Published online Oct 28, 2007. doi: 10.3748/wjg.v13.i40.5317
Revised: August 17, 2007
Accepted: September 14, 2007
Published online: October 28, 2007
AIM: To assess the safety and efficacy of antioxidant therapy for patients with chronic hepatitis C virus (HCV) infection.
METHODS: One hundred chronic HCV infection patients failed in interferon treatment were enrolled and randomly assigned to receive combined intravenous and oral antioxidants or placebo, or oral treatment alone. Primary end points were liver enzymes, HCV-RNA levels and histology.
RESULTS: Combined oral and intravenous antioxidant therapy was associated with a significant decline in ALT levels in 52% of patients who received antioxidant therapy vs 20% of patients who received placebo (P = 0.05). Histology activity index (HAI) score at the end of treatment was reduced in 48% of patients who received antioxidant therapy vs 26% of patients who received placebo (P = 0.21). HCV-RNA levels decreased by 1-log or more in 28% of patients who received antioxidant therapy vs 12% who received placebo (P = NS). In part II of the trial, oral administration of antioxidants was not associated with significant alterations in any of the end points.
CONCLUSION: Antioxidant therapy has a mild beneficial effect on the inflammatory response of chronic HCV infection patients who are non-responders to interferon. Combined antiviral and antioxidant therapy may be beneficial for these patients.
- Citation: Gabbay E, Zigmond E, Pappo O, Hemed N, Rowe M, Zabrecky G, Cohen R, Ilan Y. Antioxidant therapy for chronic hepatitis C after failure of interferon: Results of phase II randomized, double-blind placebo controlled clinical trial. World J Gastroenterol 2007; 13(40): 5317-5323
- URL: https://www.wjgnet.com/1007-9327/full/v13/i40/5317.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i40.5317
The pathogenesis of hepatitis C virus (HCV) infection involves a complex interaction between viral factors and host immune responses. A major component of the latter involves oxidative stress[1]. Oxidative stress has been attributed to both host inflammatory processes and induction by viral proteins, with the two mechanisms possibly acting in synergy. HCV non-structural proteins have been shown to induce activation of STAT-3 via oxidative stress and Ca2+ signaling[2]. This induction is influenced by the activation of cellular kinases, including p38 mitogen-activated protein kinase, JNK, JAK-2 and Src, and inhibited in vitro in the presence of antioxidant 3. It was reported that HCV core protein increases radical oxygen species (ROS) as well as lipid peroxidation products and antioxidant gene expression[3]. Increased intrahepatic lipid peroxidation products have been observed in HCV transgenic mice, following exposure to carbon tetrachloride[4]. These processes may contribute to fibrosis and carcinogenesis in chronic HCV[5]. Analysis of HCV-sub genomic RNA replicon cell lines has shown a drastic reduction in cellular glutathione peroxidase, increasing cellular susceptibility to oxidative stress[6]. Based on these data, a rationale for antioxidant treatment of chronic hepatitis C was suggested. Currently, the mainstay of treatment of chronic hepatitis C is a combination of pegylated interferons and ribavirin[7]. However, the overall response rate of its pharmacological efficacy and adverse effects is approximately 55% in genotype I patients[8].
The use of complimentary and alternative medicine is common in patients with chronic liver disease. In a recent study, 41% of patients reported use of these modalities, with 20% reporting use of herbal medicine, including sylamarin (12%), licorice root (glycyrrhizin) and St. John's wart[9]. In a review of medicinal herbs for HCV infection, the authors concluded that some of these agents may have an effect on liver enzymes but there is no firm evidence that supports their efficacy on chronic HCV[10].
In a recently published phase-I, open label clinical trial, 50 patients with chronic HCV infection were treated orally with a combination of seven antioxidative oral preparations (glycyrrhizin, schisandra, silymarin, ascorbic acid, lipoic acid, L-glutathione, and alpha-tocopherol) on a daily basis for 20 wk, along with four different intravenous preparations (glycyrrhizin, ascorbic acid, L-glutathione, B-complex) twice weekly for the first 10 wk[11]. Normalization of liver enzymes occurred in 44% of patients who had elevated pretreatment ALT levels. A decrease in viral load by one log or more was observed in 25% of the patients. Histological improvement, with two-point reduction in the HAI score, was noted in 36.1% of the patients. The SF-36 quality of life score improved in 26 of 45 patients (58%) throughout the trial. No major adverse reactions were noted. These findings suggest that antioxidant therapy may have a beneficial effect on necro-inflammatory variables in these patients.
The aim of the present trial was to determine the effect of a mixture of antioxidants on the inflammatory response of chronic HCV infection patients who were non-responders to interferon, in a double-blind placebo controlled trial. Two different treatment regimens were studied and compared. The data suggest that combined intravenous and oral antioxidant therapy mildly alleviates the inflammatory response in these patients.
A total of 100 chronic HCV infection patients were enrolled in a double-blind, placebo controlled single-center trial. The study focused on 2 treatment options, with 50 patients in each section. Part I looked at administration of intravenous and oral antioxidant preparations versus placebo. Part II tested administration of oral preparations only versus placebo. Patients were randomly assigned to treatment or placebo groups, 25 patients in each group. Antioxidants were administered as described below. All experiments were carried out in accordance with the guidelines of the Hebrew University-Hadassah Institutional Committee for Human Clinical Trials. The Israel Ministry of Health Committee for Human Trials approved all experiments.
Inclusion criteria: Eligible participants were male and female chronic HCV infection patients at the age of 18-75 years. All patients were required to display positive HCV RNA on two tests for at least 6 mo prior to enrollment. Diagnosis of chronic HCV infection was based on positive HCV RNA levels with liver biopsy (within 1 year of initiation of study) with or without elevated liver enzymes. Informed consent was obtained from patients prior to participation in the trial. Patients screened for the trial included those in whom treatment with interferon had previously failed, or was contra-indicated. Both partial responders and non-responders were enrolled. Abstinence from any alternative medications or vitamin supplements for 6 mo prior to initiation of therapy was stipulated.
Exclusion criteria: Patients excluded from the trial included those with decompensated liver disease as evidenced by Child's B or C status; those with creatinine levels above 150 μmol/L, hemoglobin levels below 100 mg/L, white blood cell count below 3 × 109/L, or platelet count below 1 × 1011/L; those with a history of varices, ascites, or encephalopathy. Also ineligible were patients who exhibited irreversible neurologic deficit or active co-infection with HBV, HAV, HDV, or HIV. Those treated with interferon or pegylated interferon or ribavirin less than 6 mo prior to enrollment or with a history of having received any systemic anti-neoplastic or immune modulatory treatment 6 mo prior to the first dose of study medication were excluded. Evidence of other causes of liver disease or other severe debilitating diseases precluded the patient from participating in this study. Patients who used alcohol or anti-viral medication during the trial, pregnancy or breast-feeding, and had a history of hepatic, renal, or other major organ transplantation were also exclude from the study.
In part I, 50 patients (30 males and 20 females) with a mean age of 56.1 (range, 22-75) years were enrolled in the trial. Sixty-four percent of patients (n = 32) had elevated liver enzymes on screening, and 36% (n = 18) had normal liver enzymes despite viremia and active inflammation on biopsy. All other markers for additional causes of liver disease were negative. HCV genotype 1, genotype 1a, and genotype 1b were found in 33, 5, and 28 patients, respectively. HCV genotype 2, genotype 3, and genotype 4 were found in 8, 6 and 3 patients, respectively.
In part II, 50 patients (34 males and 16 females) were randomly assigned to treatment (n = 25) and placebo (n = 25) groups. Their mean age was 57.4 (range 24-75) years. Thirty-five patients had genotype 1 (31 had genotype 1b and 4 had genotype 1a). Two patients had genotype 2a2c, two had genotype 3a, and one had genotype 4e.
Patients in the treatment group received a combination of seven different anti-oxidants orally at the appropriate dose (glycyrrhiza capsules, 500 mg, bid; schizandrae capsules, 500 mg, tid; ascorbate capsules, 2000 mg, tid; L-glutathione capsules, 150 mg, bid; silymarin capsules, 250 mg, tid; lipoic Acid capsules, 150 mg, bid; d-alpha tocopherol, 800 IU/d) prepared by Vital Nutrients Middletown (CT, USA), once daily for 24 wk. In addition, all patients in part I of the trial received intravenously a combination of four different preparations (120 mg glycyrrhiza, 10 g ascorbate, 750 mg L-glutathione, 1 mL B-complex) at the appropriate dose, twice a week for the first 10 wk of the study. Medications were prepared by GY&N Nutriment Pharmacology, Unique Pharmaceuticals Company, Palmdale, CA, USA. Dosages were determined based on previous trials in humans. Products for intravenous injection were added to 400 mL of normal saline for infusion. Patients were followed up for an additional 24 wk following completion of the treatment as described below. Patients in the placebo group received identical pills and normal saline intravenously.
Patients were followed up for clinical, biochemical, virological, and histological parameters. Patients underwent a bi-weekly physical examination throughout the treatment period, and every 4 wk during the follow-up period. Serum bilirubin and liver enzymes, kidney function, prothrombin time, and complete blood counts were checked bi-weekly during the treatment period and every 4 wk during follow-up. HCV RNA was detected every 4 wk throughout the study by standard assays (Roche Diagnostics Systems Inc., Branchburg, NJ). The lower limit of detection for this assay was 100 copies/L. All patients underwent a liver biopsy within 2 years prior to treatment. A repeat biopsy was performed 2 to 6 wk following completion of the antioxidant therapy. Intra-hepatic inflammatory score was evaluated by two blinded pathologists using the standard histological activity index (HAI). Evaluation of the effect of treatment on quality of life was performed using the eighth paradigm (self assessment of general health) of the SF-36 quality of life questionnaire. The questionnaire was completed 2 wk before and within 4 wk after the study.
The objectives of the study were: (1) evaluation of the safety and tolerability of intravenous and oral administration of glycyrrhizin, antioxidants, and vitamins in patients with chronic HCV infection; (2) evaluation of the effect of glycyrrhizin, antioxidants, and vitamins on inflammatory indices and HCV viral load. The two primary variables were: (1) safety and tolerability of intravenous and oral administration of glycyrrhizin, antioxidants, and vitamins in patients with chronic HCV infection; (2) amelioration of inflammation and existing liver damage, and reduction in viral load.
Data were analyzed using Fisher's exact test. P < 0.05 was considered statistically significant.
In part I, no major adverse effects related to treatment were noted. Side effects possibly related to the mineral corticoid effect of glycyrrhizin treatment included hypokalemia in 6 patients of the treatment group (24%), in 5 patients of the placebo group (20%), and mild exacerbation of hypertension in 1 patient of the treatment group. All cases were mild and treated symptomatically. Two patients in the treatment group developed pedal edema and a sense of bloating. One patient in the treatment group was hospitalized twice due to fever during the trial period which was considered unrelated to treatment.
In part II, One patient in the treatment group had exacerbation of hypertension at wk 6 of treatment, requiring treatment with enalapril and lercanidipine. Subsequent blood pressure during treatment was normal. Six patients in the treatment group had hypokalemia of 2.9 to 3.3 and one patient in the placebo group had hypokalemia of 3.4. One patient in the treatment group had a high systolic blood pressure (up to 190 mmHg) on the day of his second biopsy, and resolved after administration of oral oxazepam. One patient in the treatment group had a weight loss of 5.5 kg during treatment, but regained three kg by wk 32. One patient in the placebo group reported weakness, upper abdominal pain and arthralgia.
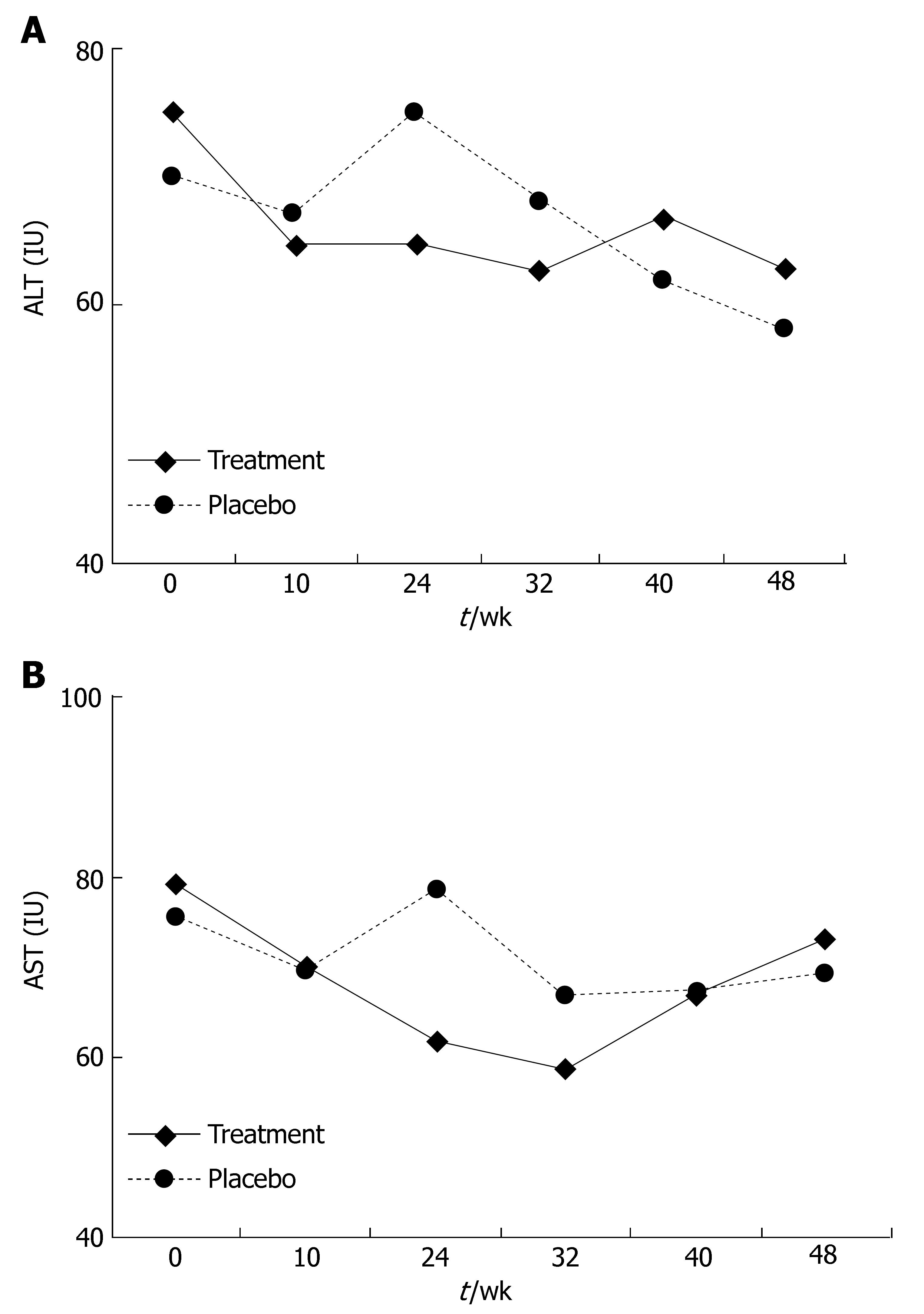
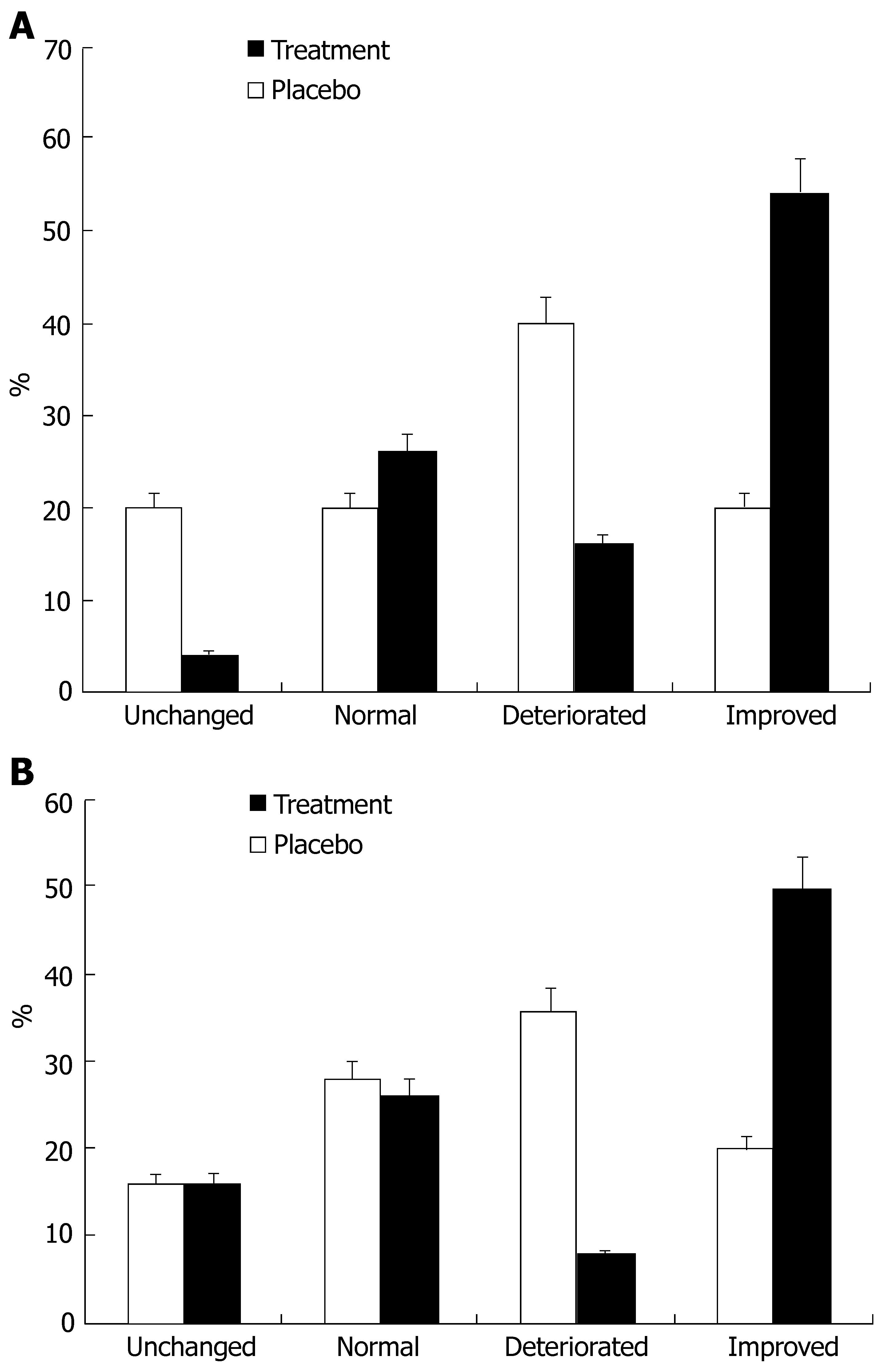
In part I, at the end of 24 wk of treatment a decline in ALT and AST levels was observed in the treatment group compared to the placebo group. The ALT level at this point was 64.8 IU in the treatment group and 75.04 IU in the placebo group (P = 0.42, Figure 1A), the AST level at this point was 61.72 IU in the treatment group and 78 IU in the placebo group (P = 0.1, Figure 1B). This effect diminished during the follow-up period. At wk 48, the ALT level was 63 IU in the treatment group and 58.27 IU in the placebo group (P = 0.68), the AST level was 73.13 IU in the treatment group and 69.33 IU in the placebo group (P = 0.75). At the end of the treatment period, the ALT level improved in 54% of treated patients and in 20% of patients receiving the placebo (P = 0.05, Figure 2A), the AST level declined in 50% of treated patients and 20% of patients receving placebo (P = 0.02, Figure 2B). At the end of the follow-up period (at wk 48), the ALT level improved in 46% of patients in the treatment group and in 45% of patients in the placebo group, the AST level improved in 35% of patients in the treatment group and 34% of patients in the placebo group compared to their baseline.
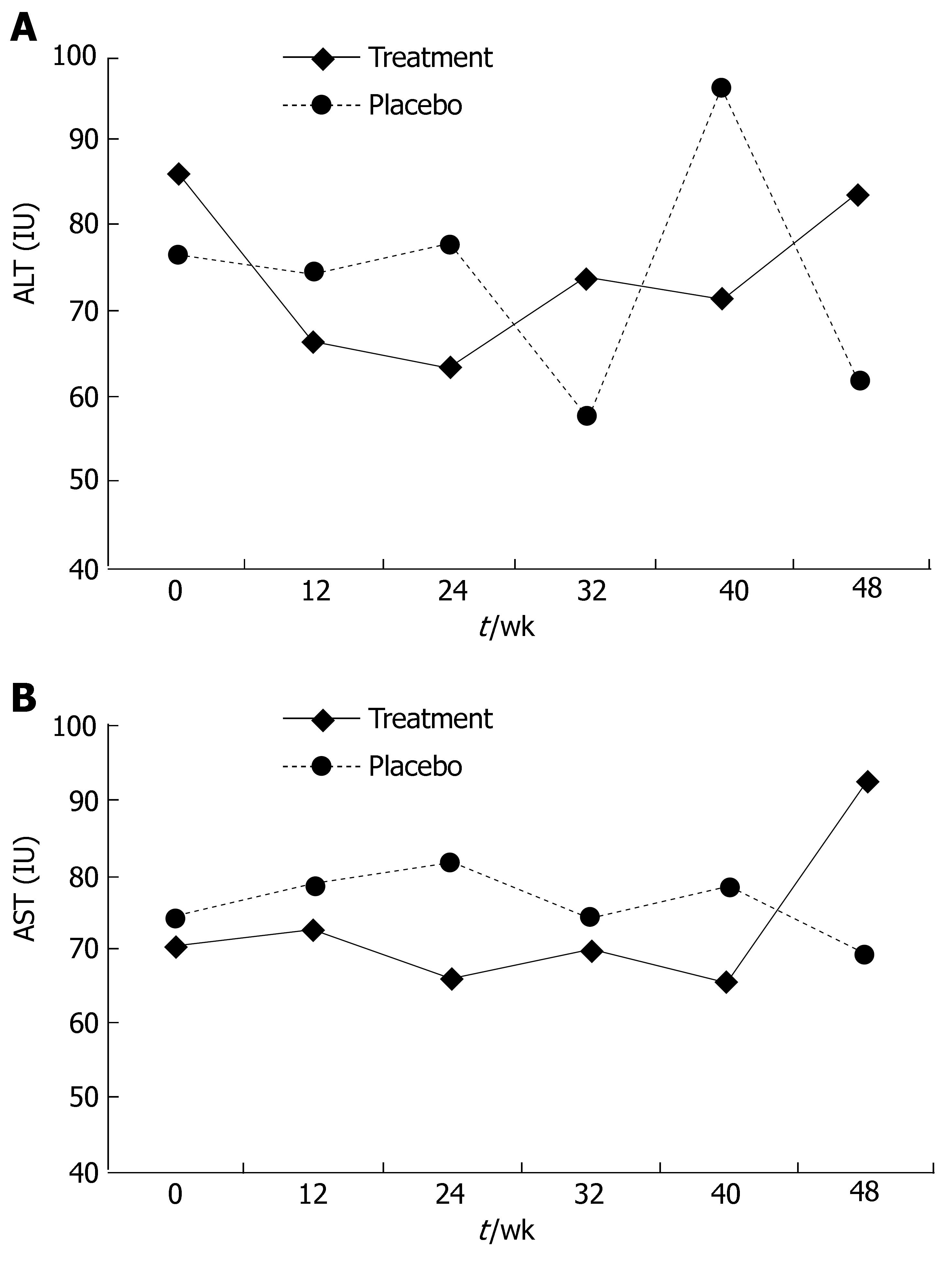
In part II, a minor non-significant decline in liver enzyme levels was noted after 24 wk of treatment. The ALT level at this point was 63.5 IU in the treatment group and 81.81 IU in the placebo group (P = 0.35, Figure 3A), the AST level was 65.71 IU in the treatment group and 81.82 IU in the placebo group (P = 0.24, Figure 3B). This effect disappeared during the follow-up period. At wk 48, the ALT level was 61.75 IU in the placebo group and 83.94 IU in the treatment group (P = 0.24, Figure 3A), the AST level was 69.5 IU in the placebo group and 92.66 IU in the treatment group (P = 0.39, Figure 3B).
In part I, the HAI reduced from 8.87 before to 8.13 after treatment in the treatment group and from 7.85 to 7.4 in the placebo group (P = 0.42). Compared to the pre-treatment score, the total biopsy HAI score at the end of treatment reduced in 48% of patients in the treatment group and 27% of patients in the placebo group, and unchanged in 26% of patients in the treatment group and 49% of patients in the placebo group (P = 0.21).
In part II, the HAI scores increased from 7.619 to 8.36 in the treatment group, and from 8.53 to 9 in the placebo group (P = 0.42).
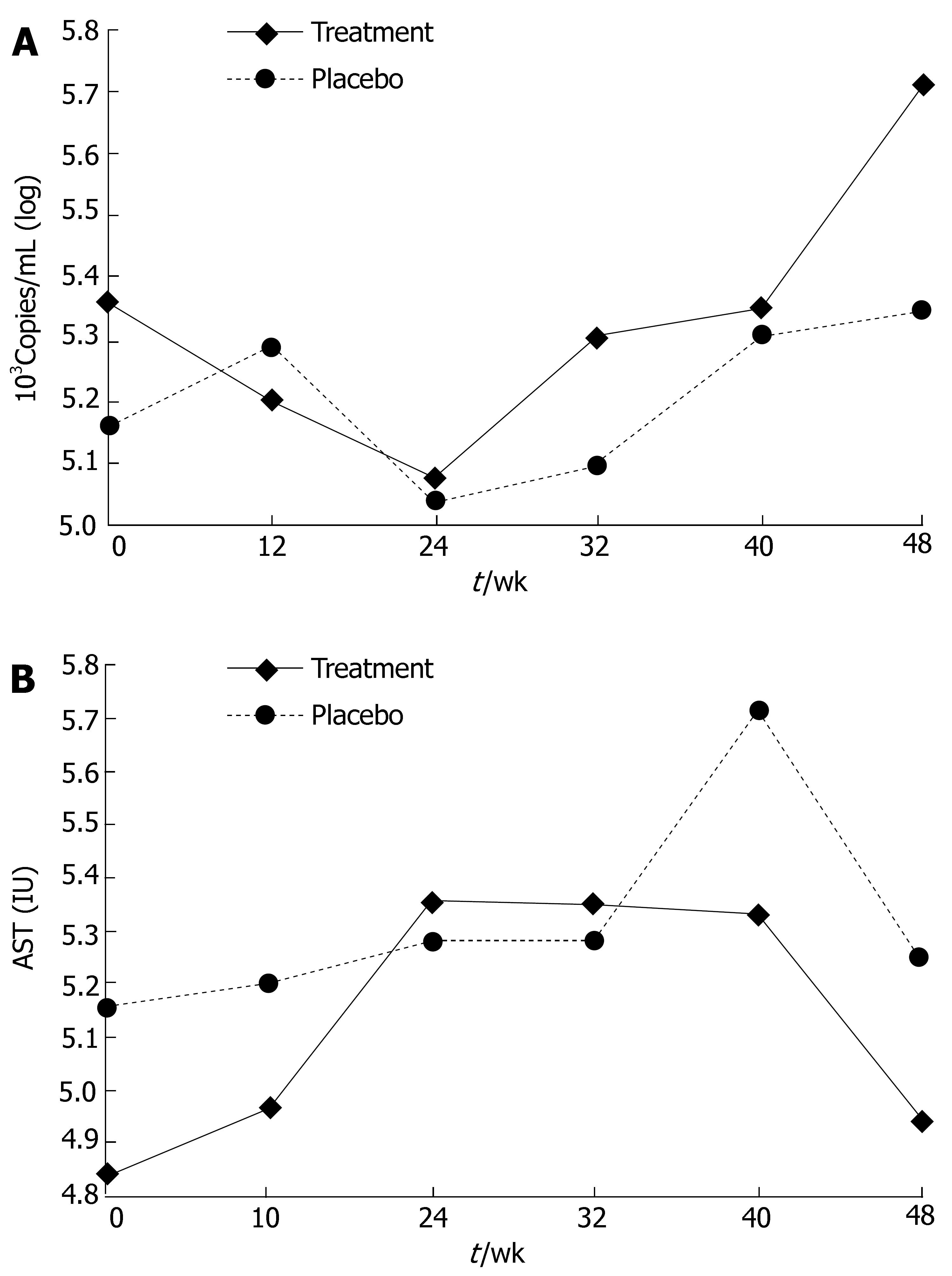
In part I, antioxidant treatment had no effect on diminishing viral load, and the average log of HCV RNA at wk 24 was 5.08 for the treatment group and 5.04 for the placebo group (Figure 4A).
In part II, similarly, no effect of treatment on viral load was observed. The average log of HCV RNA at wk 24 was 5.36 for the treatment group and 5.28 for the placebo group (Figure 4B).
In part I, data were available from thirty-eight patients. The average baseline quality of life in the treatment group was 52.88 out of a maximum of 100, and the post-treatment value was 52.12 (P = NS). The value in the placebo group was 48.5 before and 47.25 after treatment (P = NS).
In part II, data were available from thirty-seven patients. The average baseline quality of life in the treatment group was 53.1 out of a maximum of 100, and the post-treatment value was 48.8 (P = 0.50). The value in the placebo group was 43.44 before and 50.31 after treatment (P = 0.38).
A combination of intravenous and oral antioxidants could mildly alleviate the intra-hepatic inflammatory response in chronic HCV infection patients who non-responders to interferon therapy. This was manifested as a significant decrease in ALT and AST serum levels, along with improvement in the biopsy inflammatory scores. No effect was noted when oral antioxidants were used alone. The rationale for treatment of chronic HCV infection patients with antioxidants is based on the role of oxidative stress in the pathogenesis of disease progression in animal models and humans. Results of uncontrolled trials suggest that this treatment may have a beneficial effect on inflammatory indices such as liver enzymes and biopsy HAI scores, as well as quality of life[11]. Intravenous glycyrrhizin was previously tested in patients with chronic HCV infection, and lowered ALT levels (26% vs 6% with placebo) within 4 wk were noted. The effect disappears after cessation of therapy[12]. A retrospective study examining the effects of stronger neo-minophagen C (SNMC), which contains glycyrrhizin as an active component, revealed that treatment with this agent reduces the long term relative risk of developing hepatocellular carcinoma by a factor of 2.49[13]. When SNMC is used in combination with interferon, a higher rate of improvement in liver enzymes and viral clearance can be observed with no statistical significance[14]. It was reported that a combination therapy of interferon (IFN) with glycyrrhizin induces normalization of serum ALT levels in 64.3% of non-responders with serum HVC RNA disappeared in 38.5%[13]. The efficacy of glycyrrhizin in European studies was less than that reported on Japanese subjects, which can be explained by the genetic polymorphism in drug metabolism[15].
Silybum marianum (milk thistle) is a herbal medicine commonly used in treatment of liver disease. Its active component, silybinin, has been shown to have anti-fibrotic and antioxidant properties, and hepatoprotective effects in animal models of acetaminophen-induced liver damage[16]. It was reported that silymarin protects liver cells from other toxins (including ethanol, carbon tetra-chloride, and D-galactosamine) and ischemic injury, radiation, iron toxicity, and viral hepatitis[17,18]. A combination of three potent anti-oxidants [alphalipoic acid (thioctic acid), silymarin, and selenium] induces marked clinical, laboratory, and histological improvements in chronic HCV infection patients[19,20]. However, a Cochrane systematic review of trials of medicinal herbs in HCV, reported that silybin is significantly reduced in serum AST and GGT levels in only one trial, with no firm evidence for the use of herbal medicines in this condition[21]. Schizandrae chinensis, a potent anti-oxidant, lowers ALT levels in patients with chronic viral hepatitis[22]. Administration of glutathione to patients with chronic hepatitis significantly decreases GSH-Px activity of catalase (CAT), and increases superoxide dismutase (SOD) activity[23,24]. N-acetyl-cysteine, sodium selenite and vitamin E have also been studied as supplementation to interferon therapy for patients with chronic HCV infection[25]. Vitamin E supplementation increases the chance for a complete response[25]. A randomized double-blind trial of thioctic acid (alpha-lipoic acid) in chronic hepatitis patients showed that 55% patients have significant improvements in mean ALT levels, and 77% patients have histological improvements on liver biopsy[26,27]. The results of the present study showed a modest reduction in liver enzymes at the end of 24 wk of treatment in patients receiving the combined intravenous and oral protocol. The proportion of patients on the combination therapy with improvements, was statistically significant, the difference in absolute values did reach significance. In both parts of the trial, the beneficial effect on enzymes did not sustain throughout the follow-up period, which is consistent with some of the previous published data[13]. The lack of significant improvement on liver biopsy seems to reflect the relatively transient nature of the anti-inflammatory effect of antioxidants in this setting. No significant effect on viral load was noted in either part of the trial. This finding was not surprising given the lack of known direct anti-viral effects of antioxidants. A study reported that induction of gastrointestinal glutathione oxidase (which counteracts oxidative stress) by all trans retinoic acids, down-regulates HCV replicon in cell lines[6]. Adverse events that are attributable to therapy such as exacerbation of hypertension, pedal edema, and hypokalemia, are mostly related to pseudoaldosteronism associated with the 11-hydroxy-steroid dehydrogenase inhibitory activity of glycyrrhiza and L-glutathione. The excess endogenous cortisol produced by both agents, reacts with the renal mineral corticoid receptor, promoting an aldosterone-like action. Similar adverse events have also been described[11]. These findings suggest that it is important to have a close follow-up of patients' blood pressure and serum potassium levels throughout antioxidant therapy.
In the present study, the use of antioxidants had no significant effect on quality of life (QOL) as measured in the general health paradigm of the SF-36 questionnaire which is in contrast with uncontrolled trials, suggesting that antioxidants may exert a beneficial effect on quality of life in chronic HCV infection patients[11]. The combined intravenous and oral antioxidants had a better effect than oral combination alone, further supporting the potential of the intravenous antioxidants, specifically of glycyrrhiza, as a possible basis for the beneficial anti-inflammatory effect noted. Although not directly tested in the present study, the use of a combination of multiple antioxidants administered both intravenously and orally, may have enhanced the anti-oxidative effect, thus contributing to the observed effect.
The data of the present study support the role of oxidative stress in the pathogenesis of HCV-induced inflammation. The modest effect noted should be viewed in light of the fact that patients enrolled in the trial were non-responders to anti-viral therapy. Therefore, they represent a relatively "hard to treat" subset of chronic HCV infection patients. Treatment in the present study was lasted for a relatively short period of time, less than that of the current treatment with anti-viral drugs. If the main effect of antioxidants is anti-inflammatory, it would be expected that a combination of antioxidants with current anti-viral treatment may be of benefit for these patients. Indeed, previous reports suggest an added value for antioxidants in interferon-treated chronic HCV infection patients. It has been demonstrated that vitamin E-treated patients have a 2.4 times higher chance of obtaining a complete response and a more significant reduction in viral load than patients not treated with vitamin E[28].
In conclusion, a combination of intravenous and oral antioxidants can reduce intra-hepatic inflammatory response in chronic HCV infection patients who are non-responders to interferon. It is plausible to examine whether such a treatment in combination with anti-viral therapy may be useful.
The pathogenesis of hepatitis C virus (HCV) infection involves a complex interaction between viral factors and host immune responses. A major component of the latter involves oxidative stress[1].
The aim of the present trial was to determine the effect of a mixture of antioxidants on the inflammatory response of chronic HCV infection patients who were non-responders to interferon, in a double blind placebo controlled trial. Two different treatment regimens were studied and compared. The data suggest that the combined use of intravenous and oral antioxidants mildly alleviates the inflammatory response in these patients.
Melhem A, Stern M, Shibolet O, Israeli E, Ackerman Z, Pappo O, Hemed N, Rowe M, Ohana H, Zabrecky G, Cohen R, Ilan Y. Treatment of chronic hepatitis C virus infection via antioxidants: Results of a phaseIclinical trial. J Clin Gastroenterol 2005; 39: 737-342.
Antioxidant therapy has a mild beneficial effect on the inflammatory response in chronic HCV infection patients who are non-responders to interferon. A combination of antiviral and antioxidant therapy may be beneficial for these patients.
It is plausible to examine whether such a treatment in combination with anti-viral therapy may be useful.
This is the first double blind trial using a combination of several anti oxidants in patients with chronic hepatitis C who failed in interferon therapy. The study was designed well and the results were summarized and discussed appropriately.
| 1. | Koike K, Miyoshi H. Oxidative stress and hepatitis C viral infection. Hepatol Res. 2006;34:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Gong G, Waris G, Tanveer R, Siddiqui A. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-kappa B. Proc Natl Acad Sci USA. 2001;98:9599-9604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 502] [Article Influence: 20.1] [Reference Citation Analysis (1)] |
| 3. | Waris G, Turkson J, Hassanein T, Siddiqui A. Hepatitis C virus (HCV) constitutively activates STAT-3 via oxidative stress: role of STAT-3 in HCV replication. J Virol. 2005;79:1569-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, Weinman SA. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122:366-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 690] [Cited by in RCA: 680] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 5. | Koike K. Molecular basis of hepatitis C virus-associated hepatocarcinogenesis: lessons from animal model studies. Clin Gastroenterol Hepatol. 2005;3:S132-S135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Morbitzer M, Herget T. Expression of gastrointestinal glutathione peroxidase is inversely correlated to the presence of hepatitis C virus subgenomic RNA in human liver cells. J Biol Chem. 2005;280:8831-8841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Pawlotsky JM. Current and future concepts in hepatitis C therapy. Semin Liver Dis. 2005;25:72-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Hughes CA, Shafran SD. Chronic hepatitis C virus management: 2000-2005 update. Ann Pharmacother. 2006;40:74-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Seeff LB, Lindsay KL, Bacon BR, Kresina TF, Hoofnagle JH. Complementary and alternative medicine in chronic liver disease. Hepatology. 2001;34:595-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 163] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Liu JP, Manheimer E, Tsutani K, Gluud C. Medicinal herbs for hepatitis C virus infection. Cochrane Database Syst Rev. 2001;CD003183. [PubMed] |
| 11. | Melhem A, Stern M, Shibolet O, Israeli E, Ackerman Z, Pappo O, Hemed N, Rowe M, Ohana H, Zabrecky G. Treatment of chronic hepatitis C virus infection via antioxidants: results of a phase I clinical trial. J Clin Gastroenterol. 2005;39:737-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 12. | van Rossum TG, Vulto AG, Hop WC, Brouwer JT, Niesters HG, Schalm SW. Intravenous glycyrrhizin for the treatment of chronic hepatitis C: a double-blind, randomized, placebo-controlled phase I/II trial. J Gastroenterol Hepatol. 1999;14:1093-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 126] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 13. | Arase Y, Ikeda K, Murashima N, Chayama K, Tsubota A, Koida I, Suzuki Y, Saitoh S, Kobayashi M, Kumada H. The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer. 1997;79:1494-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 14. | Abe Y, Ueda T, Kato T, Kohli Y. Effectiveness of interferon, glycyrrhizin combination therapy in patients with chronic hepatitis C. Nihon Rinsho. 1994;52:1817-1822. [PubMed] |
| 15. | van Rossum TG, Vulto AG, Hop WC, Schalm SW. Pharmacokinetics of intravenous glycyrrhizin after single and multiple doses in patients with chronic hepatitis C infection. Clin Ther. 1999;21:2080-2090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Muriel P, Garciapiña T, Perez-Alvarez V, Mourelle M. Silymarin protects against paracetamol-induced lipid peroxidation and liver damage. J Appl Toxicol. 1992;12:439-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 155] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Ferenci P, Dragosics B, Dittrich H, Frank H, Benda L, Lochs H, Meryn S, Base W, Schneider B. Randomized controlled trial of silymarin treatment in patients with cirrhosis of the liver. J Hepatol. 1989;9:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 281] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 18. | Boigk G, Stroedter L, Herbst H, Waldschmidt J, Riecken EO, Schuppan D. Silymarin retards collagen accumulation in early and advanced biliary fibrosis secondary to complete bile duct obliteration in rats. Hepatology. 1997;26:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 186] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 19. | Salmi HA, Sarna S. Effect of silymarin on chemical, functional, and morphological alterations of the liver. A double-blind controlled study. Scand J Gastroenterol. 1982;17:517-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 108] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Buzzelli G, Moscarella S, Giusti A, Duchini A, Marena C, Lampertico M. A pilot study on the liver protective effect of silybin-phosphatidylcholine complex (IdB1016) in chronic active hepatitis. Int J Clin Pharmacol Ther Toxicol. 1993;31:456-460. [PubMed] |
| 21. | Liu J, Manheimer E, Tsutani K, Gluud C. Medicinal herbs for hepatitis C virus infection: a Cochrane hepatobiliary systematic review of randomized trials. Am J Gastroenterol. 2003;98:538-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Liu GT. Pharmacological actions and clinical use of fructus schizandrae. Chin Med J (Engl). 1989;102:740-749. [PubMed] |
| 23. | Loguercio C, Caporaso N, Tuccillo C, Morisco F, Del Vecchio Blanco G, Del Vecchio Blanco C. Alpha-glutathione transferases in HCV-related chronic hepatitis: a new predictive index of response to interferon therapy? J Hepatol. 1998;28:390-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Sun F, Hayami S, Ogiri Y, Haruna S, Tanaka K, Yamada Y, Tokumaru S, Kojo S. Evaluation of oxidative stress based on lipid hydroperoxide, vitamin C and vitamin E during apoptosis and necrosis caused by thioacetamide in rat liver. Biochim Biophys Acta. 2000;1500:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Look MP, Gerard A, Rao GS, Sudhop T, Fischer HP, Sauerbruch T, Spengler U. Interferon/antioxidant combination therapy for chronic hepatitis C--a controlled pilot trial. Antiviral Res. 1999;43:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 26. | Bustamante J, Lodge JK, Marcocci L, Tritschler HJ, Packer L, Rihn BH. Alpha-lipoic acid in liver metabolism and disease. Free Radic Biol Med. 1998;24:1023-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 237] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 27. | Houglum K, Venkataramani A, Lyche K, Chojkier M. A pilot study of the effects of d-alpha-tocopherol on hepatic stellate cell activation in chronic hepatitis C. Gastroenterology. 1997;113:1069-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 154] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Meydani SN, Meydani M, Blumberg JB, Leka LS, Siber G, Loszewski R, Thompson C, Pedrosa MC, Diamond RD, Stollar BD. Vitamin E supplementation and in vivo immune response in healthy elderly subjects. A randomized controlled trial. JAMA. 1997;277:1380-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 295] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
S- Editor Ma N L- Editor Wang XL E- Editor Li HY