Published online Oct 14, 2007. doi: 10.3748/wjg.v13.i38.5101
Revised: August 8, 2007
Accepted: August 26, 2007
Published online: October 14, 2007
AIM: To investigate if there is a correlation between electrical activity measured by electrogastrography (EGG) and contractile activity of the stomach as measured by antroduodenal manometry (ADM). We also studied whether the underlying motility disorder could be predicted from EGG parameters.
METHODS: We compared 21 parameters measured from EGG with 8 parameters measured from ADM. The ability of EGG to identify the underlying diagnosis was tested by comparing EGG parameters for each diagnosis group against other patients. The study comprised recordings from 148 patients and 125 females. Their median age was 45 (range 17-76) years.
RESULTS: We found few and weak correlations between EGG and ADM. Specifically the correlation between parameters reflecting the response to meal was poor (r = -0.07, P = 0.39). The discriminatory power of EGG for underlying motility disorder was also low. Patients with slow transit constipation (STC) showed a lower postprandial power in normogastric (3.7 ± 0.5 vs 4.0 ± 0.5) and tachygastric (3.5 ± 0.4 vs 3.7 ± 0.4) regions, a lower percentage of time with normogastria [87.2 (56.5-100)% vs 95.7 (0-100)%], and a higher percentage of time with tachygastria [9.3 (0-33)% vs 3.5 (0-100)%] and bradygastria [1.8 (0-20)% vs 0 (0-17.1)%]. Patients with irritable bowel syndrome had a higher percentage of time with normogastria [96.5 (62.5-100)% vs 93.3 (0-100)%] and a less unstable dominant frequency as measured by the instability coefficient [15 (3-77) vs 24 (2-72)].
CONCLUSION: EGG and ADM seem to measure different aspects of gastric motor activity but cannot show a spatial correlation. The diagnostic value of EGG is poor, but EGG may have some value for the identification of patients with STC.
- Citation: Abid S, Lindberg G. Electrogastrography: Poor correlation with antro-duodenal manometry and doubtful clinical usefulness in adults. World J Gastroenterol 2007; 13(38): 5101-5107
- URL: https://www.wjgnet.com/1007-9327/full/v13/i38/5101.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i38.5101
Electrogastrography (EGG) is a technique for recording gastric myoelectric activity using cutaneous electrodes placed on the abdominal wall overlying the stomach. EGG detects rhythm and power (amplitude) of gastric myoelectricity[1]. Studies have shown a good correlation between cutaneous EGG recordings and myoelectric signals recorded from gastric serosal leads[2]. However, there are major concerns regarding the clinical usefulness of this non-invasive procedure[3].
EGG has been advocated as a diagnostic test in the clinical evaluation of patients with unexplained nausea, vomiting and other dyspeptic symptoms to gain insight into the mechanisms of symptom generation. Conflicting results have been obtained in previous studies. In some studies, subsets of patients with upper abdominal symptoms have depicted prominent EGG abnormalities whereas healthy volunteers rarely have exhibited EGG rhythm or power disturbances[4,5]. Other studies have found no difference in EGG parameters between dyspeptic patients and healthy volunteers[6,7].
Patients with systemic diseases such as Parkinson’s disease[8], myotonic dystrophy[9], and diabetes mellitus[10] show abnormal EGG findings. However, in patients with progressive systemic sclerosis (which is usually associated with poor gut motility), EGG shows unchanged parameters[11]. Similarly, EGG shows a greater variability compared to normal volunteers but no typical EGG pattern could be identified for different surgical procedures in surgical patients after cholecystectomy, Nissen fundoplication, subtotal gastrectomy or vagotomy and gastric pull-up operations[12].
Antroduodenal manometry (ADM) records lumen-occluding contractions of the gastric antrum[13]. Under physiological conditions there is a temporal correlation between myoelectric slow waves and contractile activity of the antrum. It is less clear if there is a spatial correlation between electrical and contractile activity. A small study in healthy females showed an unstable EGG peak power during fasting and an increase in EGG peak power after a solid test meal as indicative of a correlation between electrical activity and gastric motor activity[14]. It is conceivable that there should be a correlation between electrical activity measured by EGG and contractile activity measured by ADM. It would be of interest to know if EGG can predict gastric contractile activity. Surprisingly, a direct head to head comparison between EGG and ADM has not been done previously.
The aim of the present study was to compare measures of electrical activity determined by EGG and antral contractile activity from antroduodenal manometry. We also investigated whether EGG could differentiate between different underlying diagnoses of motility disorders.
This was a retrospective analysis of data collected during 1994-2001. Adult patients suffering from various functional and motility disorders of the gastrointestinal tract were included in our study. All of them were subjected to a combined EGG and ADM study as part of their clinical work-up for suspected gastrointestinal motility disorders. The two measurements were done simultaneously using two different systems, thus synchronous analysis was not possible in this study.
This procedure has been described elsewhere[6]. Briefly, a single bipolar channel was used. The EGG signal was recorded using a specially designed digital recorder (Digitrapper-EGG, Medtronic Synectics, Stockholm, Sweden) and sampled at a rate of 1 Hz. EGG recordings were examined visually for artefacts and then categorized into excellent (no artefacts), good (< 25% of recording time excluded because of artefacts), fair (< 50% excluded) or poor quality (≥ 50% excluded). After exclusion of artefacts, the remaining parts of the recordings were analysed with a software provided by the vendor (Polygram version 6.40, Medtronic Synectics, Stockholm, Sweden). This program could analyse the EGG using fast Fourier transformations of 256-s runs with an overlap of 196 s, so-called running spectral analysis, and can also make a Fourier transformation of an entire period in order to obtain a single power spectrum for that period. The parameters derived from the automated analysis of EGG are shown in Table 1. Dominant frequency instability coefficient (DFIC) and dominant power instability coefficient (DPIC) are measures of the variability of the EGG signal, which were computed as the standard deviations divided by the mean value of the dominant frequency and dominant power, respectively, from the running spectral analysis of each period.
| 1 EGG variables | |
| (1) Preprandial (fasting) and postprandial percentage of time with dominant frequency in: | |
| Bradygastria (0.5-2 cycles per minute) | |
| Normogastria (2-4 cycles per minute) (0.5-2 cycles per minute) | |
| Tachygastria (4-10 cycles per minute) | |
| (2) Pre- and postprandial power attributed to the three frequency bands (bradygastria, tachygastria and normogastria) | |
| (3) Pre- and postprandial dominant power instability co-efficient (DPIC) | |
| (4) Pre- and postprandial dominant frequency instability co-efficient (DFIC) | |
| (5) Pre- and postprandial dominant frequency (DF) | |
| (6) Pre and postprandial period dominant power (PDP) | |
| (7) Power ratio (ratio of postprandial to preprandial power of dominant frequency) | |
| 2 ADM variables | |
| (1) Pre- and postprandial motility index (MI) | |
| (2) Pre- and postprandial contractile frequency | |
| (3) Pre- and postprandial median amplitude | |
| (4) MI ratio (ratio between postprandial and preprandial motility index) | |
| (5) Amplitude ratio (ratio between postprandial and preprandial amplitude) | |
ADM was performed using a flexible catheter with either 6 or 8 water-perfused channels. The 6-channel catheter has 3 channels spaced 2 cm apart for the recording of antral motor activity whereas the 8-channel catheter has 5 channels spaced 1 cm apart for the same purpose. We used a pneumo-hydraulic pump (Arndorfer Medical Specialties, Greendale, WI, USA) with catheters connected to external pressure transducers. Data logging was done with a Polygraph 12HR and the Polygram (Medtronic Synectics, Stockholm, Sweden). The protocol included a 3-hour fasting period followed by a test meal (500 kcal standardized mixed meal) and 2-hour post-prandial recording[15]. Numerical analysis was done on data from the most distal recording site in the gastric antrum. Data were extracted using the Polygram that was set to detect contractions with an amplitude > 9 mmHg. We determined the mean frequency and amplitude of contractions and motility index during the last hour before and the first hour after intake of the test meal. The motility index was calculated as the area under the curve above baseline per time unit. We compared 8 variables from ADM with 21 variables from EGG (Table 1).
Normally distributed and lognormal data were expressed as mean ± SD and data with a non-normal distribution were given as a median and full range. We used Student’s t-test and Mann-Whitney U-test for assessing differences between the groups. Correlations were studied using Pearson’s product moment correlation. Logistic regression analysis was done to test EGG parameters for their discriminatory value in predicting underlying diagnoses. P < 0.05 was considered statistically significant.
A total of 185 patients were eligible for the study but 37 were excluded either because of previous gastric resection (n = 6), poor quality of the EGG recording (n = 23), or because of a technical failure (n = 8) in the EGG system. Another 4 patients were excluded from the comparison between EEG and ADM because ADM recordings did not show a recording from the antrum during the fasting or the fed period or both. Thus, the study population finally included 148 patients for comparing EGG parameters among various diagnosis groups and 144 patients for assessing the correlation between EGG and ADM parameters. The median age of the patients was 45 (range 17-76) years and 122 (82%) were females. There were 52 patients (35%) with IBS, 22 (15%) with enteric dysmotility (ED), i.e. abnormal small bowel motor activity but no bowel dilatation[16], 26 (18%) with slow transit constipation (STC), 11 (7%) with chronic intestinal pseudo-obstruction (CIP), 13 (9%) with functional dyspepsia with or without gastroparesis and 24 (16%) with other diagnoses, including various systemic and neurological diseases.
Few correlations were found between EGG and ADM. The correlation matrix was analyzed between 21 EGG parameters and 8 ADM parameters in this study (Brady = bradygastria, Normo = normogastria, Tachy = tachygastria, DF = dominant frequency, DFIC = dominant frequency instability coefficient, DPIC = dominant power instability coefficient, PDP = period dominant power, Amp = amplitude, MI = motility index, Freq = frequency, Pre = preprandial, Post = postprandial) (Table 2).
| Parameters | Pre MI1 | Post MI1 | MI ratio§ | Pre Freq | Post Freq | Pre Amp1 | Post Amp1 | Amp Ratio1 |
| Pre-Brady power1 | 0.15 | 0.01 | -0.10 | 0.10 | 0.01 | 0.08 | 0.06 | -0.01 |
| Pre-Normo power1 | 0.06 | 0.03 | -0.01 | 0.10 | 0.05 | -0.01 | -0.01 | 0.00 |
| Pre-Tachy power1 | 0.10 | 0.05 | -0.03 | 0.13 | 0.07 | 0.01 | 0.00 | -0.01 |
| Pre-Brady DF% | 0.06 | -0.01 | -0.05 | 0.05 | 0.01 | -0.09 | 0.08 | 0.12 |
| Pre-Normo DF% | 0.05 | 0.09 | 0.06 | 0.08 | 0.08 | 0.06 | 0.14 | 0.06 |
| Pre-Tachy DF% | -0.03 | -0.13 | -0.11 | -0.07 | -0.12 | -0.02 | -0.18a | -0.11 |
| Pre-DFIC | -0.04 | 0.04 | 0.06 | -0.12 | 0.02 | 0.07 | 0.03 | -0.04 |
| Pre-DPIC | -0.01 | -0.13 | -0.12 | -0.12 | -0.13 | 0.09 | -0.10 | -0.14 |
| Pre-DF | -0.08 | -0.11 | -0.05 | -0.10 | -0.10 | -0.04 | -0.17a | -0.09 |
| Pre-PDP1 | -0.09 | 0.01 | 0.08 | 0.00 | 0.03 | -0.02 | -0.04 | -0.02 |
| Post-Brady power1 | 0.04 | -0.04 | -0.07 | 0.13 | -0.04 | -0.10 | 0.02 | 0.09 |
| Post-Normo power1 | 0.09 | -0.01 | -0.07 | 0.13 | 0.04 | -0.03 | -0.04 | -0.01 |
| Post-Tachy power1 | 0.08 | 0.01 | -0.05 | 0.15 | 0.03 | -0.06 | -0.02 | 0.03 |
| Post-Brady DF% | 0.00 | 0.01 | 0.01 | -0.02 | -0.07 | -0.06 | 0.01 | 0.05 |
| Post-Normo DF% | -0.03 | -0.03 | -0.01 | -0.06 | -0.04 | 0.06 | 0.06 | 0.00 |
| Post-Tachy DF% | 0.02 | -0.04 | -0.05 | 0.05 | -0.02 | -0.05 | -0.09 | -0.02 |
| Post-DFIC | 0.04 | 0.03 | -0.01 | 0.11 | 0.08 | -0.09 | -0.11 | -0.01 |
| Post-DPIC | -0.06 | -0.1a | -0.14 | -0.09 | -0.17a | 0.02 | -0.18a | -0.15 |
| Post-DF | 0.04 | -0.01 | -0.04 | 0.06 | 0.00 | -0.05 | 0.00 | 0.04 |
| Post-PDP1 | 0.06 | -0.01 | -0.04 | 0.08 | 0.04 | 0.01 | -0.02 | -0.03 |
| Power ratio1 | 0.04 | -0.04 | -0.07 | 0.04 | -0.01 | -0.02 | -0.04 | -0.01 |
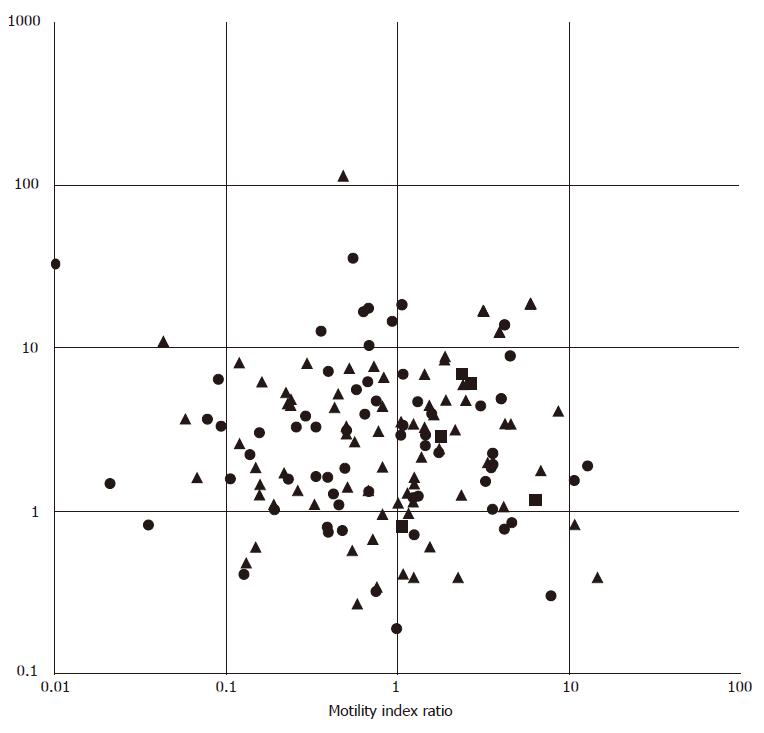
Postprandial contractile activity (amplitude, frequency and motility index) showed weak, negative correlations with the postprandial dominant power instability coefficient. Preprandial tachygastria (reflected by the percentage of time with a dominant frequency in the tachygastric region and dominant frequency) was also negatively correlated with postprandial contractile amplitude. The comparison between EGG response to meal ingestion (ratio between postprandial and fasting power) and ADM response to ingestion of a 500 kcal test meal (ratio between postprandial and fasting motility index) revealed no correlation (r = -0.07, P = 0.44, Figure 1).
The ability of EGG to identify diagnostic groups was tested by comparing EGG parameters for each group against all other patients included. Table 3 shows the discriminatory power of EGG for the larger patient groups. Variables with a normal or log-normal distribution were expressed as mean ± SD, else as median and full range. Patients with other diagnoses (n = 24) were not listed but included in the calculation of differences. Significant P-values were expressed as aP < 0.05, bP < 0.01, and dP < 0.001 (Brady: bradygastria, Tachy: tachygastria, Normo = normogastria, DFIC: dominant frequency instability coefficient, DPIC: dominant power instability coefficient, DF: dominant frequency, PDP: period dominant power).
| EGG parameter | Chronic intestinal pseudo-obstruction (n = 11) | Enteric dysmotility(n = 22) | Irritable bowel syndrome(n = 52) | Functional dyspepsia/gastroparesis (n = 13) | Slow transit constipation(n = 26) |
| Preprandial | |||||
| Log Brady power | 2.9 ± 0.4 | 2.8 ± 0.5 | 2.7 ± 0.5 | 2.7 ± 0.4 | 2.6 ± 0.4 |
| Log Normo power | 3.8 ± 0.5a | 3.7 ± 0.5 | 3.6 ± 0.5 | 3.6 ± 0.5 | 3.5 ± 0.5 |
| Log Tachy power | 3.7 ± 0.4a | 3.5 ± 0.5 | 3.3 ± 0.4 | 3.4 ± 0.4 | 3.3 ± 0.4 |
| Brady DF% | 2.7 (0-16.2) | 1.8 (0-17.5) | 0 (0-15.2) | 0 (0-10.3) | 2 (0-14.3) |
| Normo DF% | 89.7 (0-100) | 88.2 (36.2-100) | 92.0 (38.6-100) | 89.8 (59-100) | 87.7 (30.8-100) |
| Tachy DF% | 6.0 (0-100) | 8.8 (0-61.7) | 5.1 (0-61.4) | 6.5 (0-73.7) | 6.1 (0-69.2) |
| DFIC | 22 (3-51) | 32 (5-68) | 23 (3-75)a | 31 (1-70) | 30 (3-69) |
| DPIC | 77 (40-165) | 67 (33-169) | 64 (39-145) | 65 (31-101) | 60 (47-159) |
| DF | 3.05 (2.58-11.25) | 3.05 (2.34-3.98) | 3.05 (2.11-6.05) | 3.05 (2.58-3.75) | 3.05 (2.11-8.91) |
| Log PDP | 2.4 ± 0.6a | 2.0 ± 0.5 | 2.1 ± 0.6 | 2.1 ± 0.5 | 1.9 ± 0.5 |
| Postprandial | |||||
| Log Brady power | 3.0 ± 0.4 | 3.0 ± 0.5 | 2.9 ± 0.4 | 2.9 ± 0.5 | 2.8±0.5 |
| Log Normo power | 4.1 ± 0.3 | 4.1 ± 0.5 | 4.0 ± 0.5 | 4.1 ± 0.7 | 3.7 ± 0.5b |
| Log Tachy power | 3.9 ± 0.4 | 3.8 ± 0.5a | 3.6 ± 0.4 | 3.8 ± 0.6 | 3.5 ± 0.4a |
| Brady DF% | 0 (0-5.8) | 0 (0-5.0) | 0 (0-14.5) | 0 (0-17.1) | 1.8 (0-20)b |
| Normo DF% | 92.3 (0-100) | 96.1 (32.1-100) | 96.5 (62.5-100)a | 95.5 (32-100) | 87.2 (56.5-100)d |
| Tachy DF% | 5.8 (0-100) | 1.8 (0-66) | 3.1 (0-18.8) | 1.9 (0-21.1) | 9.3 (0-33)b |
| DFIC | 18 (4-63) | 16 (2-62) | 15 (3-77)a | 27 (5-56) | 27 (8-72)b |
| DPIC | 67 (59-81) | 56 (35-110) | 70 (34-129) | 61 (30-82) | 64 (39-108) |
| DF | 3.05 (2.58-9.14) | 3.28 (2.34-9.61) | 3.05 (2.58-3.75) | 3.28 (2.11-10.08) | 3.05 (2.34-3.75) |
| Log PDP | 2.5 ± 0.5 | 2.5 ± 0.5 | 2.4 ± 0.5 | 2.5 ± 0.8 | 2.1 ± 0.6b |
| Log Power ratio | 0.2 ± 0.5 | 0.5 ± 0.4 | 0.4 ± 0.4 | 0.5 ± 0.6 | 0.2 ± 0.5 |
In general, the discriminatory power of EGG was low. Only patients with STC in one diagnostic group, showed a reasonable number of differences in postprandial EGG parameters compared to those in all other diagnosis groups. STC patients had a lower postprandial power in the normogastric region (3.7 ± 0.5 vs 4.0 ± 0.5, P < 0.01) and in the tachygastric region (3.5 ± 0.4 vs 3.7 ± 0.4, P < 0.05). Similarly, the postprandial percentage of time with a dominant frequency was higher in bradygastric and tachygastric frequency bands and lower in the normogastric frequency band of STC patients (Table 3). Patients with STC also demonstrated a significantly lower postprandial dominant power (2.1 ± 0.6 vs 2.4 ± 0.5) compared to other patients.
In patients with CIP, EGG during fasting showed a significantly higher power in the tachygastric region (3.7 ± 0.4 vs 3.3 ± 0.4, P < 0.05) and a higher period dominant power (2.4 ± 0.7 vs 2.0 ± 0.5) compared to patients without CIP. In patients with IBS there was a higher percentage of time with a normogastric dominant frequency in the postprandial period and a more stable dominant frequency measured by DFIC during both preprandial and postprandial periods (Table 3).
Twenty patients underwent gastric emptying studies. Five of them had delayed gastric emptying. No significant differences were found in EGG parameters among patients with or without delayed gastric emptying.
To the best of our knowledge, this is the largest series of simultaneous recordings of EGG and ADM in patients with motility disorders of the gut. In the present study, we studied the relationship between EGG parameters and antral manometric findings. We also tried to evaluate the role of EGG in discriminating between various motility disorders of the gastrointestinal tract. EGG is not capable to record spike activity, hence the major electrical indicator of contractile activity is absent in its recordings. However, an increase in wave amplitude on EGG (referred to as power from the spectral analysis) can be asserted as a reflection of contractile activity. It has been observed that EGG power increases after certain drugs or meals that stimulate motility and decreases after drugs or meals that inhibit motility[12]. Therefore, to measure the significance of EGG power, it is best to compare EGG changes before and after the test meal given during EGG recording[14]. We found few and weak correlations between ADM findings and EGG parameters, especially no correlation between the motility index ratio and the power ratio. This is contrary to the findings of another study[17], where the EGG power ratio was significantly but weakly correlated with the motility index ratio. The number of patients in that study was, however, only 16. A partial correlation has also been found in children between the postprandial increase in amplitude of gastric electrical activity and antral motor activity[18]. In the present study, a more stable dominant power, i.e. lower DPIC, was associated with a higher contractile activity and tachygastria during the preprandial period was associated with lower amplitudes of contraction during the postprandial period, but the correlations were weak in both cases.
One important reason for a poor correlation between EGG-recorded electrical activity and ADM-recorded motor activity of the stomach could be that EGG recordings are sensitive to the degree of gastric wall distension and its approximation to the anterior abdominal wall. An underlying motility disorder of the gut might cause the gastric wall to distend inappropriately in response to the test meal and therefore lead to an inadequate EGG recording of gastric myoelectric activity. In light of the weak correlation between ADM and EGG found in other studies[17,18] and our observations, we think that EGG and ADM can measure two different aspects of gastric motor activity and a spatial relation cannot be fully determined between EGG and ADM.
Surprisingly, STC in the only diagnostic group stood out as clearly different from the other diagnostic groups on the basis of EGG parameters. Patients with STC exhibited differences mainly in the postprandial EGG parameters. Many studies have demonstrated a decrease in the density of interstitial cells of Cajal (ICC) in patients with slow transit constipation[19-21]. It is possible that observed postprandial changes in EGG parameters might be the reflection of a more generalized gastrointestinal myoelectrical dysfunction in STC patients secondary to depletion of ICC and other neuroenteric abnormalities. Further studies are needed to validate the EGG findings in patients with STC in the present study.
It was reported that EGG is diagnostically useful in adult patients with CIP[22]. Moreover, a comparison between normal subjects and children with CIP could reveal a significant increase of preprandial values for tachygastria and period dominant frequency and a decrease in normogastria and an increase in total tachygastria values in postprandial period[23]. In our study, the CIP patients had a significantly higher power on EGG in the tachygastric region and a higher PDP during fasting.
Another field of interest among researchers interested in evaluating the clinical usefulness of EGG is functional dyspepsia with or without gastroparesis. Studies have shown abnormal EGG patterns such as abnormal slow wave propagation and coupling[24], lower power ratio, lower postprandial level of increment in dominant power and a higher instability coefficient[25,26] in patients with functional dyspepsia. However, abnormal parameters are not the same in different studies. Interestingly, significantly more gastric arrhythmias have been found in oesophageal reflux patients with dyspeptic symptoms than in those with no dyspeptic symptoms[27].
Contradictory findings can ben found when capability of EGG is assessed to predict delayed gastric emptying. It was reported that patients with impaired gastric emptying show a significantly lower percentage of normal gastric slow waves and a lower postprandial increment in dominant power[28]. On the other hand, another study conducted in patients with systemic sclerosis has not found any correlation between delayed gastric emptying and EGG abnormalities[11]. In our study, no difference in EGG parameters was found between dyspeptic patients with delayed gastric emptying and those with normal gastric emptying, possibly due to a small number of patients with gastric emptying.
Our study has certain limitations. One of them was the retrospective design of the present series, which is unlikely to have influenced the results of the present study concerning the correlation between EGG and ADM. On the other hand, the comparison of EGG parameters between different diagnostic groups was hampered by a small number of patients in certain groups. Another limitation was the absence of a control group to compare with patients with motility disorders. In the present series, however, comparison of both EGG and ADM comparison was available in each patient. In our opinion, the assessment of correlation between EGG and ADM is not affected by the absence of a control group. We used a single channel EGG instead of a multiple channel EGG in our study. Novel multi-channel EGG can study the propagation and coupling of slow waves in the stomach, and is perhaps more helpful than single channel EGG in the diagnosis of gastric dysfunction[24]. It is possible that registrations from multiple channels would have a better chance to detect correlations with contractile activity, not least because sampling error from multiple channels would be smaller than those from a single channel. However, no data are available from comparisons between ADM and multi-channel EGG.
A high exclusion rate of patients in our series was due to EGG recordings that were not interpretable. Motion artefacts were common despite the stationary condition of the patients. Care was taken in preparing the skin so that the impedance between any two electrodes was less than 10 ohms. Nevertheless, many recordings exhibited low frequency aberrations and shifts of baseline. Moreover, various technical problems, such as faulty electrode cables or malfunction due to static electricity, also resulted in exclusion of a significant number of patients from analysis. We have not come across any account for such failures of EGG recording in previously published studies.
In short, no consistent relationship was observed between EGG and ADM in this series. No evidence was found in this study to favour a spatial correlation between the parameters measured by EGG and ADM. Moreover, the ability of EGG to identify motility disorders was generally poor in this study.
In conclusion, EGG findings in STC patients are interesting but further studies are required to validate the role of EGG in STC patients.
Electrogastrography is to measure the electrical activity of the stomach from electrodes attached to the abdominal surface. The usefulness of this technique for the diagnosis of gastrointestinal diseases is yet unknown. However, since electrical activity is pivotal for motor function, it is believed that measurement of electrical activity should reveal important diagnostic information in patients with motility disorders.
The correlation between measures derived from electrogastrography and other techniques such as manometry has received little attention. Such correlations are important for understanding the relation between different measurement techniques.
This is a head-to-head comparison of electrogastrography and antroduodenal manometry, i.e. measurement of contractile activity of the stomach done at the same time from a large number of patients with gastrointestinal motility disorders.
This study could not show any correlation between antroduodenal manometry and electrogastrography, suggesting that the two techniques can measure different aspects on gastric motor function. The study also showed that the ability of electrogastrography to identify patients with different diagnoses was poor. To the surprise of the authors, patients with slow transit constipation, a severe form of intractable constipation, were the only group of patients identified by electrogastrography. The reason for this is not clear, but it is known from previous research that patients with slow transit constipation have a lack of pacemaker cells in the gut, which could explain why they also had disturbed electrical rhythm in the stomach.
Correlation is a measure of the relative correspondence of two sets of data. Manometry is the measurement of (in this case, intraluminal) pressure over time. Motility disorders comprise a large number of disorders that affect gastrointestinal motor activity. The most common disorder is irritable bowel syndrome.
This is a well written paper on a subject with a considerable debate. Whether electrogastrography and antroduodenal manometry can measure different aspects of gastric motor activity needs to be further studied.
| 1. | Chang FY. Electrogastrography: basic knowledge, recording, processing and its clinical applications. J Gastroenterol Hepatol. 2005;20:502-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Lin Z, Chen JD, Schirmer BD, McCallum RW. Postprandial response of gastric slow waves: correlation of serosal recordings with the electrogastrogram. Dig Dis Sci. 2000;45:645-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Bortolotti M. Electrogastrography: a seductive promise, only partially kept. Am J Gastroenterol. 1998;93:1791-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Ogawa A, Mizuta I, Fukunaga T, Takeuchi N, Honaga E, Sugita Y, Mikami A, Inoue Y, Takeda M. Electrogastrography abnormality in eating disorders. Psychiatry Clin Neurosci. 2004;58:300-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Rezende Filho J, De Rezende JM, Melo JR. Electrogastrography in patients with Chagas' disease. Dig Dis Sci. 2005;50:1882-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Holmvall P, Lindberg G. Electrogastrography before and after a high-caloric, liquid test meal in healthy volunteers and patients with severe functional dyspepsia. Scand J Gastroenterol. 2002;37:1144-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Oba-Kuniyoshi AS, Oliveira Jr JA, Moraes ER, Troncon LE. Postprandial symptoms in dysmotility-like functional dyspepsia are not related to disturbances of gastric myoelectrical activity. Braz J Med Biol Res. 2004;37:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Lin Z, Eaker EY, Sarosiek I, McCallum RW. Gastric myoelectrical activity and gastric emptying in patients with functional dyspepsia. Am J Gastroenterol. 1999;94:2384-2389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Rönnblom A, Hellström PM, Holst JJ, Theodorsson E, Danielsson A. Gastric myoelectrical activity and gut hormone secretion in myotonic dystrophy. Eur J Gastroenterol Hepatol. 2001;13:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Koch KL. Electrogastrography: physiological basis and clinical application in diabetic gastropathy. Diabetes Technol Ther. 2001;3:51-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Franck-Larsson K, Hedenström H, Dahl R, Rönnblom A. Delayed gastric emptying in patients with diffuse versus limited systemic sclerosis, unrelated to gastrointestinal symptoms and myoelectric gastric activity. Scand J Rheumatol. 2003;32:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Kauer WK, Stein HJ, Balint A, Siewert JR. Transcutaneous electrogastrography: a non-invasive method to evaluate post-operative gastric disorders? Hepatogastroenterology. 1999;46:1244-1248. [PubMed] |
| 13. | Soffer EE, Thongsawat S, Ellerbroek S. Prolonged ambulatory duodeno-jejunal manometry in humans: normal values and gender effect. Am J Gastroenterol. 1998;93:1318-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Chen JD, Richards RD, McCallum RW. Identification of gastric contractions from the cutaneous electrogastrogram. Am J Gastroenterol. 1994;89:79-85. [PubMed] |
| 15. | Stanghellini V, Camilleri M, Malagelada JR. Chronic idiopathic intestinal pseudo-obstruction: clinical and intestinal manometric findings. Gut. 1987;28:5-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 226] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Wingate D, Hongo M, Kellow J, Lindberg G, Smout A. Disorders of gastrointestinal motility: towards a new classification. J Gastroenterol Hepatol. 2002;17 Suppl:S1-S14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 100] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Lee JS, Jang JY, Hong SJ, Im HH, Ryu CB, Kim JO, Cho JY, Lee MS, Shim CS, Kim BS. Clinical significance of cutaneous electrogastrography (EGG) compared with antroduodenal manometry (ADM). Neurogastroenterol Motil. 2002;14:308. |
| 18. | Faure C, Wolff VP, Navarro J. Effect of meal and intravenous erythromycin on manometric and electrogastrographic measurements of gastric motor and electrical activity. Dig Dis Sci. 2000;45:525-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Lee JI, Park H, Kamm MA, Talbot IC. Decreased density of interstitial cells of Cajal and neuronal cells in patients with slow-transit constipation and acquired megacolon. J Gastroenterol Hepatol. 2005;20:1292-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 20. | Bassotti G, Villanacci V, Maurer CA, Fisogni S, Di Fabio F, Cadei M, Morelli A, Panagiotis T, Cathomas G, Salerni B. The role of glial cells and apoptosis of enteric neurones in the neuropathology of intractable slow transit constipation. Gut. 2006;55:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 156] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 21. | Tong WD, Liu BH, Zhang LY, Xiong RP, Liu P, Zhang SB. Expression of c-kit messenger ribonucleic acid and c-kit protein in sigmoid colon of patients with slow transit constipation. Int J Colorectal Dis. 2005;20:363-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 22. | Debinski HS, Ahmed S, Milla PJ, Kamm MA. Electrogastrography in chronic intestinal pseudoobstruction. Dig Dis Sci. 1996;41:1292-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Bracci F, Iacobelli BD, Papadatou B, Ferretti F, Lucchetti MC, Cianchi D, Francalanci P, Ponticelli A. Role of electrogastrography in detecting motility disorders in children affected by chronic intestinal pseudo-obstruction and Crohn's disease. Eur J Pediatr Surg. 2003;13:31-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Lin X, Chen JZ. Abnormal gastric slow waves in patients with functional dyspepsia assessed by multichannel electrogastrography. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1370-G1375. [PubMed] |
| 25. | Lu CL, Chen CY, Chang FY, Kang LJ, Lee SD, Wu HC, Kuo TS. Impaired postprandial gastric myoelectrical activity in Chinese patients with nonulcer dyspepsia. Dig Dis Sci. 2001;46:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | van der Voort IR, Osmanoglou E, Seybold M, Heymann-Mönnikes I, Tebbe J, Wiedenmann B, Klapp BF, Mönnikes H. Electrogastrography as a diagnostic tool for delayed gastric emptying in functional dyspepsia and irritable bowel syndrome. Neurogastroenterol Motil. 2003;15:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Chen CL, Lin HH, Huang LC, Huang SC, Liu TT. Electrogastrography differentiates reflux disease with or without dyspeptic symptoms. Dig Dis Sci. 2004;49:715-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Chen JD, Lin Z, Pan J, McCallum RW. Abnormal gastric myoelectrical activity and delayed gastric emptying in patients with symptoms suggestive of gastroparesis. Dig Dis Sci. 1996;41:1538-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 194] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
S- Editor Liu Y L- Editor Wang XL E- Editor Li JL