Published online Nov 14, 2007. doi: 10.3748/wjg.v13.i42.5598
Revised: July 31, 2007
Accepted: August 17, 2007
Published online: November 14, 2007
While restorative proctocolectomy with ileal pouch-anal anastomosis has significantly improved the quality of life in patients with underlying ulcerative colitis who require surgery, complications can occur. Pouchitis as the most common long-term complication represents a spectrum of disease processes ranging from acute, antibiotic-responsive type to chronic antibiotic-refractory entity. Accurate diagnosis using a combined assessment of symptoms, endoscopy and histology and the stratification of clinical phenotypes is important for treatment and prognosis the disease. The majority of patients respond favorably to antibiotic therapy. However, management of chronic antibiotic-refractory pouchitis remains a challenge.
- Citation: Yu ED, Shao Z, Shen B. Pouchitis. World J Gastroenterol 2007; 13(42): 5598-5604
- URL: https://www.wjgnet.com/1007-9327/full/v13/i42/5598.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i42.5598
Restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA) has become a part of standard surgical treatment for patients with ulcerative colitis (UC) or familial adenomatous polyposis (FAP). Despite advances in medical therapy, approximately 30% of patients with UC eventually require total proctocolectomy[1]. Restorative proctocolectomy with IPAA has the following advantages: (1) gastrointestinal continuity is reestablished with IPAA, (2) the procedure helps improve symptoms of patients and health-related quality of life, (3) the majority of patients with IPAA can avoid UC-related medications, particularly immunomodulators and biological agents and their associated potential adverse effects, and (4) IPAA with proctocolectomy substantially reduces the risk for dysplasia or cancer. However, adverse outcomes or complications often occur after surgery. Common long-term inflammatory and functional complications of restorative proctocolectomy are pouchitis, Crohn's
disease (CD) of the pouch, cuffitis (inflammation in the rectal muscular cuff), and irritable pouch syndrome (IPS). Pouchitis likely represents a spectrum of disease processes ranging from acute antibiotic-responsive entity to chronic antibiotic-refractory type. Accurate diagnosis and classification of pouchitis are important for its proper management and prognosis.
Pouchitis, a nonspecific inflammatory condition at the ileal pouch reservoir, is the most common long-term complication in patients with IPAA which significantly affects patients' quality of life[2]. Reported cumulative frequency rates of pouchitis 10-11 years after IPAA surgery range from 23% to 46%[3,4]. It is estimated that approximately 50% of patients who have undergone IPAA surgery for UC will develop at least one episode of pouchitis[5]. The estimated incidence within 12 mo after ileostomy was as high as 40% in a European study[6]. The discrepancy in the reported cumulative frequencies from different institutions likely results from diagnostic criteria used (e.g., diagnosis made based on symptom assessment alone or on a combined assessment of symptoms, endoscopy, with or without histology), intensity of follow-up with pouch endoscopy, and inclusion or exclusion of other inflammatory or functional disorders of the pouch and surgery related conditions (such as abscess, fistula, and sinus of the pouch).
Pouchitis almost exclusively occurs in patients with underlying UC and is rarely seen in patients with FAP[7,8]. Although the etiology and pathogenesis of pouchitis are not entirely clear, bulk of evidence points towards an abnormal mucosal immune response (innate and adaptive) to altered microflora in the pouch leading to acute and/or chronic inflammation[6,9,10,11,12,13]. The prevailing theory holds that pouchitis results from an overgrowth of certain commensal bacteria[9,13,14,15]. Pouchitis only develops after ileostomy, i.e., the pouch mucosa starts to expose fecal stream. Manipulation of microflora with antibiotic or probiotic therapy resulting in improvement in patients with pouchitis provides additional evidence of involvement of microflora in the pathogenesis of pouchitis.
Immune mechanisms for pouchitis have been extensively studied in a similar fashion to that for inflammatory bowel disease. There are overlaps in tissue cytokine profiles between pouchitis and UC. However, pouchitis is not simply a duplication of the disease process seen in UC. The role of T-cell-mediated intestinal immunity in the pathogenesis of pouchitis is not entirely clear and is likely secondary to alterations in pouch microflora. Alterations in the macrophage and T cell subpopulations have been postulated in the process of pouchitis[16,17,18]. Increased T-cell activation and proliferation have been demonstrated in pouchitis, as evidenced by an increased expression in activation markers, such as CD25, CD30, and CD27[18]. As a result of activation of T cells and other immune cells, production of cytokines is up-regulated. Abnormal cytokine profiles have been reported in pouchitis including a deregulated production of proinflammatory and immunoregulatory cytokines[19]. Proinflammatory cytokines, such as TNF-α, are released at a great extent in the inflamed mucosa by macrophages and monocytes, leading to tissue injury, and are considered to be involved in pouchitis as a secondary pathophysiologic mechanism[19]. As in UC, the production of other inflammatory mediators including cytokines (such as IL-1β, IL-6, and IL-8)[20,21,22,30], cell adhesion molecules (such as E selectin and intercellular adhesion molecule-1)[23], platelet-activating factor[24], lipoxygenase products of arachidonic acids (such as leukotriene B4 and prostaglandin E2)[25], proinflammatory neuropeptides[26], macrophage inflammatory protein (MIP) 2α, matrix metalloproteinase (MMP)-1[21,27], MMP-2[21,27,28], MMP-9[28], MRP-14[21], and inducible nitric oxide[28], is also increased. Abnormalities in immunoregulatory cytokines such as IL-2, and interferon-γ[18,29], IL-4[29], and IL-10 are also seen in pouchitis. Imbalance between proinflammatory and immunoregulatory cytokines has been described in patients with pouchitis[30]. Abnormalities of T cells and other immune cells may not explain the whole mechanism of pouchitis. It is likely that such abnormalities are nonspecific and secondary in nature. Inconsistent results in the studies of immune cells and inflammatory mediators in pouchitis reflect the complexity in pathogenesis of the disease.
There are few published studies addressing the interplay between microflora and mucosal immune system in pouchitis. Exposure of peripheral blood and lamina propria lymphocytes ex vivo to sonicated flora from pouchitis induces more intense proliferation as compared with sonicates from healthy pouches. In vitro pretreatment of the sonicate preparation of pouch flora with metronidazole abolishes the stimulating ability[31]. Bacterial sonicates from a heterologous but healthy pouch do not stimulate lymphocyte proliferation[31]. The greater stimulatory effect of sonicates from pouchitis suggests that certain microflora may predominantly present in inflamed pouch mucosa and these microflora may be potentially pathogenic in activation of local mononuclear cells[31].
One of the most intriguing aspects of pouchitis is that it occurs almost exclusively in patients with underlying UC. Interestingly, there are similarities in terms of clinical presentations and immunological abnormalities between pouchitis and UC, suggesting that a subset of pouchitis may actually represent the recurrence of a UC-like disease in the ileal pouch. The theory of recurrent UC is supported by several lines of evidence. With the presence of stasis in the pouch, exposure to fecal contents and an increased microbial load could cause inflammatory changes leading to morphological alterations in the ileal pouch mucosa mimicking colon epithelia in UC[24]. Colonic metaplasia of the pouch mucosa seems to be a nonspecific adaptive response to the new luminal environment[24]. Colonic metaplasia characterized by villous blunting, crypt cell hyperplasia, and colon epithelium-specific antigens such as human tropomyosin 5, may increase the risk for pouchitis[32]. A similar alteration in mucin glycoproteins occurs in pouchitis as seen in UC[33]. It is possible that the altered glycoproteins are more susceptible to enzymatic degradation by bacteria, making the mucus barrier less resistant[34]. Additionally, some patients with pouchitis have the same extra-intestinal manifestations (such as arthralgia and primary sclerosing cholangitis or PSC) as those seen in patients with UC[35]. Smoking tends to have a protective effect against the development of pouchitis as it does against UC[36].
Risk factors and potential predictors for pouchitis have been extensively studied. The implications of these studies include identification of etiopathogenetic factors, provision of strategies for modification of certain risk factors, and prediction of pouch outcome. Genetic polymorphisms such as those of IL-1 receptor antagonist[38,39,40] and NOD2/CARD15[40] may increase the risk for pouchitis. The reported risk factors for pouchitis also include non-carrier status of TNF allele 2[39], extensive UC[4,41,42], backwash ileitis[41], pre-proctocolectomy thrombocytosis[43], extra-intestinal manifestations, especially PSC[3,35,44,45], the presence of serum perinuclear anti-neutrophil cytoplasmic antibodies (p-ANCA)[46,47], being a non-smoker[36,42,48], and use of non-steroidal anti-inflammatory drugs (NSAID)[42,48]. In addition to p-ANCA, the presence of serologic markers, anti-Saccharomyces cervesiae antibodies to CD-related antigen from Pseudomonas fluorescens or outer membrane porin C of Escherichia coli in patients with pre-operative indeterminate colitis appears to be associated with persistent inflammation of the pouch after restorative proctocolectomy[49]. Acute and chronic pouchitis may have different risk factors[42].
It appears that few studies came up with the same risk factors. This inherent discrepancy among the studies may be contributed to the following factors: (1) small vs large sample sizes were analyzed, (2) the number of variables and outcomes was studied, (3) univariable analyses vs multivariable analyses were used, (4) diagnostic criteria for pouchitis were used, (5) pouchitis was stratified into acute vs chronic entities, and (6) type of controls was compared.
Patients with pouchitis have a wide range of clinical presentations, including increased stool frequency, urgency, tenesmus, incontinence, nocturnal seepage, abdominal cramping, and pelvic discomfort. While bloody bowel movements are uncommon in typical pouchitis, patients with IPAA with or without pouchitis can have iron deficiency anemia[50,51]. Patients with severe pouchitis occasionally present with fever, dehydration, malnutrition which may require hospitalization. Patients may have predominant extra-intestinal symptoms such as arthralgia and uveitis. These symptoms, however, are not specific and can present in disorders of the pouch other than pouchitis, such as cuffitis, CD of the pouch, proximal small bowel bacterial overgrowth, and IPS.
Making diagnosis of pouchitis should not solely rely on presenting symptoms. The severity of symptoms does not necessarily correlate with the degree of endoscopic or histologic inflammation of the pouch[52,53]. A combined assessment of symptoms, endoscopic and histologic features is the key to making an accurate diagnosis and it is necessary to differentiate pouchitis from other inflammatory and non-inflammatory disorders of the pouch such as cuffitis, pouch stricture, pouch sinus, and IPS. There are no universally accepted diagnostic criteria for pouchitis. For clinical trials, the 18-point pouchitis disease activity index (PDAI) is most commonly used in the diagnosis of pouchitis and measurement of disease activity[54].
Pouch endoscopy yields valuable information on severity and extent of mucosal inflammation, presence or absence of concurrent ileitis or cuffitis, and structural abnormalities such as strictures, sinuses, and fistula openings. In addition, pouch endoscopy with segmental biopsy is the tool for dysplasia surveillance and can deliver effective therapy, including stricture dilation. Histopathology is invaluable for the detection of dysplasia, viral inclusion bodies of cytomegalovirus infection, granulomas, pyloric gland metaplasia, mucosal prolapse, and ischemic changes. It should be pointed out that villous blunting and an increased number of mononuclear cells in the lamina propria can be a part of "normal" adaptive changes of pouch mucosa to fecal stasis in the pouch which does not necessarily indicate pouchitis.
In cases of suspected complicated pouchitis, imaging studies such as contrasted pouchography, CT and MRI are typically used to assess the presence of mucosal and transmural disease activity within and around the pouch[55]. Wireless capsule endoscopy appears safe in patients with IPAA, which has been used for diagnostic evaluation in patients with chronic pouchitis[56] or anemia[57]. For patients with symptoms of dyschezia and feeling of incomplete evacuation, anal pouch manometry may be used to identify functional abnormalities such as paradoxical contractions.
Pouchitis likely represents a disease spectrum from acute, antibiotic-responsive type to chronic, antibiotic-refractory entity. From various perspectives, pouchitis can be categorized into: (1) idiopathic vs secondary based on etiology, (2) remission vs active based on disease status, (3) acute vs chronic based on disease duration, (4) infrequent episodes vs relapsing or continuous based on disease course, and (5) responsive vs refractory based on response to antibiotic therapy[58]. A subpopulation of patients has pouchitis associated with identifiable and modifiable causes (namely secondary pouchitis), such as Clostridium difficile[59,60] or cytomegalovirus[61,62] infection, and regular use of NSAID[63].
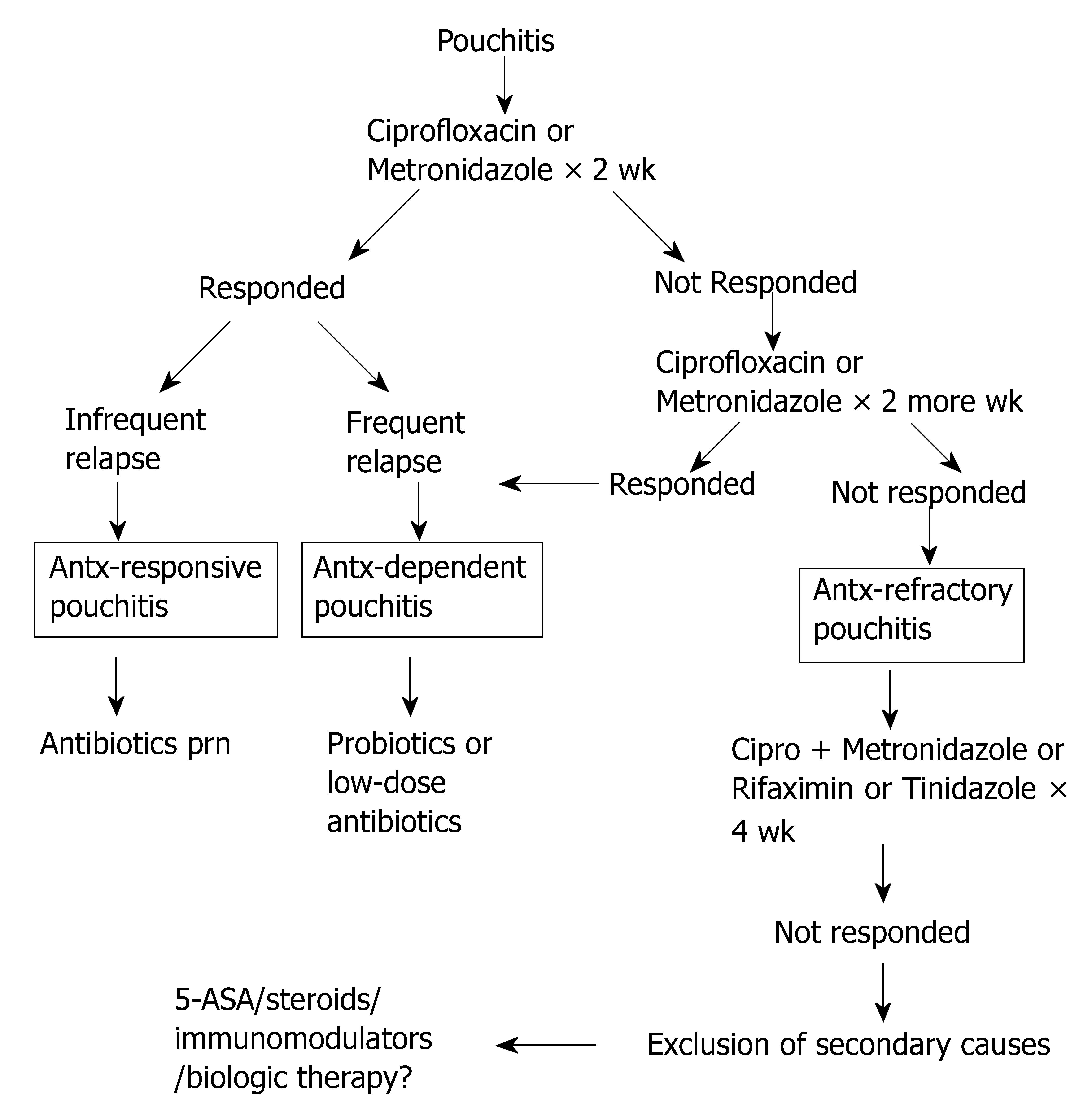
While the majority of patients with pouchitis respond favorably to antibiotic therapy particularly at initial stages of the disease, some patients develop pouchitis refractory to regular antibiotic treatment. This leads to another useful clinical classification based on the response to antibiotic therapy[64]. Analogous to the classification of UC according to the response to or dependency on corticosteroids, pouchitis can be classified as antibiotic-responsive, antibiotic-dependent, and antibiotic-refractory pouchitis[48,64] based on the manner of the patients' response to antibiotics (Figure 1).
As the majority of patients develop acute pouchitis within the first year after IPAA[65], VSL#3® containing 4 strains of Lactobacillus, 3 Bifidobacterium species, and Streptococcus salivarius subsp. Thermophillus was evaluated for the primary prophylaxis for the initial episode of pouchitis. Two of 20 patients (10%) treated with VSL#3® developed pouchitis within 12 mo after IPAA, while 8 of 20 patients (40%) experienced pouchitis in the placebo group during the same period of time[6].
The management and prognosis vary in different types of pouchitis (Figure 1). For antibiotic-responsive pouchitis, the first-line therapy includes a 14-d course of oral metronidazole (15-20 mg/kg per day) or ciprofloxacin (1000 mg/d)[66,67]. A randomized trial of ciprofloxacin and metronidazole showed that patients treated with ciprofloxacin experience significantly greater reductions in the PDAI scores and fewer adverse effects than those treated with metronidazole[67]. Other agents have been reported in open-labeled trials including tetracycline, clarithromycin, amoxicillin/clavulanic acid, doxycycline, rifaximin, and budesonide enemas[68], alicaforsen enemas, an anti-sense inhibitor of intercellular adhesion molecule-1[69], and AST-120, a highly adsorptive, porous, carbon microspheres[70].
Patients with antibiotic-dependent pouchitis often require long-term maintenance therapy with either antibiotics or probiotics to keep disease in remission. A randomized trial of VSL#3® at a dose of 6 g/d was conducted for the secondary prophylaxis for relapse of pouchitis, after remission was induced by oral ciprofloxacin (1000 mg/d) and rifaximin (2000 mg/d). During the 9-mo trial in 40 patients with relapsing pouchitis, only 15% in the probiotic group relapsed while 100% in the placebo group relapsed[11]. A separate randomized trial of VSL#3® in patients with antibiotic-dependent pouchitis showed that 17 of 20 patients (85%) in the VSL#3® group maintained clinical remission, compared to remission in 1 of 16 patients (6%) in the placebo group[12]. However, in a recent post-market open-labeled trial of VSL#3® in 31 patients with antibiotic-dependent pouchitis, patients received 2 wk of treatment with ciprofloxacin followed by VSL#3®[71]. After 8 mo, 6 of the 31 patients (19%) were still taking VSL#3® and the remaining 25 patients (81%) stopped the agent mainly because of lack of efficacy or development of adverse effects[71].
Antibiotic-refractory pouchitis which is often difficult to treat, is a common cause of pouch failure. Since the patients typically do not respond to full-dose, single-agent antibiotic therapy, it is important to investigate contributing causes (in secondary pouchitis) related to failure to antibiotic therapy. Secondary causes of refractory disease include use of NSAID, concurrent Clostridium difficile or cytomegalovirus infection, celiac disease and other autoimmune disorders, cuffitis, CD of the pouch, pouch ischemia, and inflammatory polyps of the pouch[72]. There are no randomized trials in the literature for this category of pouchitis. For patients without obvious causes, treatment options include a prolonged course of combined antibiotic therapy, 5-aminosalicylates, corticosteroids, immunosuppressive agents or even biological therapy. Regimens reported in open-labeled trials include combined ciprofloxacin (1000 mg/d) with rifaximin (2000 mg/d)[73] or metronidazole (1000 mg/d)[74] or tinidazole (1000-1500 mg/d) for 4 wk[75]. However, maintenance of remission in this group of patients after the induction therapy with dual antibiotics remains a challenge[76]. Anti-inflammatory agents, immunomodulators, and biological therapy have been used to treat pouchitis. These agents include bismuth carbomer enemas, short-chain fatty acid enemas, and glutamine enemas, mesalamine enemas, oral budesonide[77], 6-mercaptopurine, and infliximab.
The natural history of pouchitis is not entirely clear. In a study consisting of 100 consecutive UC patients who had restorative proctocolectomy with IPAA, 32 patients developed pouchitis, 5 had chronic refractory pouchitis, 2 of them had pouch failure after pouch resection[58]. Few studies were performed to identify the natural history of pouch and pouchitis. Patients with initial pouchitis almost uniformly respond to antibiotic therapy. However, relapse of pouchitis is common. Of the patients with acute pouchitis, 39% have a single acute episode that responds to antibiotic therapy whereas the remaining 61% of patients develop at least one recurrence[35]. Approximately 5% to 19% patients with acute pouchitis develop refractory or rapidly relapsing symptoms[78-80]. Here is a common scenario: the more frequent the episodes of pouchitis a patient has, the more often the antibiotic therapy is administered, the less likely the patient maintains favorable response to the treatment. The course of antibiotic-responsive pouchitis could evolve into antibiotic-dependent pouchitis followed by antibiotic-refractory pouchitis. Chronic refractory pouchitis is one of the most common causes for pouch failure. Although PSC is a risk factor for pouchitis[3,44,45], liver transplantation with post-transplant use of immunosuppressive agents does not appear to have adverse effects on the course of pouchitis[81,82]. In addition, chronic inflammation of the pouch and cuff may pose an increased risk of developing dysplasia or cancer [83,84].
In summary, pouchitis is the most common long-term adverse sequela of IPAA after restorative proctocolectomy. The natural history of pouchitis is yet to be defined. Patients with pouchitis can have a wide range of clinical presentations, disease courses, and prognoses. Accurate diagnosis and classification of pouchitis are the keys to appropriate management. Treatment of pouchitis is largely antibiotic-based. Maintenance of remission in antibiotic-dependent pouchitis and management of antibiotic-refractory pouchitis are a challenge. Secondary causes for refractory pouchitis should be excluded.
| 1. | Dhillon S, Loftus EV Jr, Tremaine WJ, Jewell DA, Harmsen WS, Zinsmeister AR, Melton LJ, Pemberton JH, Wolff BG, Dozois EJ. The natural history of surgery for ulcerative colitis in a population-based cohort from Olmsted County, Minnesota. Am J Gastroenterol. 2005;100:A819. |
| 2. | Shen B, Fazio VW, Remzi FH, Delaney CP, Bennett AE, Achkar JP, Brzezinski A, Khandwala F, Liu W, Bambrick ML. Comprehensive evaluation of inflammatory and noninflammatory sequelae of ileal pouch-anal anastomoses. Am J Gastroenterol. 2005;100:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 159] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Penna C, Dozois R, Tremaine W, Sandborn W, LaRusso N, Schleck C, Ilstrup D. Pouchitis after ileal pouch-anal anastomosis for ulcerative colitis occurs with increased frequency in patients with associated primary sclerosing cholangitis. Gut. 1996;38:234-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 351] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 4. | Fazio VW, Ziv Y, Church JM, Oakley JR, Lavery IC, Milsom JW, Schroeder TK. Ileal pouch-anal anastomoses complications and function in 1005 patients. Ann Surg. 1995;222:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 947] [Cited by in RCA: 859] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 5. | Stocchi L, Pemberton JH. Pouch and pouchitis. Gastroenterol Clin North Am. 2001;30:223-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 75] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, Vitali B, Poggioli G, Miglioli M, Campieri M. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 807] [Cited by in RCA: 712] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 7. | Penna C, Tiret E, Kartheuser A, Hannoun L, Nordlinger B, Parc R. Function of ileal J pouch-anal anastomosis in patients with familial adenomatous polyposis. Br J Surg. 1993;80:765-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 55] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Tjandra JJ, Fazio VW, Church JM, Oakley JR, Milsom JW, Lavery IC. Similar functional results after restorative proctocolectomy in patients with familial adenomatous polyposis and mucosal ulcerative colitis. Am J Surg. 1993;165:322-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 79] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Sandborn WJ. Pouchitis following ileal pouch-anal anastomosis: definition, pathogenesis, and treatment. Gastroenterology. 1994;107:1856-1860. [PubMed] |
| 10. | Gosselink MP, Schouten WR, van Lieshout LM, Hop WC, Laman JD, Ruseler-van Embden JG. Delay of the first onset of pouchitis by oral intake of the probiotic strain Lactobacillus rhamnosus GG. Dis Colon Rectum. 2004;47:876-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 137] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, Poggioli G, Miglioli M, Campieri M. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1077] [Cited by in RCA: 961] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 12. | Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, Nicholls RJ, Gionchetti P, Campieri M, Kamm MA. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53:108-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 618] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 13. | Komanduri S, Gillevet PM, Sikaroodi M, Mutlu E, Keshavarzian A. Dysbiosis in pouchitis: evidence of unique microfloral patterns in pouch inflammation. Clin Gastroenterol Hepatol. 2007;5:352-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Duffy M, O'Mahony L, Coffey JC, Collins JK, Shanahan F, Redmond HP, Kirwan WO. Sulfate-reducing bacteria colonize pouches formed for ulcerative colitis but not for familial adenomatous polyposis. Dis Colon Rectum. 2002;45:384-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 102] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Nasmyth DG, Godwin PG, Dixon MF, Williams NS, Johnston D. Ileal ecology after pouch-anal anastomosis or ileostomy. A study of mucosal morphology, fecal bacteriology, fecal volatile fatty acids, and their interrelationship. Gastroenterology. 1989;96:817-824. [PubMed] |
| 16. | de Silva HJ, Jones M, Prince C, Kettlewell M, Mortensen NJ, Jewell DP. Lymphocyte and macrophage subpopulations in pelvic ileal pouches. Gut. 1991;32:1160-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Hirata I, Austin LL, Blackwell WH, Weber JR, Dobbins WO. Immunoelectron microscopic localization of HLA-DR antigen in control small intestine and colon and in inflammatory bowel disease. Dig Dis Sci. 1986;31:1317-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Stallmach A, Schäfer F, Hoffmann S, Weber S, Müller-Molaian I, Schneider T, Köhne G, Ecker KW, Feifel G, Zeitz M. Increased state of activation of CD4 positive T cells and elevated interferon gamma production in pouchitis. Gut. 1998;43:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Goldberg PA, Herbst F, Beckett CG, Martelli B, Kontakou M, Talbot IC, Ciclitira PJ, Nicholls RJ. Leucocyte typing, cytokine expression, and epithelial turnover in the ileal pouch in patients with ulcerative colitis and familial adenomatous polyposis. Gut. 1996;38:549-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Patel RT, Bain I, Youngs D, Keighley MR. Cytokine production in pouchitis is similar to that in ulcerative colitis. Dis Colon Rectum. 1995;38:831-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Schmidt C, Giese T, Ludwig B, Menges M, Schilling M, Meuer SC, Zeuzem S, Stallmach A. Increased cytokine transcripts in pouchitis reflect the degree of inflammation but not the underlying entity. Int J Colorectal Dis. 2006;21:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Gionchetti P, Campieri M, Belluzzi A, Bertinelli E, Ferretti M, Brignola C, Poggioli G, Miglioli M, Barbara L. Mucosal concentrations of interleukin-1 beta, interleukin-6, interleukin-8, and tumor necrosis factor-alpha in pelvic ileal pouches. Dig Dis Sci. 1994;39:1525-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Patel RT, Pall AA, Adu D, Keighley MR. Circulating soluble adhesion molecules in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1995;7:1037-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Chaussade S, Denizot Y, Valleur P, Nicoli J, Raibaud P, Guerre J, Hautefeuille P, Couturier D, Benveniste J. Presence of PAF-acether in stool of patients with pouch ileoanal anastomosis and pouchitis. Gastroenterology. 1991;100:1509-1514. [PubMed] |
| 25. | Gertner DJ, Rampton DS, Madden MV, Talbot IC, Nicholls RJ, Lennard-Jones JE. Increased leukotriene B4 release from ileal pouch mucosa in ulcerative colitis compared with familial adenomatous polyposis. Gut. 1994;35:1429-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Stucchi AF, Shebani KO, Leeman SE, Wang CC, Reed KL, Fruin AB, Gower AC, McClung JP, Andry CD, O'Brien MJ. A neurokinin 1 receptor antagonist reduces an ongoing ileal pouch inflammation and the response to a subsequent inflammatory stimulus. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1259-G1267. [PubMed] |
| 27. | Stallmach A, Chan CC, Ecker KW, Feifel G, Herbst H, Schuppan D, Zeitz M. Comparable expression of matrix metalloproteinases 1 and 2 in pouchitis and ulcerative colitis. Gut. 2000;47:415-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Ulisse S, Gionchetti P, D'Alò S, Russo FP, Pesce I, Ricci G, Rizzello F, Helwig U, Cifone MG, Campieri M. Expression of cytokines, inducible nitric oxide synthase, and matrix metalloproteinases in pouchitis: effects of probiotic treatment. Am J Gastroenterol. 2001;96:2691-2699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 134] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | Thomas PD, Forbes , Nicholls RJ, Ciclitira PJ. Increased mucosal IFN-γ production in pouchitis despite normal functional responses of isolated CD4 cells. Gut. 1999;44 suppl 4:32A. |
| 30. | Bulois P, Tremaine WJ, Maunoury V, Gambiez L, Hafraoui S, Leteurtre L, Cortot A, Sandborn WJ, Colombel JF, Desreumaux P. Pouchitis is associated with mucosal imbalance between interleukin-8 and interleukin-10. Inflamm Bowel Dis. 2000;6:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Bell AJ, Nicholls RJ, Forbes A, Ellis HJ, Ciclitira PJ. Human lymphocyte stimulation with pouchitis flora is greater than with flora from a healthy pouch but is suppressed by metronidazole. Gut. 2004;53:1801-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Biancone L, Palmieri G, Lombardi A, Colantoni A, Tonelli F, Das KM, Pallone F. Tropomyosin expression in the ileal pouch: a relationship with the development of pouchitis in ulcerative colitis. Am J Gastroenterol. 2003;98:2719-2726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Tysk C, Riedesel H, Lindberg E, Panzini B, Podolsky D, Järnerot G. Colonic glycoproteins in monozygotic twins with inflammatory bowel disease. Gastroenterology. 1991;100:419-423. [PubMed] |
| 34. | Merrett MN, Soper N, Mortensen N, Jewell DP. Intestinal permeability in the ileal pouch. Gut. 1996;39:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Lohmuller JL, Pemberton JH, Dozois RR, Ilstrup D, van Heerden J. Pouchitis and extraintestinal manifestations of inflammatory bowel disease after ileal pouch-anal anastomosis. Ann Surg. 1990;211:622-627; discussion 627-629. [PubMed] |
| 36. | Merrett MN, Mortensen N, Kettlewell M, Jewell DO. Smoking may prevent pouchitis in patients with restorative proctocolectomy for ulcerative colitis. Gut. 1996;38:362-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 119] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Carter MJ, Di Giovine FS, Cox A, Goodfellow P, Jones S, Shorthouse AJ, Duff GW, Lobo AJ. The interleukin 1 receptor antagonist gene allele 2 as a predictor of pouchitis following colectomy and IPAA in ulcerative colitis. Gastroenterology. 2001;121:805-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 92] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 38. | Brett PM, Yasuda N, Yiannakou JY, Herbst F, Ellis HJ, Vaughan R, Nicholls RJ, Ciclitira PJ. Genetic and immunological markers in pouchitis. Eur J Gastroenterol Hepatol. 1996;8:951-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Aisenberg J, Legnani PE, Nilubol N, Cobrin GM, Ellozy SH, Hegazi RA, Yager J, Bodian C, Gorfine SR, Bauer JJ. Are pANCA, ASCA, or cytokine gene polymorphisms associated with pouchitis? Long-term follow-up in 102 ulcerative colitis patients. Am J Gastroenterol. 2004;99:432-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Meier CB, Hegazi RA, Aisenberg J, Legnani PE, Nilubol N, Cobrin GM, Duerr RH, Gorfine SR, Bauer JJ, Sachar DB. Innate immune receptor genetic polymorphisms in pouchitis: is CARD15 a susceptibility factor? Inflamm Bowel Dis. 2005;11:965-971. [PubMed] |
| 41. | Schmidt CM, Lazenby AJ, Hendrickson RJ, Sitzmann JV. Preoperative terminal ileal and colonic resection histopathology predicts risk of pouchitis in patients after ileoanal pull-through procedure. Ann Surg. 1998;227:654-662; discussion 663-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 107] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Achkar JP, Al-Haddad M, Lashner B, Remzi FH, Brzezinski A, Shen B, Khandwala F, Fazio V. Differentiating risk factors for acute and chronic pouchitis. Clin Gastroenterol Hepatol. 2005;3:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 43. | Okon A, Dubinsky M, Vasiliauskas EA, Papadakis KA, Ippoliti A, Targan SR, Fleshner PR. Elevated platelet count before ileal pouch-anal anastomosis for ulcerative colitis is associated with the development of chronic pouchitis. Am Surg. 2005;71:821-826. [PubMed] |
| 44. | Shepherd NA, Hultén L, Tytgat GN, Nicholls RJ, Nasmyth DG, Hill MJ, Fernandez F, Gertner DJ, Rampton DS, Hill MJ. Pouchitis. Int J Colorectal Dis. 1989;4:205-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 111] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Hata K, Watanabe T, Shinozaki M, Nagawa H. Patients with extraintestinal manifestations have a higher risk of developing pouchitis in ulcerative colitis: multivariate analysis. Scand J Gastroenterol. 2003;38:1055-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 46. | Fleshner PR, Vasiliauskas EA, Kam LY, Fleshner NE, Gaiennie J, Abreu-Martin MT, Targan SR. High level perinuclear antineutrophil cytoplasmic antibody (pANCA) in ulcerative colitis patients before colectomy predicts the development of chronic pouchitis after ileal pouch-anal anastomosis. Gut. 2001;49:671-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 173] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 47. | Kuisma J, Järvinen H, Kahri A, Färkkilä M. Factors associated with disease activity of pouchitis after surgery for ulcerative colitis. Scand J Gastroenterol. 2004;39:544-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 48. | Shen B, Fazio VW, Remzi FH, Brzezinski A, Bennett AE, Lopez R, Hammel JP, Achkar JP, Bevins CL, Lavery IC. Risk factors for diseases of ileal pouch-anal anastomosis after restorative proctocolectomy for ulcerative colitis. Clin Gastroenterol Hepatol. 2006;4:81-89; quiz 2-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 148] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 49. | Hui T, Landers C, Vasiliauskas E, Abreu M, Dubinsky M, Papadakis KA, Price J, Lin YC, Huiying Y, Targan S. Serologic responses in indeterminate colitis patients before ileal pouch-anal anastomosis may determine those at risk for continuous pouch inflammation. Dis Colon Rectum. 2005;48:1254-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 50. | Pastrana RJ, Torres EA, Arroyo JM, Rivera CE, Sánchez CJ, Morales L. Iron-deficiency anemia as presentation of pouchitis. J Clin Gastroenterol. 2007;41:41-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 51. | Oikonomou IK, Fazio VW, Remzi FH, Lopez R, Lashner BA, Shen B. Risk factors for anemia in patients with ileal pouch-anal anastomosis. Dis Colon Rectum. 2007;50:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Shen B, Achkar JP, Lashner BA, Ormsby AH, Remzi FH, Bevins CL, Brzezinski A, Petras RE, Fazio VW. Endoscopic and histologic evaluation together with symptom assessment are required to diagnose pouchitis. Gastroenterology. 2001;121:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 176] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 53. | Moskowitz RL, Shepherd NA, Nicholls RJ. An assessment of inflammation in the reservoir after restorative proctocolectomy with ileoanal ileal reservoir. Int J Colorectal Dis. 1986;1:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 245] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 54. | Sandborn WJ, Tremaine WJ, Batts KP, Pemberton JH, Phillips SF. Pouchitis after ileal pouch-anal anastomosis: a Pouchitis Disease Activity Index. Mayo Clin Proc. 1994;69:409-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 550] [Cited by in RCA: 525] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 55. | Nadgir RN, Soto JA, Dendrinos K, Lucey BC, Becker JM, Farraye FA. MRI of complicated pouchitis. AJR Am J Roentgenol. 2006;187:W386-W391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 56. | Calabrese C, Fabbri A, Gionchetti P, Rizzello F, Morselli C, Liguori G, Poggioli G, Campieri M, Di Febo G. Controlled study using wireless capsule endoscopy for the evaluation of the small intestine in chronic refractory pouchitis. Aliment Pharmacol Ther. 2007;25:1311-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 57. | Shen B, Remzi FH, Santisi J, Lashner BA, Brzezinski A, Fazio VW. Application of wireless capsule endoscopy for the evaluation of iron deficiency anemia in patients with ileal pouches. J Clin Gastroenterol. 2008;42:897-902. [PubMed] |
| 58. | Sandborn WJ. Pouchitis: Risk factors, frequency, natural history, classification and public health prospective. Trends in Inflammatory Bowel Disease 1996. Lancaster: Kluwer Academic Publishers 1997; 51-63. |
| 59. | Mann SD, Pitt J, Springall RG, Thillainayagam AV. Clostridium difficile infection--an unusual cause of refractory pouchitis: report of a case. Dis Colon Rectum. 2003;46:267-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 60. | Shen B, Goldblum JR, Hull TL, Remzi FH, Bennett AE, Fazio VW. Clostridium difficile-associated pouchitis. Dig Dis Sci. 2006;51:2361-2364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 61. | Muñoz-Juarez M, Pemberton JH, Sandborn WJ, Tremaine WJ, Dozois RR. Misdiagnosis of specific cytomegalovirus infection of the ileoanal pouch as refractory idiopathic chronic pouchitis: report of two cases. Dis Colon Rectum. 1999;42:117-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 67] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 62. | Moonka D, Furth EE, MacDermott RP, Lichtenstein GR. Pouchitis associated with primary cytomegalovirus infection. Am J Gastroenterol. 1998;93:264-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 63. | Shen B, Fazio VW, Remzi FH, Bennett AE, Lopez R, Lavery IC, Brzezinski A, Sherman KK, Lashner BA. Effect of withdrawal of nonsteroidal anti-inflammatory drug use on ileal pouch disorders. Dig Dis Sci. 2007;52:3321-3328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 64. | Shen B. Diagnosis and treatment of patients with pouchitis. Drugs. 2003;63:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 65. | Ståhlberg D, Gullberg K, Liljeqvist L, Hellers G, Löfberg R. Pouchitis following pelvic pouch operation for ulcerative colitis. Incidence, cumulative risk, and risk factors. Dis Colon Rectum. 1996;39:1012-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 175] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 66. | Madden MV, McIntyre AS, Nicholls RJ. Double-blind crossover trial of metronidazole versus placebo in chronic unremitting pouchitis. Dig Dis Sci. 1994;39:1193-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 187] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 67. | Shen B, Achkar JP, Lashner BA, Ormsby AH, Remzi FH, Brzezinski A, Bevins CL, Bambrick ML, Seidner DL, Fazio VW. A randomized clinical trial of ciprofloxacin and metronidazole to treat acute pouchitis. Inflamm Bowel Dis. 2001;7:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 237] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 68. | Sambuelli A, Boerr L, Negreira S, Gil A, Camartino G, Huernos S, Kogan Z, Cabanne A, Graziano A, Peredo H. Budesonide enema in pouchitis--a double-blind, double-dummy, controlled trial. Aliment Pharmacol Ther. 2002;16:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 113] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 69. | Miner P, Wedel M, Bane B, Bradley J. An enema formulation of alicaforsen, an antisense inhibitor of intercellular adhesion molecule-1, in the treatment of chronic, unremitting pouchitis. Aliment Pharmacol Ther. 2004;19:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 70. | Shen B, Pardi DS, Bennett AE, Sherman KK. A pilot study of the efficacy and tolerability of AST-120 in the treatment of active pouchitis. Am J Gastroenterol. 2007;102:(Abstract). |
| 71. | Shen B, Brzezinski A, Fazio VW, Remzi FH, Achkar JP, Bennett AE, Sherman K, Lashner BA. Maintenance therapy with a probiotic in antibiotic-dependent pouchitis: experience in clinical practice. Aliment Pharmacol Ther. 2005;22:721-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 72. | Schaus BJ, Fazio VW, Remzi FH, Bennett AE, Lashner BA, Shen B. Clinical features of ileal pouch polyps in patients with underlying ulcerative colitis. Dis Colon Rectum. 2007;50:832-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 73. | Gionchetti P, Rizzello F, Venturi A, Ugolini F, Rossi M, Brigidi P, Johansson R, Ferrieri A, Poggioli G, Campieri M. Antibiotic combination therapy in patients with chronic, treatment-resistant pouchitis. Aliment Pharmacol Ther. 1999;13:713-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 162] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 74. | Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, Nicholls RJ, Gionchetti P, Campieri M, Kamm MA. Four-week open-label trial of metronidazole and ciprofloxacin for the treatment of recurrent or refractory pouchitis. Aliment Pharmacol Ther. 2002;16:909-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 134] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 75. | Shen B, Fazio VW, Remzi FH, Bennett AE, Lopez R, Brzezinski A, Oikonomou I, Sherman KK, Lashner BA. Combined ciprofloxacin and tinidazole therapy in the treatment of chronic refractory pouchitis. Dis Colon Rectum. 2007;50:498-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 76. | Viscido A, Kohn A, Papi C, Caprilli R. Management of refractory fistulizing pouchitis with infliximab. Eur Rev Med Pharmacol Sci. 2004;8:239-246. [PubMed] |
| 77. | Gionchetti P, Rizzello F, Poggioli G, Pierangeli F, Laureti S, Morselli C, Tambasco R, Calabrese C, Campieri M. Oral budesonide in the treatment of chronic refractory pouchitis. Aliment Pharmacol Ther. 2007;25:1231-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 78. | Mowschenson PM, Critchlow JF, Peppercorn MA. Ileoanal pouch operation: long-term outcome with or without diverting ileostomy. Arch Surg. 2000;135:463-465; discussion 465-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 79. | Hurst RD, Chung TP, Rubin M, Michelassi F. The implications of acute pouchitis on the long-term functional results after restorative proctocolectomy. Inflamm Bowel Dis. 1998;4:280-284. [PubMed] |
| 80. | Madiba TE, Bartolo DC. Pouchitis following restorative proctocolectomy for ulcerative colitis: incidence and therapeutic outcome. J R Coll Surg Edinb. 2001;46:334-337. [PubMed] |
| 81. | Zins BJ, Sandborn WJ, Penna CR, Landers CJ, Targan SR, Tremaine WJ, Wiesner RH, Dozois RR. Pouchitis disease course after orthotopic liver transplantation in patients with primary sclerosing cholangitis and an ileal pouch-anal anastomosis. Am J Gastroenterol. 1995;90:2177-2181. [PubMed] |
| 82. | Freeman K, Shao Z, Remzi FH, Fazio VE, Lopez R, Shen B. Orthotopic liver Transplantation for primary sclerosing cholangitis in patients with ulcerative colitis: Impact on occurrence of chronic pouchitis. Gastroenterology. 2007;132:A516. |
| 83. | Das P, Johnson MW, Tekkis PP, Nicholls RJ. Risk of dysplasia and adenocarcinoma following restorative proctocolectomy for ulcerative colitis. Colorectal Dis. 2007;9:15-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 84. | Thompson-Fawcett MW, Marcus V, Redston M, Cohen Z, McLeod RS. Risk of dysplasia in long-term ileal pouches and pouches with chronic pouchitis. Gastroenterology. 2001;121:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
S- Editor Liu Y L- Editor Wang XL E- Editor Li HY