Published online Jan 28, 2007. doi: 10.3748/wjg.v13.i4.612
Revised: September 23, 2006
Accepted: October 23, 2006
Published online: January 28, 2007
AIM: To assess whether trace metal concentrations (which influence metabolism as both essential and non-essential elements) are increased or decreased in cancerous tissues and to understand the precise role of these metals in carcinogenesis.
METHODS: Concentrations of trace metals including Cd, Ni, Cu, Zn, Fe, Mg and Ca in both cancerous and non-cancerous stomach tissue samples were determined by atomic absorption spectrometry (AAS). Tissue samples were digested using microwave energy. Slotted tube atom trap was used to improve the sensitivity of copper and cadmium in flame AAS determinations.
RESULTS: From the obtained data in this study, the concentrations of nickel, copper and iron in the cancerous human stomach were found to be significantly higher than those in the non-cancerous tissues, by using t-test for the paired samples. Furthermore, the average calcium concentrations in the cancerous stomach tissue samples were found to be significantly lower than those in the non-cancerous stomach tissue samples by using t-test. Exceedingly high Zn concentrations (207-826 mg/kg) were found in two paired stomach tissue samples from both cancerous and non-cancerous parts.
CONCLUSION: In contrast to the literature data for Cu and Fe, the concentrations of copper, iron and nickel in cancerous tissue samples are higher than those in the non-cancerous samples. Furthermore, the Ca levels are lower in cancerous tissue samples than in non-cancerous tissue samples.
- Citation: Yaman M, Kaya G, Yekeler H. Distribution of trace metal concentrations in paired cancerous and non-cancerous human stomach tissues. World J Gastroenterol 2007; 13(4): 612-618
- URL: https://www.wjgnet.com/1007-9327/full/v13/i4/612.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i4.612
The importance of essential trace metals in health and disease is indisputable because of their essential role in specific concentration ranges and toxic role at relatively high levels. Essential trace elements have four major functions as stabilizers, elements of structure, essential elements for hormonal function and cofactors in enzymes. As a result, the lack of essential trace elements influences structure alone or alters function of structure through the lack of stabilization, change of charge properties or allosteric configuration[1]. It may be expected that deficiency of essential trace elements as cofactors of enzymes could severely impair the host's resistance against carcinogenic stress[2]. Among these elements, zinc is a component of more than 3000 zinc-associated transcription factors including DNA-binding proteins with zinc fingers, and more than 300 enzymes including Cu/Zn superoxide dismutase (CuZnSOD) (SOD is an important antioxidant enzyme for cellular protection against reactive oxygen species (ROS) and several proteins involved in DNA repair[3-5]. Metallothioneins, being intracellular polypeptides have a remarkable ability to bind to metallic ions including both essential and also toxic metals such as cadmium or lead. Copper is a component of more than 30 enzymes including caeruloplasmine, cytochrome oxidase, lysine oxidase, dopamine-hydroxylase, ascorbate oxidase and tyrosinase in human body, some of which are involved in collagen synthesis, as well as being necessary for the healthy development of connective tissue, nerve coverings and bone[6,7].
The role of metals in the development and inhibition of cancer has a complex character and raises many questions. In the past 25 years, some metals including cadmium, nickel, arsenic, beryllium and chromium (VI), have been recognized as human or animal carcinogens in addition to primary carcinogens such as radiation, viruses and other chemicals[7,8]. Their carcinogenic potential depend largely on factors such as oxidation states and chemical species[9]. It is supposed that oxidative DNA lesions play an important role in various diseases including cancer and premature aging. The increase in oxidative DNA lesions are frequently described as being attributable to metal exposure. Metal carcinogenesis is mediated either by the increased generation of highly ROS on the basis of ESR spin trapping studies[10] and/or by interference with DNA repair processes[11]. Almost all metals are able to generate ROS, which can explain a great part of both their carcinogenicity and their aptitude in the treatment of cancer. Induction of oxidative DNA damage and interaction with DNA repair processes can lead to an enhancement of genotoxicity in combination with a variety of DNA-damaging agents. Nucleotide excision repair (NER) which is the major repair system, is inhibited at low levels as well as at non-cytotoxic concentrations of Ni (II), Cd (II), Co (II) and As (III). The repair of oxidative DNA base modifications is disturbed by Ni (II) and Cd (II) ions. One reason for repair inhibition appears to be the displacement of Zn (II) and Mg (II)[12]. Magnesium and Zn, that are cofactors for DNA polymerase, are effective protectors against carcinogenesis in vivo. Although Zn and Cu concentrations in serum and tissues of cancer patients have been studied extensively, the precise role of these metals in carcinogenesis is not clearly understood. While a great depth of literature is available regarding the alterations in the levels of trace elements in serum, relatively few studies are available on trace element levels in cancerous and non-cancerous human stomach tissue. Reddy and coworkers reported that the concentrations of essential metals including Fe, Zn and Cu are significantly lower in cancerous stomach tissue than in normal tissues[13]. Similarly, the lower Fe and Zn levels in cancerous stomach tissues than in normal tissues are also supported by von Czarnowski and coworkers[14]. Few studies have simultaneously determined both toxic and essential trace elements in cancerous and non-cancerous stomach tissues. On the other hand, most studies have been performed on dried and occasionally homogenized samples, that disturb the tissue from its natural physiological state. Ng and coworkers[15] reported that the wet-to-dry ratio of tumor (malignant) breast tissues is higher (more 2-times) than that of the normal tissues. Therefore the elevation of elemental contents in tumors is significantly different from that in normal tissues when concentrations are adjusted by using the wet-to-dry ratio of the samples. The same study noted that the wet-to-dry ratio varies significantly amongst specimens, not only of different types but also between samples of the same group[15]. Therefore, evaluation of trace element levels in dried samples should be regarded as incomplete in the absence of wet-to-dry ratios for individual specimens. It would appear that study of fresh and unprocessed specimens is preferable. On the other hand, the ratio of Cu to Zn (Cu/Zn) intake is widely utilized to assist diagnosis of various cancers or tumors[16]. The usefulness of the tissue-metal determination in cancer prevention, detection, monitoring, treatment and prognosis requires further investigation.
In our laboratory, atomic absorption spectrometry (AAS) being the most common analytical technique has been successfully used for trace metal analysis in biological samples[17-20]. To improve the sensitivity of flame atomic absorption spectrometry (FAAS), a slotted tube atom trap (STAT) has been used for some metals such as Cd, Pb and Cu in biological matrices[19-22]. In the current study, the concentrations of various minor and trace metals, including Cd, Ni, Cu, Zn, Fe, Mg and Ca in cancerous and non-cancerous stomach tissues, were determined by atomic absorption spectrophotometry. For digestion of the tissues, a microwave oven was used.
An ATI UNICAM 929 flame atomic absorption spectrophotometer (FAAS) equipped with ATI UNICAM and KOTTO hollow cathode lamps was used for metal determinations. The optimum conditions for FAAS are given in Table 1. A STAT was used to improve the Cd and Cu sensitivities by FAAS. A domestic microwave oven (Kenwood) was used for digestion of the tissues. Unless stated otherwise, all chemicals used were of analytical grade. Throughout the analysis, doubly distilled water was used. All glass apparatus (Pyrex) were kept permanently full of 1 mol/L nitric acid when they were not used. In the digestion procedures, concentrated nitric acid (65%, Merck) and hydrogen peroxide (35%, Merck) were used. Stock solutions of metals (1000 mg/L) were prepared by dissolving their salts (Merck) in 1.0 mol/L nitric acid.
| Parameter | Cd | Ni | Cu | Zn | Fe | Mg | Ca |
| Wavelength (nm) | 228.8 | 232 | 324.8 | 213.9 | 248.3 | 285.2 | 422.7 |
| HCL current (mA) | 4 | 7.5 | 3 | 9.5 | 15 | 15 | 6 |
| Acetylene flow rate (L/min) | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 4.2 |
| N2O flow rate (L/min) | - | - | - | - | - | - | 4.7 |
| Air flow rate (L/min) | 4 | 4 | 4 | 4 | 4 | 4 | - |
| Slit (nm) | 0.5 | 0.2 | 0.5 | 0.5 | 0.2 | 0.5 | 0.5 |
Fresh stomach tissue samples were taken since fresh and formalin-fixed tissues have been demonstrated to yield virtually the same results for essential and toxic metals including Ca, Mg, Fe, Cu, Zn, As, Cd, Hg and Pb[23]. In the current study, the samples were obtained in the formaldehyde solution from private Pathology Laboratories and the pathology laboratories of Firat University in Elazig, Turkey, after surgery and histopathologic examination. A total of eighteen samples were taken, of which four cancerous (malign) stomach tissue samples were taken from patients of different sex, age and living conditions, described as independent samples in this study, the other fourteen samples were taken from both cancerous (malign) and non-cancerous (normal) stomach tissues, described as paired samples in this study. All the patients were diagnosed as grade II-III or III-IV adenocarcinoma except that one patient at the age of 80 years had grade I and poorly differentiated adenocarcinoma. Furthermore, most patients had metastatic and differentiated adenocarcinoma. The tissue samples were cut into small pieces with a stainless steel knife and transferred to a beaker.
Exactly 2.0 mL of the mixture of HNO3/H2O2 (2:1) was added to 0.7 g of the tissue samples. The mixture was placed into the water bath at 70°C for 30 min and stirred occasionally. Then, 1.0 mL of the same acid mixture was added, and the mixture was transferred into a Teflon vessel bomb for the microwave oven. The bomb was closed, and the solution was placed inside the microwave oven. Radiation was applied for 3 min at 450 W. After addition of 0.5 mL of the same acid mixture, radiation was repeated for 3 min. After cooling for 5 min, 2.0 mL of 0.1 mol/L HNO3 was added, and the solution was transferred into a Pyrex tube. After centrifugation, the clear solution was measured by FAAS. Three different portions of each sample were digested and the average value was calculated for the same tissue. Blank digests were carried out in the same way.
Calibration curves were obtained by using solutions of the studied elements at different concentrations. The graphs obtained were linear in the concentration range and the equations of the curves are described in Table 2.
| Equation | ||
| Y = 2.4972x + 1.12 | R2 = 0.99 | For Cd (4-100 ng/mL by STAT-AAS) |
| Y = 0.3278x - 0.2083 | R2 = 1 | For Cu (25-400 ng/mL by STAT-AAS) |
| Y = 85x + 0.5 | R2 = 0.99 | For Ni (0.2-2.0 mg/L) |
| Y = 302x + 0.75 | R2 = 0.99 | For Zn (0.1-1.0 mg/L) |
| Y = 64x + 0.43 | R2 = 1 | For Fe (0.20-3.0 mg/L) |
| Y = 515x + 7.0 | R2 = 0.99 | For Mg (0.25-2.0 mg/L) |
| Y = 305x + 39 | R2 = 1 | For Ca (0.25-2.0 mg/L) |
The accuracy of the method was studied by examining the recovery of metals from stomach tissue samples fortified with various amounts of the studied metals. The following metal amounts were added: 30 ng/g of Cd, 200 ng/g of Ni, 0.3 mg/kg of Cu, 10 mg/kg of Zn, 10 mg/kg of Fe, 100 mg/kg of Mg and 300 mg/kg of Ca. After digestion in microwave oven, the recoveries were found to be at least 90% for all studied metals. Furthermore, the standard addition method was used to remove possible interferences caused by the matrix. The slopes of the calibration curves for all studied elements were compared with those obtained by the standard addition method. The slopes of the calibration curves were found to be the same as those obtained with the standard addition method. In other words, all of the standard addition curves were parallel to the calibration curves. These results indicated the absence of chemical interference.
Levels of the metals including Cd, Ni, Cu, Zn, Fe, Mg and Ca in the reagent blanks in the analytical steps were found to be 0.5, 25, 10, 50, 50, 105 and 190 ng/mL with the standard deviations being 0.1, 4.0, 1.5, 9.0, 8.0, 20 and 35, respectively. Therefore, the detection limits for these elements defined as three times the s values of blanks were calculated as 0.3, 12, 4.5, 27, 24, 60 and 105 ng/mL. The precision of the standard deviations for 10 samples of the same tissue was found to be less than 10% for all studied elements.
Metals are considered to act not only as carcinogens but also as co-carcinogens that activate carcinogenic chemicals. In evaluating the differences in cancerous and non-cancerous tissue samples, two comparisons were conducted: one between paired cancerous and non-cancerous tissue samples, the other between total cancerous and non-cancerous samples. P values (obtained by using t-test) less than 0.05 were considered significantly different between the two groups. The samples with exceptionally high values were disregarded in calculating the average and range.
Data related with carcinogenic effects of cadmium are available from the literature[24]. Multiple studies have linked occupational exposure to Cd with pulmonary cancer in humans, whereas a few studies showed that Cd exposure is associated with cancers of stomach and other sites in humans[24]. As it can be seen from Table 3, there was no significant difference in Cd concentrations between cancerous and non-cancerous stomach tissue samples, in the present study.
| Tissue | Cd (ng/g) | Ni (ng/g) | Cu (mg/kg) | Zn (mg/kg) | Fe (mg/kg) | Mg (mg/kg) | Ca (mg/kg) | |||||||
| (Age) | Cancerous | non-can-cerous | Cancerous | non-cancerous | Cancer-ous | non-cancerous | Cancer-ous | non-cancerous | Cancerous | non-cancerous | Cancer-ous | non-cancerous | Cancer-ous | non-cancerous |
| Stomach | 30 ± 9 | 230 ± 55 | 0.6 ± 0.1 | 11 ± 1 | 24 ± 3 | 508 ± 752 | 526 ± 85 | |||||||
| (65) | ||||||||||||||
| Stomach | 156 ± 16 | 260 ± 30 | 0.9 ± 0.1 | 26 ± 2 | 8 ± 1 | 203 ± 18 | 545 ± 90 | |||||||
| (62) | ||||||||||||||
| Stomach | 50 ± 9 | 740 ± 84 | 0.9 ± 0.2 | 23 ± 24 | 65 ± 30 | 44 ± 172 | 1010 ± 930 | |||||||
| (80) | ||||||||||||||
| Stomach | 29 ± 16 | 820 ± 113 | 0.7 ± 0.1 | 7 ± 1 | 60 ± 17 | 112 ± 30 | 480 ± 130 | |||||||
| (66) | ||||||||||||||
| Stomach | 10 ± 3 | 33 ± 6 | 905 ± 690 | 328 ± 55 | 1.2 ± 0.1 | 1.0 ± 0.3 | 11 ± 1 | 23 ± 0.1 | 25 ± 4 | 19 ± 8 | 80 ± 10 | 200 ± 14 | 390 ± 76 | 834 ± 155 |
| (58)1 | ||||||||||||||
| Stomach | 33 ± 10 | 42 ± 7 | 1120 ± 265 | 840 ± 93 | 1.8 ± 0.1 | 1.5 ± 0.2 | 12 ± 2 | 17 ± 1 | 16 ± 2 | 21 ± 2 | 150 ± 12 | 84 ± 10 | 335 ± 41 | 450 ± 86 |
| (60)1 | ||||||||||||||
| Stomach | 68 ± 26 | 130 ± 21 | 510 ± 80 | 439 ± 57 | 1.5 ± 0.2 | 1.2 ± 0.1 | 16 ± 2 | 21 ± 4 | 37 ± 9 | 18 ± 5 | 210 ± 23 | 190 ± 15 | 432 ± 96 | 1047 ± 120 |
| (57)1 | ||||||||||||||
| Stomach | 90 ± 15 | 63 ± 8 | 2010 ± 356 | 1240 ± 112 | 1.7 ± 0.2 | 0.9 ± 0.1 | 25 ± 3 | 19 ± 1 | 40 ± 5 | 23 ± 3 | 106 ± 11 | 45 ± 52 | 235 ± 34 | 492 ± 75 |
| (59)1 | ||||||||||||||
| Stomach | 107 ± 19 | 55 ± 5 | 335 ± 164 | 321 ± 237 | 1.5 ± 0.6 | 1.1 ± 0.3 | 22 ± 2 | 19 ± 1 | 34 ± 14 | 36 ± 21 | 210 ± 15 | 300 ± 128 | 911 ± 215 | 1199 ± 53 |
| (52)1 | ||||||||||||||
| Stomach | 22 ± 9 | 58 ± 8 | 740 ± 145 | 720 ± 95 | 1.1 ± 0.2 | 0.6 ± 0.1 | 18 ± 2 | 16 ± 1 | 35 ± 4 | 34 ± 3 | 24 ± 32 | 30 ± 52 | 403 ± 55 | 523 ± 41 |
| (51)1 | ||||||||||||||
| Stomach | 31 ± 3 | 33 ± 6 | 527 ± 116 | 241 ± 32 | 2.8 ± 0.1 | 0.9 ± 0.5 | 41 ± 4 | 30 ± 15 | 20 ± 2 | 23 ± 7 | 270 ± 15 | 241 ± 30 | 530 ± 10 | 624 ± 78 |
| (66)1 | ||||||||||||||
| Stomach | 30 ± 6 | 55 ± 6 | 360 ± 92 | 270 ± 32 | 0.5 ± 0.07 | 0.7 ± 0.1 | 25 ± 2 | 30 ± 3 | 9 ± 2 | 10 ± 3 | 200 ± 28 | 215 ± 25 | 600 ± 110 | 615 ± 65 |
| (60)1 | ||||||||||||||
| Stomach | 10 ± 1 | 33 ± 3 | 182 ± 72 | 190 ± 5 | 4.3 ± 0.5 | 2.1 ± 1.6 | 19 ± 4 | 23 ± 2 | 17 ± 3 | 20 ± 4 | 130 ± 21 | 110 ± 17 | 460 ± 62 | 484 ± 56 |
| (49)1 | ||||||||||||||
| Stomach | 30 ± 5 | 32 ± 4 | 500 ± 98 | 240 ± 28 | 2.8 ± 0.1 | 1.2 ± 0.2 | 33 ± 3 | 35 ± 4 | 12 ± 3 | 18 ± 4 | 190 ± 20 | 135 ± 15 | 533 ± 67 | 624 ± 60 |
| (55)1 | ||||||||||||||
| Stomach | 65 ± 17 | 85 ± 10 | 700 ± 85 | 750 ± 103 | 1.7 ± 0.1 | 0.8 ± 0.1 | 207 ± 192 | 826 ± 552 | 25 ± 2 | 13 ± 2 | 314 ± 30 | 184 ± 22 | 746 ± 86 | 713 ± 66 |
| (50)1 | ||||||||||||||
| Stomach | 60 ± 12 | 80 ± 11 | 300 ± 45 | 130 ± 12 | 2.4 ± 0.3 | 0.8 ± 0.1 | 410 ± 282 | 380 ± 362 | 26 ± 3 | 15 ± 2 | 207 ± 31 | 275 ± 10 | 685 ± 74 | 715 ± 61 |
| (53)1 | ||||||||||||||
| Stomach | 70 ± 12 | 105 ± 10 | 730 ± 98 | 850 ± 120 | 2.2 ± 0.2 | 1.9 ± 0.1 | 15 ± 2 | 16 ± 2 | 42 ± 4 | 25 ± 3 | 215 ± 10 | 200 ± 40 | 375 ± 42 | 417 ± 36 |
| (40)1 | ||||||||||||||
| Stomach | 32 ± 5 | 100 ± 12 | 410 ± 65 | 800 ± 112 | 2.2 ± 0.2 | 1.3 ± 0.1 | 17 ± 1 | 16 ± 2 | 42 ± 5 | 24 ± 2 | 327 ± 30 | 210 ± 25 | 476 ± 26 | 508 ± 52 |
| (51)1 | ||||||||||||||
| Average | 51 ± 37 | 65 ± 31 | 632 ± 430 | 526 ± 335 | 1.7 ± 1 | 1.1 ± 0.4 | 20 ± 9 | 22 ± 6 | 30 ± 16 | 21 ± 7 | 195 ± 72 | 195 ± 63 | 537 ± 196 | 660 ± 230 |
| Range | 10-156 | 32-130 | 230-2010 | 130-1240 | 0.5-4.3 | 0.6-2.1 | 7-41 | 16-30 | 8-65 | 10-36 | 24-508 | 30-300 | 235-1010 | 417-1199 |
Nickel: The International Agency for Research on Cancer (IARC) has classified the carcinogenic substances in two groups. Group 1 includes substances “known to be human carcinogens or sufficient evidence of carcinogenicity from studies in humans”, group 2 includes substances “reasonably anticipated to be a human carcinogen”. The chemicals in group 2 are classified in two subgroups by IARC. Group 2A includes substances “probably carcinogenic to humans”, and group 2B includes substances “possibly carcinogenic to humans”[8]. Kasprazak and coworkers[25] have made an overall evaluation of the carcinogenicity of nickel and found that Ni compounds are carcinogenic to humans (group 1), and metallic nickel is possibly carcinogenic to humans (group 2B).
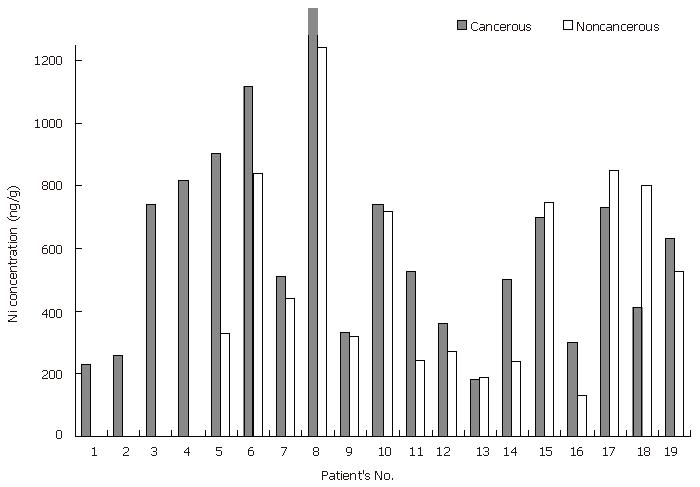
vonCzarnowski et al[14] showed that Ni levels are lower in cancerous (malign) stomach tissues than in normal stomach tissues, whereas Reddy et al[13] reported that nickel concentrations are 6-time higher in cancerous stomach tissues than in normal stomach tissues. In this study, the Ni levels in cancerous stomach tissue samples were significantly higher (P < 0.05 for the paired samples) than those in the non-cancerous stomach tissue samples (Tables 3, 4 and Figure 1).
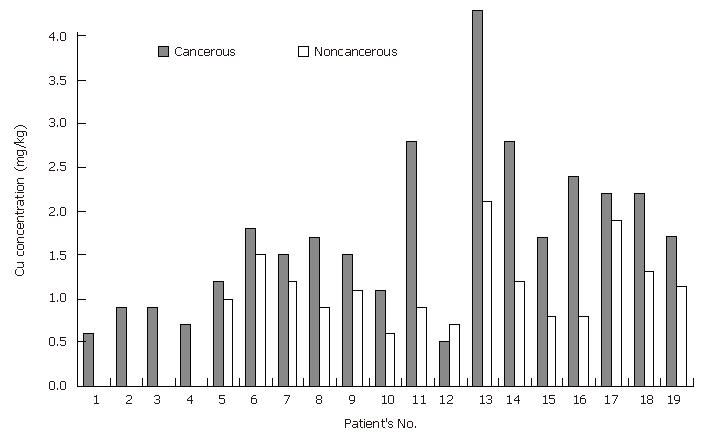
Copper: Although copper is an essential element for humans and animals, high concentrations of Cu (above normal) could induce growth proliferation and cancer by damaging DNA with toxic free hydroxyl radicals[26]. Conflicting results regarding Cu concentrations have been observed in cancerous and normal stomach tissues[13,14]. VonCzarnowski and coworkers[14] reported that there are no differences in Cu concentrations between cancerous (malign) and normal stomach tissue samples, whereas Reddy and coworkers[13] described that Cu levels in cancerous stomach tissue samples are 3-time lower than those in normal stomach tissue samples. In the present study, the Cu levels in cancerous stomach tissues were significantly higher (P = 8.10-4 for the paired samples and P < 0.05 for total samples) than those in non-cancerous stomach samples (Tables 3, 4 and Figure 2). The mechanism of copper elevation in cancerous tissues may be explained by modifications in the relationships among trace elements with reduced catabolism or by increased neoplastic synthesis of ceruoplasmin. Metal carcinogenesis is mediated either by the increased generation of highly ROS (Fenton reaction) and/or by interference with DNA repair processes[11,26]. Since almost all metals are able to generate ROS, further studies on the determination of trace element levels together with ROS production are needed. Consequently, the physiological processes underlying tumor development can lead to uptake of trace elements by neoplastic cells because of the increased cellular and enzymatic activity.
Zinc: It was reported that Zn concentrations in cancerous stomach tissue are lower than those in normal tissues[13-14]. It can be seen from Table 3, there was no significant difference in Zn concentrations between cancerous and non-cancerous stomach tissue the paired samples. Excessive zinc concentrations were found in both cancerous and non-cancerous stomach tissues from two patients. Unfortunately, we could not explain these excessive Zn levels.
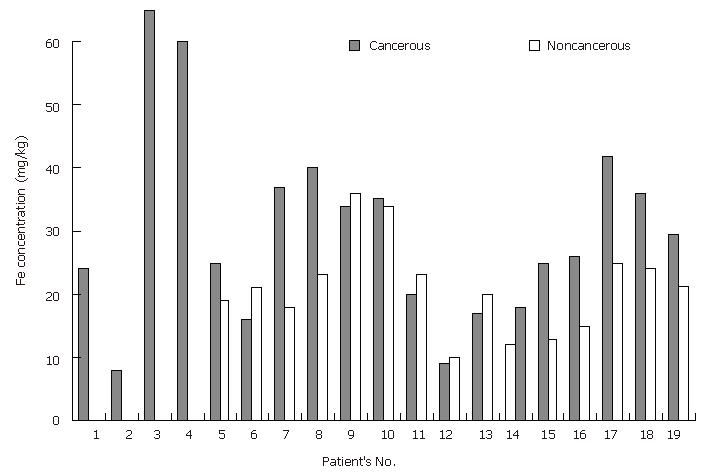
Iron: Although Fe is an essential nutritional element for all life forms, it is known that excess iron and iron deficiency also lead to oxidative DNA damage[27]. It was reported that iron levels are significantly decreased in cancerous stomach tissue in comparison with those in normal stomach tissue[13-14]. On the other hand, Hercberg and coworkers[28] reported that serum ferritin concentration >160 ng/mL is an increased risk of developing cancer in women but not in men. In this study, Fe levels in the cancerous stomach tissue samples were significantly higher (P < 0.05 for the paired samples and P = 0.065 for all samples) than those in the non-cancerous tissue samples (Tables 3, 4 and Figure 3). These findings can also be explained by the Fenton reaction described above.
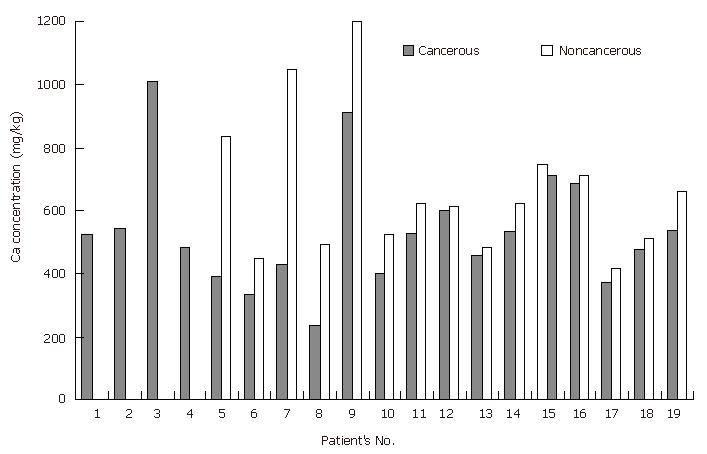
Calcium: It was reported that calcium concentrations in cancerous stomach tissues are lower than those in the normal tissues[13], whereas steady Ca concentrations are observed in cancerous and normal stomach tissues[14]. In this study, Ca levels in the cancerous stomach tissue samples were significantly lower (P < 0.01 for the paired samples) than those in the non-cancerous tissue samples, similar to the results of Reddy coworkers (Tables 3, 4 and Figure 4). It was described that Ni+2 can block Ca+2 channels and hence, nickel releases the stored intracellular Ca+2 via a mechanism underlying the interaction between Ni+2 ions and the cell surface Ca+2 receptor[25]. The results of this study, involving the lower Ca concentrations in cancerous stomach tissue samples than in the non-cancerous tissue samples, agree with the observed higher Ni levels in cancerous tissues than in non-cancerous tissues.
Magnesium: There were no significant differences in the Mg concentrations between cancerous and non-cancerous stomach tissues (Table 3).
The significant and tendentious elements are listed in Table 4. The positive sign was used to illustrate accumulation of the elements in cancerous tissue, and the minus sign was used to indicate the depletion of elements in the cancerous stomach tissue samples in comparison to the non-cancerous tissue samples.
| Status | Stomach | P (for paired samples) | P (for total samples) |
| Significant | Ca (-) | 0.009 | 0.122 |
| Cu (+) | 0.001 | 0.036 | |
| Fe (+) | 0.046 | 0.065 | |
| Tendentious | Ni (+) | 0.092 | 0.409 |
| 0.025 |
Reddy and coworkers[13] found that the concentrations of trace metals in normal/cancerous tissues (mg/kg) on dry weight basis are as follows: Fe = 2408/684, Cu = 63.5/21.2, Zn = 818/229, Ni = 10.5/60, Pb = 8.8/8.1 and Ca = 647/433. They described that the low iron level observed in carcinoma tissue of stomach might not initiate carcinoma in stomach, but the low absorption of iron may be due to the lack of HCl which in turn may be due to the carcinogenic nature of stomach. The lower iron levels observed in cancer tissue of stomach is supported by vonCzarnowski and coworkers[14]. In the present study, copper, iron and nickel concentrations in cancerous stomach tissue samples were higher than those in non-cancerous stomach tissue samples. Although ROS were not measured in this study, these results are in agreement with the reported data[29]. Furthermore, we found that Ca levels in the cancerous stomach tissue samples were lower than those in the non-cancerous stomach tissue samples. It was reported that calcium and magnesium concentrations, similar to iron, nickel and zinc in cancerous prostate tissue are higher than those in non-cancerous prostate tissue[18]. The increase in calcium concentration and its heterogeneous distribution in malign prostate tissue in contrast to the data obtained in stomach tissue may be attributed to calcium functions and behaviors depending on the organ type. The organ-dependency on the changes in trace metal concentrations in cancerous and endometrial tissues[30] also supports these explanations. We think that the decreased Ca levels and the increased Ni concentrations in cancerous stomach tissues as well as its heterogeneous distribution in comparison to non-cancerous samples are very important for the investigation of cancer mechanism in this organ due to the displacement of Ni with Ca. The results in disagreement with the explanations above may be attributed to their subgroups of cancerous properties because the different mechanisms may be effective in such conditions.
In conclusion, STAT can be used to improve the sensitivity of copper and cadmium. In addition, the tissue digested in a microwave oven has very low blank values and can reduce the risk of metal loss or contaminations. The closed microwave digestion offers an easy and reliable method for the complete dissolution of tissues prior to the determination of trace metals.
| 1. | Feinendegen LE, Kasperek K. Medical aspects of trace element research. Trace Elem Anal Chem Med Biol. 1980;1-17. |
| 2. | Schrauzer GN. The role of trace elements in the etiology of cancer. Trace Elem Anal Chem Med Biol. 1980;183-195. |
| 3. | Beyersmann D. Homeostasis and cellular functions of zinc. Materıalwıssenschaft und werkstofftechnık. 2002; 33:764-769. [DOI] [Full Text] |
| 4. | Auld DS. Zinc coordination sphere in biochemical zinc sites. Biometals. 2001;14:271-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 468] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 5. | Ho E. Zinc deficiency, DNA damage and cancer risk. J Nutr Biochem. 2004;15:572-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 317] [Article Influence: 15.1] [Reference Citation Analysis (1)] |
| 6. | Cai L, Li XK, Song Y, Cherian MG. Essentiality, toxicology and chelation therapy of zinc and copper. Curr Med Chem. 2005;12:2753-2763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Mertz W. Trace elements in human and animal nutrition. 5th ed. San Diego, California: Academic Press 1987; 1-2. |
| 8. | Tomatis L. The identification of human carcinogens and primary prevention of cancer. Mutat Res. 2000;462:407-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Rojas E, Herrera LA, Poirier LA, Ostrosky-Wegman P. Are metals dietary carcinogens? Mutat Res. 1999;443:157-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 93] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Wang Y, Fang J, Leonard SS, Rao KM. Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic Biol Med. 2004;36:1434-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 514] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 11. | Merzenich H, Hartwig A, Ahrens W, Beyersmann D, Schlepegrell R, Scholze M, Timm J, Jöckel KH. Biomonitoring on carcinogenic metals and oxidative DNA damage in a cross-sectional study. Cancer Epidemiol Biomarkers Prev. 2001;10:515-522. [PubMed] |
| 12. | Hartwig A. Recent advances in metal carcinogenicity. Pure Appl Chem. 2000;72:1007-1014. [RCA] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Reddy SB, Charles AJ, Raju GJN, Vijayan V, Reddy BS, Kumar MR, Sundareswar B. Trace elemental analysis of carcinoma kidney and stomach by PIXE method. Nucl Instr Meth Phys Res B. 2003;207:345-355. [DOI] [Full Text] |
| 14. | VonCzarnowski D, Denkhaus E, Lemke K. Determination of trace element distribution in cancerous and normal human tissues by total X-ray fluorescence analysis. Spectrochim Acta B. 1997;52:1047-1052. [DOI] [Full Text] |
| 15. | Ng KH, Bradley DA, Looi LM. Elevated trace element concentrations in malignant breast tissues. Br J Radiol. 1997;70:375-382. [PubMed] |
| 16. | Zhai H, Chen X, Hu Z. Study on the relationship between intake of trace elements and breast cancer mortality with chemometric methods. Comput Biol Chem. 2003;27:581-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Yaman M. Nickel speciation in soil and the relationship with its concentration in fruits. Bull Environ Contam Toxicol. 2000;65:545-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Yaman M, Atici D, Bakirdere S, Akdeniz I. Comparison of trace metal concentrations in malign and benign human prostate. J Med Chem. 2005;48:630-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Yaman M. The improvement of sensitivity in lead and cadmium determinations using flame atomic absorption spectrometry. Anal Biochem. 2005;339:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Yaman M, Akdeniz I. Sensitivity enhancement in flame atomic absorption spectrometry for determination of copper in human thyroid tissues. Anal Sci. 2004;20:1363-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Yaman M. Determination of Cd and Pb in Human Urine by STAT-FAAS after enrichment on Activated Carbon. J Anal At Spectrom. 1999;14:275-278. [DOI] [Full Text] |
| 22. | Yaman M, Dilgin Y. AAS Determination of Cadmium in Fruits and Soils. At. Spectros. 2002;23:59-64. |
| 23. | Bush VJ, Moyer TP, Batts KP, Parisi JE. Essential and toxic element concentrations in fresh and formalin-fixed human autopsy tissues. Clin Chem. 1995;41:284-294. [PubMed] |
| 24. | Waisberg M, Joseph P, Hale B, Beyersmann D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology. 2003;192:95-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1032] [Cited by in RCA: 987] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 25. | Kasprzak KS, Sunderman FW, Salnikow K. Nickel carcinogenesis. Mutat Res. 2003;533:67-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 456] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 26. | Theophanides T, Anastassopoulou J. Copper and carcinogenesis. Crit Rev Oncol Hematol. 2002;42:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 228] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 27. | Ames BN. DNA damage from micronutrient deficiencies is likely to be a major cause of cancer. Mutat Res. 2001;475:7-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 362] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 28. | Hercberg S, Estaquio C, Czernichow S, Mennen L, Noisette N, Bertrais S, Renversez JC, Briançon S, Favier A, Galan P. Iron status and risk of cancers in the SU.VI.MAX cohort. J Nutr. 2005;135:2664-2668. [PubMed] |
| 29. | Yaman M. Comprehensive comparison of trace metal concentrations in cancerous and non-cancerous human tissues. Curr Med Chem. 2006;13:2513-2525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Yaman M, Kaya G, Simsek M. Comparison of trace element concentrations in cancerous and noncancerous human endometrial and ovary tissues. Int J Gynecol Cancer. 2007;17:220-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
S- Editor Wang GP L- Editor Wang XL E- Editor Ma WH