater reduction in the histopathological scores, while allopurinol alone did not produce satisfactory histological recovery. The histological abnormalities in the combined treatment group were significantly less compared with the use of allopurinol and HBO alone, indicating a potentiation of effect. Allopurinol also decreased the oxidative stress parameters, as it has been reported previously[13,14,27,28], although allopurinol was found to have no effect on the incidence and severity of endoscopic retrograde cholangiopancreatography (ERCP)-induced pancreatitis in studies on human subjects by Budzynska et al[29]. When allopurinol was co-administered with HBO at the same doses, the overall antioxidant effect did not increase. These results correlate well with the histological recovery seen in animals treated with individual drugs and combination therapy. However, when the data regarding oxidative stress was examined, it was interesting to note that combination therapy was more effective in increasing the anti-oxidant system. These findings suggest that the improvement in pancreatic morphology was related to the increase in the anti-oxidant system.
Bacterial translocation was very similar in the individual treatment groups. Again, bacterial contamination of the pancreatic tissue was significantly less in the combined treatment group, indicating a potentiating effect. Although xanthine oxidase, an important source of endothelial cell-derived superoxide and hydrogen peroxide, plays a primary role in ischemia-reperfusion injury, which contributes to the failure of the intestinal barrier[11], it can be postulated that even with the addition of antioxidant activity of allopurinol, inhibition of xanthine oxidase was not superior compared to the use of HBO alone. However, a combination of these two agents may produce remarkable inhibition of bacterial translocation, perhaps through different mechanisms including not only xanthine oxidase inhibition and antioxidant activity but also direct antibacterial, immunological, angiogenic and cellular-subcellular effects.
Finally, the present study confirmed our previous observations on the efficacy of HBO and allopurinol in experimental acute necrotizing pancreatitis and also demonstrated that a combination of these treatment options prevented more effectively the progression of pancreatic injury. Nevertheless, the activity and potency of xanthine oxidase, the importance of blocking its activity, and the detailed effects of HBO on this enzyme in the intestines and in the pancreas in acute pancreatitis need further examination.
The severity of acute pancreatitis may range from a mild, self-limited illness to a catastrophic disease with multiple potentially severe complications and risk of death. Translocation of bacteria from the intestines is one of the most important factors in the development of septic complications and mortality in acute pancreatitis
Most of the experimental and clinical studies designed to reduce morbidity and mortality in acute pancreatitis are focused on minimizing the extent of necrosis and the prevention of bacterial contamination of necrotic pancreatic tissue.
Several studies have assessed the effect of allopurinol and hyperbaric oxygen on bacterial translocation, oxidative stress, and histology in experimental acute necrotizing pancreatitis. The present study was carried out a rat model to evaluate the effect of combined allopurinol and hyperbaric oxygen treatment on bacterial translocation, oxidative stress and the course of acute necrotizing pancreatitis.
If these results are confirmed on further studies, combination treatment with allopurinol and hyperbaric oxygen can be applied clinically in patients with acute necrotizing pancreatitis to prevent oxidative stress and bacterial translocation.
This paper examines the effects of allopurinol and hyperbaric oxygen on taurocholate infusion-induced acute necrotic pancreatitis in rats. It was observed that both treatments improved the pathological abnormalities, and combination of the two modalities provided further improvement.
| 1. | Renner IG, Savage WT 3rd, Pantoja JL, Renner VJ. Death due to acute pancreatitis. A retrospective analysis of 405 autopsy cases. Dig Dis Sci. 1985;30:1005-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 302] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 2. | Schmid SW, Uhl W, Friess H, Malfertheiner P, Büchler MW. The role of infection in acute pancreatitis. Gut. 1999;45:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 144] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | DeMeo MT, Mutlu EA, Keshavarzian A, Tobin MC. Intestinal permeation and gastrointestinal disease. J Clin Gastroenterol. 2002;34:385-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 184] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 4. | Leach RM, Rees PJ, Wilmshurst P. Hyperbaric oxygen therapy. BMJ. 1998;317:1140-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 244] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Nylander G, Lewis D, Nordström H, Larsson J. Reduction of postischemic edema with hyperbaric oxygen. Plast Reconstr Surg. 1985;76:596-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 108] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Tibbles PM, Edelsberg JS. Hyperbaric-oxygen therapy. N Engl J Med. 1996;334:1642-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 563] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 7. | Mas N, Isik AT, Mas MR, Comert B, Tasci I, Deveci S, Ozyurt M, Ates Y, Yamanel L, Doruk H. Hyperbaric oxygen-induced changes in bacterial translocation and acinar ultrastructure in rat acute necrotizing pancreatitis. J Gastroenterol. 2005;40:980-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Sakoda M, Ueno S, Kihara K, Arikawa K, Dogomori H, Nuruki K, Takao S, Aikou T. A potential role of hyperbaric oxygen exposure through intestinal nuclear factor-kappaB. Crit Care Med. 2004;32:1722-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Lin HC, Wan FJ, Wu CC, Tung CS, Wu TH. Hyperbaric oxygen protects against lipopolysaccharide-stimulated oxidative stress and mortality in rats. Eur J Pharmacol. 2005;508:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Bosco G, Yang ZJ, Nandi J, Wang J, Chen C, Camporesi EM. Effects of hyperbaric oxygen on glucose, lactate, glycerol and anti-oxidant enzymes in the skeletal muscle of rats during ischaemia and reperfusion. Clin Exp Pharmacol Physiol. 2007;34:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Toyama MT, Lewis MP, Kusske AM, Reber PU, Ashley SW, Reber HA. Ischaemia-reperfusion mechanisms in acute pancreatitis. Scand J Gastroenterol Suppl. 1996;219:20-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Deitch EA, Specian RD, Berg RD. Endotoxin-induced bacterial translocation and mucosal permeability: role of xanthine oxidase, complement activation, and macrophage products. Crit Care Med. 1991;19:785-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 93] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Schoenberg MH, Büchler M, Gaspar M, Stinner A, Younes M, Melzner I, Bültmann B, Beger HG. Oxygen free radicals in acute pancreatitis of the rat. Gut. 1990;31:1138-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 147] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Isik AT, Mas MR, Yamanel L, Aydin S, Comert B, Akay C, Erdem G, Mas N. The role of allopurinol in experimental acute necrotizing pancreatitis. Indian J Med Res. 2006;124:709-714. [PubMed] |
| 15. | Chen HM, Shyr MH, Ueng SW, Chen MF. Hyperbaric oxygen therapy attenuates pancreatic microcirculatory derangement and lung edema in an acute experimental pancreatitis model in rats. Pancreas. 1998;17:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Czakó L, Takács T, Varga IS, Tiszlavicz L, Hai DQ, Hegyi P, Matkovics B, Lonovics J. Oxidative stress in distant organs and the effects of allopurinol during experimental acute pancreatitis. Int J Pancreatol. 2000;27:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Gulec B, Yasar M, Yildiz S, Oter S, Akay C, Deveci S, Sen D. Effect of hyperbaric oxygen on experimental acute distal colitis. Physiol Res. 2004;53:493-499. [PubMed] |
| 18. | Schmidt J, Rattner DW, Lewandrowski K, Compton CC, Mandavilli U, Knoefel WT, Warshaw AL. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:44-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 694] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 19. | Sharp SE, Bowler PG. Wound and soft tissue cultures. Clinical Microbiology Procedures Hand Book. Washington DC: ASM Press 2004; 3.13.1.1-3.13.1.16. |
| 20. | York MK. Quantitative cultures of wound tissues. Clinical Microbiology Procedures Hand Book. Washington DC: ASM Press 2004; 3.13.2 .1-3.13.2.4. |
| 21. | Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17627] [Cited by in RCA: 19253] [Article Influence: 409.6] [Reference Citation Analysis (1)] |
| 22. | Chiou JF, Hu ML. Elevated lipid peroxidation and disturbed antioxidant enzyme activities in plasma and erythrocytes of patients with uterine cervicitis and myoma. Clin Biochem. 1999;32:189-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Pleban PA, Munyani A, Beachum J. Determination of selenium concentration and glutathione peroxidase activity in plasma and erythrocytes. Clin Chem. 1982;28:311-316. [PubMed] |
| 24. | Sawa H, Ueda T, Takeyama Y, Yasuda T, Shinzeki M, Nakajima T, Kuroda Y. Blockade of high mobility group box-1 protein attenuates experimental severe acute pancreatitis. World J Gastroenterol. 2006;12:7666-7670. [PubMed] |
| 25. | Isenmann R, Rünzi M, Kron M, Kahl S, Kraus D, Jung N, Maier L, Malfertheiner P, Goebell H, Beger HG. Prophylactic antibiotic treatment in patients with predicted severe acute pancreatitis: a placebo-controlled, double-blind trial. Gastroenterology. 2004;126:997-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 299] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 26. | Isik AT, Mas MR, Comert B, Yasar M, Korkmaz A, Akay C, Deveci S, Tasci I, Mas N, Ates Y. The effect of combination therapy of hyperbaric oxygen, meropenem, and selective nitric oxide synthase inhibitor in experimental acute pancreatitis. Pancreas. 2004;28:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Sanfey H, Sarr MG, Bulkley GB, Cameron JL. Oxygen-derived free radicals and acute pancreatitis: a review. Acta Physiol Scand Suppl. 1986;548:109-118. [PubMed] |
| 28. | Spahr L, Bresson-Hadni S, Amann P, Kern I, Golaz O, Frossard JL, Hadengue A. Allopurinol, oxidative stress and intestinal permeability in patients with cirrhosis: an open-label pilot study. Liver Int. 2007;27:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Budzyńska A, Marek T, Nowak A, Kaczor R, Nowakowska-Dulawa E. A prospective, randomized, placebo-controlled trial of prednisone and allopurinol in the prevention of ERCP-induced pancreatitis. Endoscopy. 2001;33:766-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
S- Editor Zhu LH L- Editor Anand BS E- Editor Wang HF