Published online Oct 7, 2007. doi: 10.3748/wjg.v13.i37.5009
Revised: July 16, 2007
Accepted: July 26, 2007
Published online: October 7, 2007
AIM: To evaluate the predictive value of hepatocyte proliferation and hepatic angiogenesis for the occurrence of Hepatocellular carcinoma (HCC) in hepatitis C virus (HCV) cirrhotic patients.
METHODS: One hundred-five patients (69 males, 36 females; age range, 51-90 year; median 66 year) with biopsy proven HCV cirrhosis were prospectively monitored for HCC occurrence for a median time of 64 mo. Angiogenesis was assessed by using microvessel density (MVD), hepatocyte turnover by MIB1 and PCNA indexes at inclusion in liver biopsies.
RESULTS: Forty six patients (43.8%) developed HCC after a median time of 55 (6-120) mo while 59 (56.2%) did not. Patients were divided into two groups according to the median value of each index. The difference between patients with low (median MVD = 3; range 0-20) and high (median MVD = 7; range 1-24) MVD was statistically significant (χ2 = 22.06; P < 0.0001) which was not the case for MIB1 or PCNA (MIB-1: χ2 = 1.41; P = 0.2351; PCNA: χ2 = 1.27; P = 0.2589). The median MVD was higher in patients who developed HCC than in those who did not. HCC-free interval was significantly longer in patients with the MVD ≤ 4 (P = 0.0006). No relationship was found between MIB1 or PCNA and MVD (MIB-1 r2 = 0.00007116, P = 0.9281; PCNA: r2 = 0.001950; P = 0.6692). MVD only was able to predict the occurrence of HCC in these patients. Among other known risk factors for HCC, only male sex was statistically associated with an increased risk.
CONCLUSION: Liver angiogenesis has a role for in HCV-related liver carcinogenesis and for defining patients at higher risk.
- Citation: Mazzanti R, Messerini L, Comin CE, Fedeli L, Gannè-Carrie N, Beaugrand M. Liver angiogenesis as a risk factor for hepatocellular carcinoma development in hepatitis C virus cirrhotic patients. World J Gastroenterol 2007; 13(37): 5009-5014
- URL: https://www.wjgnet.com/1007-9327/full/v13/i37/5009.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i37.5009
Hepatocellular carcinoma (HCC) is nowadays the leading cause of death in patients with cirrhosis and its incidence is continuously increasing in the west[1]. Although cirrhosis is present in about 80%-90% of cases in western countries, other risk factors have been well defined such as age, sex, platelet count, alpha-fetoprotein (AFP) level and the cause of the underlying liver disease[2]. Cirrhosis is considered to cause HCC independently of etiology and HCC from different individuals may have different phenotypes[3]. This could be due to individual host response to etiologic agent, but there are features that unify HCC occurring in a background of a specific etiologic agent[3]. HCC is the result of a multi step process that proceeds from the initiation of one or more hepatocytes to a diffuse disease[4]. HCC due to chronic viral infection may be an indirect result of hepatocyte proliferation that occurs during chronic hepatitis in an effort to replace infected cells that have been immunologically attacked[5]. Changes due to viral factors may also play a more direct role in liver oncogenesis[6,7]. Most cases of HCC in the West occur in patients with cirrhosis mainly due to hepatitis C virus (HCV) infection which is the second cause of HCC in the world[8]. HCV-infected patients have a high risk for developing HCC but mechanisms leading to the increased risk for liver cancer are still matter of debate.
Chronic HCV infection may lead to a high hepatocyte turnover, resulting in replicative senescence of liver cells, accumulation of DNA alterations[9,10] and cancer. This phenomenon appears to be hardly specific to HCV infection[3].
Angiogenesis is known to play a pivotal role in almost every kind of malignancy favoring growth and metastasis of several types of cancer including HCC[11]. Many angiogenesis-related factors such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), platelet-derived endothelial cell growth factor (PD-ECGF), thrombospondin (TSP), angiogenin, pleiotrophin and endostatin levels have been evaluated and shown to be of prognostic significance in various types of cancer. Quantification of microvessel density (MVD) inside the tumour has been widely demonstrated to be a reliable tool of evaluating angiogenesis[12]. The exact role of these factors is still unclear although the crucial importance of VEGF, cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) overexpression are widely recognized[13].
The prognostic significance of MVD independently of other pathologic predictors was demonstrated in several malignancies, i.e. breast, gastric, colorectal, pancreatic carcinoma, testicular germ tumour, malignant melanoma and even hematological malignancies[14-17].
HCC is a highly vascular tumour characterized by a propensity for vascular invasion. Angiogenesis increases in the early phases of HCC development[18]. MVD is reported to be correlated to the size of the tumour and may predict early recurrence after resection[19,20]. Therefore, it is conceivable that the high density of new vessels observed in the liver of patients with chronic HCV infection may contribute to the early development of cancer cells and may explain the high incidence of HCC in these patients[18].
In a previous study, we demonstrated that liver angiogenesis as measured by MVD was significantly more common and intense in patients with chronic HCV infection in comparison to matched patients with chronic HBV infection or controls[21]. Experiments in vitro using endothelial cells exposed to liver homogenates and sera of patients with different hepatic diseases confirmed that HCV positive biologic tissues stimulated in a greater extent migration and proliferation of endothelial cells[21]. Based on these results, liver angiogenesis due to chronic HCV infection was argued to be independent of inflammation. These observations indicated that liver angiogenesis is most intense in HCV infected patients, suggesting a possible mechanism linking HCV infection to the increased risk of developing HCC.
The present study aimed at investigating whether increased liver angiogenesis as estimated by the MVD in liver biopsies could be predictive of the future occurrence of HCC in patients with HCV related cirrhosis. Furthermore, since PCNA staining, a marker of hepatocyte proliferation, was previously reported to be predictive of HCC, in this study also two proliferative indexes, PCNA and MIB1 and their correlation to MVD were studied together other known risk factors for HCC.
One hundred and five cirrhotic patients (69 males and 36 females; age range, 51-90 year; mean 62.5 ± 8.3 year) were included and monitored (120 mo) from January 1993 to January 2003. All enrolled patients met the following criteria: (1) chronic HCV infection documented by the presence of anti-HCV antibodies and HCV RNA in the serum; (2) histologically proven HCV related cirrhosis; (3) absence of other causes of viral or non-viral liver disease including alcohol abuse or any kind of genetic disease; (4) absence of contemporary or previous antiviral treatment. All patients were investigated at the beginning of the study by routine liver function test assessment, liver ultrasound scan, and determination of serological markers of viral infections and autoimmune diseases. All patients had a homogenous liver at ultrasound (US) examination and serum alfafetoprotein (AFP) level under 20 μg/L. All patients were affected by Child-Pugh A well compensated liver cirrhosis according to international criteria[22,23]. They were thereafter included in a screening program for HCC consisting in periodic US examination and determination of serum AFP levels at least every 6 mo.
This study was approved by the local Ethical Committees of both Azienda Ospedaliero-Universtaria Careggi, Firenze, Italy and Hopital Jean Verdier, Assistance Publique-Hopitaux de Paris et UFR SMBH-Universite Paris XIII, Bondy, France. All enrolled subjects gave their informed consent to undergo liver biopsy for diagnostic purposes and to participate in the study.
All patients were tested for anti-HCV antibodies by a second generation enzyme-linked immunosorbant assay (Ortho Diagnostic Systems, Inc., Raritan, NJ) and radio immunoblotting assay (Chiron Corporation, Emeryville, CA). Confirmation of the presence of serum viral HCV-RNA in anti-HCV positive patients was performed by a standard polymerase chain reaction technique. Serum hepatitis B markers and HBV DNA (Abbott Laboratories, North Chicago, IL) were performed in addition.
Liver biopsies were coded, formaldehyde-fixed, paraffin-embedded, cut into 4 μm thick sections, and stained with standard techniques. Immunohistochemical stainings were performed on 4 μm thick formaldehyde-fixed paraffin embedded sections, using the streptavidin-biotin immunoperoxidase technique. Antigen retrieval with microwave treatment with citrate buffer, pH 6.0, for 10 min was used. After blocking endogenous peroxidase the primary antibodies were applied.
Monoclonal antibody anti-CD34 (Immunotech, Marsiglia, Fr) was used at a 1:100 dilution at 4°C for 12 h. Sections were incubated for 12 h at room temperature with MIB-1 monoclonal antibody (Immunotech, Marseille, Fr) at 1:50 dilution or were incubated for 2 h at room temperature with PCNA monoclonal antibody (Dako, Carpinteria CA) at 1:200 dilution. For negative controls, sections were treated the same way except they were incubated with Tris-buffered saline instead of the primary antibody.
The percentage of tumor cells positive for MIB-1 as well as PCNA was determined semiquantitatively by assessing the whole tumor section, and each sample was scored by 0.1 points for every 1% of positive cells.
MVD was assessed by light microscopy using the counting method introduced by Weidner et al[24], CD34 was used to identify newly formed microvessels. As explained elsewhere[21] liver tissue sections were scanned at low magnification (× 40 and × 100) to find the areas that showed the most intense vascularization (hot spots). Individual microvessels were counted in three fields at 200 × magnification (20 × objective lens and 10 × ocular lens; 0.7386 mm2 per field). The final MVD was the mean value obtained from counts of the 3 fields. MVD was expressed as mean ± standard deviation (vessels per mm2). Any immunostained endothelial cell or endothelial cell cluster that was clearly separated from hepatocytes and other connective tissue elements was considered a single and countable microvessel. Vessel lumens were not necessary for a structure to be defined as a microvessel, and red cells were not used to define a vessel lumen. Evaluation of MVD was performed without knowledge of any clinical and pathological data.
Statistical analysis was performed using Yates’ corrected analysis to compare proportions. Correlation between continuous variables was performed with linear regression analysis. Disease-free survival rates were computed by the Kaplan-Meier method and were compared by the log-rank test. Student’s t-test, one-way ANOVA, χ2 analysis were also used where indicated. P≤ 0.05 was considered significant. Cox regression method was used to analyze the effect of single risk factors on HCC occurrence risk. SPSS 10.0 software (SPSS, USA) was used to analyze the data.
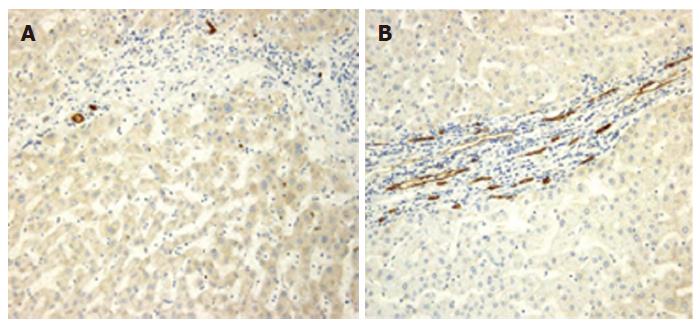
Serum AFP levels of patients were 7.55 ± 4.87 μg/L, and their platelets levels of patients were 113 ± 25 × 108/L (mean ± SD). The median time of follow-up in the whole group of patients was 64 months. Fourty-six out of the 105 (43.8%) patients developed HCC during the follow-up. The median time to HCC occurrence was 55 (range 6-120) mo. Fifty-nine patients (56.2%) did not develop HCC the observation time. Year incidence of HCC was 4.4 %. CD 34 staining as a marker of newly formed microvessels was observed within hepatic tissue along vascular channels, in groups of cells that apparently did not border vascular lumina and in groups of cells located in portal tracts and septa. Figure 1 shows examples of low (MVD ≤ 4, Panel A) and high (MVD > 4, Panel B) CD 34 expression, assessed by QB-END/10 monoclonal antibody. The highest number of newly formed microvessels observed in one single microscopic field (250 ×) was 24. A microvessel density score less than or equal to 4 was considered to be low angiogenesis expression while one greater than 4 as high. The cut-off score of 4 was chosen as it was the median of the MVD scores in our study group.
When considering the whole group of 105 patients, 32 out of the 46 patients who developed HCC showed a high (> 4) MVD score while only 14 patients of the 59 patients who did not develop HCC showed a high MVD score (χ2 = 22.26; P < 0.0001).
The median MVD score of those patients developing HCC was 7 (range 1-24) while the median score of those who remained HCC-free was 3 (range 0-20). Proliferating cells were identified by immunohistochemistry for monoclonal antibodies, MIB-1 and PCNA. No difference was observed when comparing immunostaining for Ki-67 (MIB-1 high score: > 1 and low score: ≤ 1 and PCNA: high score: > 4 and low score: ≤ 4 as score 1 and 4 were the medians of the MIB-1 and PCNA scores in our study group, respectively) in the group of patients which developed HCC during the study as compared to other patients who did not develop HCC (MIB-1: χ2 = 1.24; P = 0.2351; PCNA: χ2 = 1.27; P = 0. 2589) (Table 1).
| HCC incidenceduring follow-up | No HCCduring follow-up | Total | |
| MIB-1 > 1 | 40 | 46 | 86 |
| MIB-1 ≤ 1 | 6 | 13 | 19 |
| PCNA > 4 | 23 | 23 | 46 |
| PCNA ≤ 4 | 23 | 36 | 59 |
| Total | 46 | 59 | 105 |
No correlation was found between the MVD score and MIB-1(Panel A, MIB-1: r2 = 0.00007116; P = 0.9281) or PCNA score (Panel B; PCNA: r2 = 0.001950; P = 0.6692). As previously shown, the presence of cirrhosis had no significant effect on immunostaining results for either QB-END/10 or MIB-1 or PCNA antibodies (data not shown)[21].
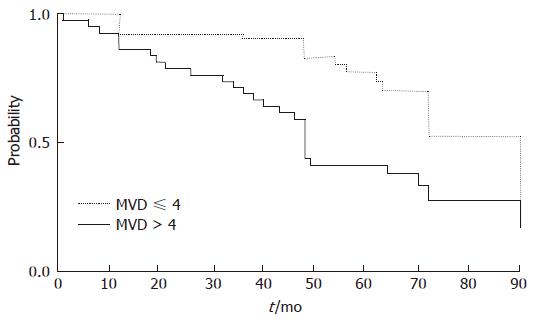
The HCC-free interval during follow-up turned out to be significantly longer in the group of patients having an MVD score < 4 (P = 0.0006) (Figure 2). On the contrary, no difference was shown by chi-squared analysis of the 105 patients who were divided in two groups according to the PCNA score (χ2 = 1.27; P = 0.2589). Among known risk factors for HCC such as sex, age, platelets and AFP only male sex was a risk factor for HCC development
(χ2 = 3.91; P = 0.048).
HCC is a lethal malignancy and its incidence ranks fifth but fourth in terms of mortality[2,4]. It is well known that the growth of tumours and metastatization depend on adequate angiogenesis. HCC is a hyper-vascular tumour and neo-vascularization is a hallmark of HCC. At least two studies demonstrated that angiogenesis, as assessed by counting of microvessels, should be considered as an important prognostic factor in HCC surgical therapy (resection) outcome[25,26]. Intense angiogenesis expressed as high microvessel count is associated with neoplastic disease spread and poor survival. The present study is the first one that analyzes the predictive value of MVD for occurrence of HCC in patients with HCV cirrhosis. Results shown here suggest that HCV positive cirrhotic patients with more intense hepatic angiogenesis are at higher risk for HCC occurrence. This data contribute to a more accurate definition of risk factors in HCV positive cirrhotic patients together with other simple epidemiological parameters such as sex and age. According to this findings, hepatic MVD could be added to other of histologic parameters such as large cell dysplasia that could help to a more accurate definition of high risk groups of patients for HCC. Furthermore, it gives a contribute to our understanding of liver cancerogenesis in HCV positive patients.
Several studies considered angiogenesis in HCC patients, even in early stages, but none provided evidence of a relationship between angiogenesis and the probability of HCC developing during chronic HCV infection. Although a relationship between MVD and HCC free survival time and prognosis in patient surgically treated for HCC was already proven, this is the first study that analyze the prognostic value of MVD in patients with chronic HCV infection and liver cirrhosis. Many angiogenesis-related immunohistochemical markers, such as VEGF, b-FGF, PD-ECGF, TSP, pleiotrophin and endostatin levels as well as the identification of angiogenesis measured as MVD have been found to be of prognostic significance in cancer disease. Particularly, MVD has been widely demonstrated to be a suitable assay to evaluate cancer related angiogenesis[12,15].
A prognostic value of MVD independently from conventional pathologic predictors was demonstrated in many types of cancer. It was shown that angiogenesis as measured by MVD correlates significantly with tumour size in patients undergoing HCC resection and may predict early recurrence after liver resection. Increased angiogenesis assessed by MVD can be considered as an hallmark of HCV chronic hepatitis as we emphasized years ago[21]. In that study, it was shown that MVD was 14 fold increased in patients with chronic HCV hepatitis compared to controls and 5 fold compared to patients with matched chronic HBV hepatitis. Patients with non viral liver diseases such as primary biliary cirrhosis and inflammatory pseudo tumour were considered as controls and had a mild and non significant increase in MVD. Studies performed in vitro confirmed those findings showing enhanced HUVEC migration and proliferation when endothelial cells were exposed to sera or liver homogenates from HCV infected patients.
In the present study we used the MVD count to quantify angiogenesis in the liver of 105 patients with HCV related cirrhosis. Forty-six of them have developed HCC after a median time of 55 mo whilst 59 patients did not develop HCC. The whole group of patients was divided in two groups according to the MVD count. Patients with a MVD count higher than 4 were exposed to a higher risk of developing HCC during follow-up. A high MVD score in fact, was associated with a significantly higher probability to develop HCC during follow-up. It is noteworthy that the median time of follow-up of those who remained free from HCC was significantly longer than that of the group of patients that would have developed HCC during follow-up. The mean annual incidence of HCC occurrence was 4.4% corresponding to what is usually observed in this type of patients.
Among other known risk factors for HCC, only male sex was associated with a higher risk to develop HCC. The difference between male and female in the occurrence of HCC was statistically significant (69 males vs 36 females; P = 0.048). However, it must be said that to establish risk factors for multifactorial diseases as is cancer are usually needed a much larger number of subjects included in a survey study than what included in the present study. In addition, in the present study only patients with histology proven HCV related liver cirrhosis with a mean age older than what is commonly reported in the literature as mean age of HCC occurrence were considered. We think that these facts played a big role in explaining why some of other known risk factors for HCC did not appear statistically related to liver cancer in the present study. Interestingly, different geographic areas did not play any role in affecting HCC risk in the present work, in fact French and Italian patients who developed HCC did not show a significant difference in mean age (68.5 years vs 65.3 years, French vs Italian, respectively) at the moment of diagnosis. In addition, dividing French from Italian patients the MVD score remained predictive for HCC development (χ2 = 13.18; P < 0.0001; and 5.60; P < 0.0179 French and Italians, respectively).
In this study, besides the MVD counts, we considered the predictive value of 2 proliferative indexes, PCNA and MIB-1, that have been proposed as risk factors for the occurrence of HCC[12]. In our hands, PCNA and MIB-1 staining were not predictive of the occurrence of HCC.
No one of patients included in the present study had had treatment with antiviral drugs before entering the study neither had during it. After that it was shown[27,28] that antiviral treatment resulted in a reduction of HCC risk the study could not go on for ethical reasons. These findings, although have brought to the end our study, on the same time, they indirectly confirmed the validity of the observation we did in the present study. In fact, lymphoblastoid IFN alpha has antiangiogenic activity in addition to its antiviral activity and therefore our findings support the role of IFN alpha treatment in patients with HCV positive liver cirrhosis.
Recent epidemiological studies have showed that the annual incidence of HCC is greater in HCV related cirrhosis than in other types of liver diseases due to non viral agents but also HBV liver disease at least in Western countries and in Japan[29]. In geographic areas such as Africa and South-East Asia the occurrence of HCC is greatly influenced by aflatoxin exposure and p53 mutations in addition to HBV and HCV infection. In Western countries and in Japan where exposure to aflatoxin is negligible the incidence of HCC in patients with HBV cirrhosis has always been found lower than in patients with HCV cirrhosis leading to endless speculations about the oncogenic role of a RNA virus unable to integrate in the hepatocyte genome[29]. We think that intense liver angiogenesis might provide a plausible explanation of this phenomenon and explains why patients with mixed causes of chronic liver diseases such as alcoholic or HBV liver disease are at very high risk of HCC when super-HCV infection occurs. The mechanism by which increased angiogenesis in HCV patients is unknown but COX-2 expression in hepatocytes might play a role as it is correlated to the degree of angiogenesis in liver tumours and is up-regulated by viral core and NS5A protein in patients with chronic hepatitis[30]. This hypothesis is consistent with data where cancer related angiogenesis was evaluated. In particular, it was demonstrated the association between HCC vascularity and COX-2 and iNOS expression[31]. The Authors concluded that higher COX-2 and iNOS expression in HCC was correlated to angiogenesis and to a worst prognosis of patients[31].
Novel therapies have been proposed for HCC[32-34]. Based on in vitro data, chemoprevention of HCC by COX-2 inhibitors has been recently proposed although further studies should be carried out.
In conclusion, the increased hepatic angiogenesis assessed by the MVD counting is a hallmark of HCV liver disease, it can play a role in liver carcinogenesis and, most importantly, can be considered as a risk factor for HCC occurrence in patients with HCV cirrhosis. If confirmed, this observation could help to make a better definition of high risk patients, giving a clue to understand the mechanism leading to high incidence of cancer in these patients and a potential therapeutic target in the future.
Prof. Paolo Gentilini, to whom this article is dedicated, for his continuous support to our research throughout the last 30 years. Prof. Vieri Boddi for statistical supervision and Dr. Nadia Lasagna for manuscript revision.
Chronic HCV infection is one of the most important causes for HCC, however hepatic oncogenic mechanisms related to HCV infection are still not fully elucidated. Since it was shown that HCV is angiogenetic, we hypothesised that HCV related angiogenesis is a risk factor for HCC development.
Years ago we showed that patients with chronic HCV hepatitis had more common and intense liver angiogenesis as compared to matched patients with chronic HBV hepatitis and patients such as those with PBC or pseudo-inflammatory tumor who were used as controls for inflammation (1). Angiogenesis is a well recognized risk factor for cancer, at least for its growth and diffusion (2). Patients with chronic HCV infection show expression of COX-2 an inducible enzyme that is angiogenetic and anti apoptotic. Thus, the goal of our manuscript was to show that in cirrhotic patients, with chronic HCV infection, those with the most intense hepatic angiogenesis were at greater risk for HCC development.
The manuscript improves our understanding on how chronic HCV infection may be responsible for the higher incidence of HCC as compared to other patients with chronic liver diseases. In fact, the demonstration that active liver angiogenesis increases the risk for HCC not only can explain why interferon treatment reduce the risk of HCC acting directly on angiogenesis (interferons are compounds with substantial anti angiogenetic activity), but it also opens new perspectives for designing new therapeutic strategies to prevent HCC in cirrhotic HCV patients. With regards this point, for instance, the recent demonstration that sorafenib (ASCO 2007, abstract book), a new anti tyrosine kinase agent whose main action is antiangiogenetic, can be very active and helpful in the treatment of advanced HCC, support data of the present manuscript.
Angiogenesis is the phenomenon that occurs in several physiological and pathological conditions in the body and it is characterized by the production of new vessels starting from pre-existing endothelial cells. The micro vessel density counting (MVD) is a quite diffuse manner to measure angiogenetic activity and it correlates quite well with bad prognosis of cancer patients. PCNA and MIB-1 are two indexes used to measure proliferative activity in a tissue.
The manuscript deals with an important issue in clinical oncology and gastroenterology: To improve our understanding on how chronic HCV infection increases the risk of HCC in addition to the fact to cause chronic inflammation and liver cirrhosis.
| 1. | El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002;35:S72-S78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 471] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 2. | Di Bisceglie AM. Epidemiology and clinical presentation of hepatocellular carcinoma. J Vasc Interv Radiol. 2002;13:S169-S171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Block TM, Mehta AS, Fimmel CJ, Jordan R. Molecular viral oncology of hepatocellular carcinoma. Oncogene. 2003;22:5093-5107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 375] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 4. | Romeo R, Colombo M. The natural history of hepatocellular carcinoma. Toxicology. 2002;181-182:39-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Hayashi J, Aoki H, Arakawa Y, Hino O. Hepatitis C virus and hepatocarcinogenesis. Intervirology. 1999;42:205-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Schulze zur Wiesch J, Schmitz H, Borowski E, Borowski P. The proteins of the Hepatitis C virus: their features and interactions with intracellular protein phosphorylation. Arch Virol. 2003;148:1247-1267. [PubMed] |
| 7. | Hino O, Kajino K, Umeda T, Arakawa Y. Understanding the hypercarcinogenic state in chronic hepatitis: a clue to the prevention of human hepatocellular carcinoma. J Gastroenterol. 2002;37:883-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Seeff LB, Hoofnagle JH. Appendix: The National Institutes of Health Consensus Development Conference Management of Hepatitis C 2002. Clin Liver Dis. 2003;7:261-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 128] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Velázquez RF, Rodríguez M, Navascués CA, Linares A, Pérez R, Sotorríos NG, Martínez I, Rodrigo L. Prospective analysis of risk factors for hepatocellular carcinoma in patients with liver cirrhosis. Hepatology. 2003;37:520-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 307] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 10. | Persico M, Palmentieri B, Coppola L, Di Giacomo Russo G, De Marino F, De Sio I, Torella R. Occurrence of HCC in asymptomatic HCV-related chronic hepatitis. Dig Dis Sci. 2002;47:2407-2410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2986] [Cited by in RCA: 3069] [Article Influence: 133.4] [Reference Citation Analysis (8)] |
| 12. | Qin LX, Tang ZY. The prognostic molecular markers in hepatocellular carcinoma. World J Gastroenterol. 2002;8:385-392. [PubMed] |
| 13. | Sugimachi K, Tanaka S, Terashi T, Taguchi K, Rikimaru T, Sugimachi K. The mechanisms of angiogenesis in hepatocellular carcinoma: angiogenic switch during tumor progression. Surgery. 2002;131:S135-S141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Hlatky L, Hahnfeldt P, Folkman J. Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn't tell us. J Natl Cancer Inst. 2002;94:883-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 568] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 15. | Vermeulen PB, Gasparini G, Fox SB, Colpaert C, Marson LP, Gion M, Beliën JA, de Waal RM, Van Marck E, Magnani E. Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. Eur J Cancer. 2002;38:1564-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 348] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 16. | McDonald DM, Choyke PL. Imaging of angiogenesis: from microscope to clinic. Nat Med. 2003;9:713-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 671] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 17. | Hasan J, Byers R, Jayson GC. Intra-tumoural microvessel density in human solid tumours. Br J Cancer. 2002;86:1566-1577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 221] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 18. | Park YN, Kim YB, Yang KM, Park C. Increased expression of vascular endothelial growth factor and angiogenesis in the early stage of multistep hepatocarcinogenesis. Arch Pathol Lab Med. 2000;124:1061-1065. [PubMed] |
| 19. | Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 681] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 20. | Wei AC, Tung-Ping Poon R, Fan ST, Wong J. Risk factors for perioperative morbidity and mortality after extended hepatectomy for hepatocellular carcinoma. Br J Surg. 2003;90:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 238] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 21. | Mazzanti R, Messerini L, Monsacchi L, Buzzelli G, Zignego AL, Foschi M, Monti M, Laffi G, Morbidelli L, Fantappié O. Chronic viral hepatitis induced by hepatitis C but not hepatitis B virus infection correlates with increased liver angiogenesis. Hepatology. 1997;25:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2558] [Cited by in RCA: 2519] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 23. | Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1582] [Cited by in RCA: 1517] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 24. | Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4014] [Cited by in RCA: 4111] [Article Influence: 117.5] [Reference Citation Analysis (0)] |
| 25. | Poon RT, Ng IO, Lau C, Yu WC, Yang ZF, Fan ST, Wong J. Tumor microvessel density as a predictor of recurrence after resection of hepatocellular carcinoma: a prospective study. J Clin Oncol. 2002;20:1775-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 228] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 26. | El-Assal ON, Yamanoi A, Soda Y, Yamaguchi M, Igarashi M, Yamamoto A, Nabika T, Nagasue N. Clinical significance of microvessel density and vascular endothelial growth factor expression in hepatocellular carcinoma and surrounding liver: possible involvement of vascular endothelial growth factor in the angiogenesis of cirrhotic liver. Hepatology. 1998;27:1554-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 242] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 27. | Bruno S, Battezzati PM, Bellati G, Manzin A, Maggioni M, Crosignani A, Borzio M, Solforosi L, Morabito A, Ideo G. Long-term beneficial effects in sustained responders to interferon-alfa therapy for chronic hepatitis C. J Hepatol. 2001;34:748-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Colombo M, Sangiovanni A. Etiology, natural history and treatment of hepatocellular carcinoma. Antiviral Res. 2003;60:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Oon CJ, Chen WN. Lymphoblastoid alpha-interferon in the prevention of hepatocellular carcinoma (HCC) in high-risk HbsAg-positive resected cirrhotic HCC cases: a 14-year follow-up. Cancer Invest. 2003;21:394-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Anzola M. Hepatocellular carcinoma: role of hepatitis B and hepatitis C viruses proteins in hepatocarcinogenesis. J Viral Hepat. 2004;11:383-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 137] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 31. | Rahman MA, Dhar DK, Yamaguchi E, Maruyama S, Sato T, Hayashi H, Ono T, Yamanoi A, Kohno H, Nagasue N. Coexpression of inducible nitric oxide synthase and COX-2 in hepatocellular carcinoma and surrounding liver: possible involvement of COX-2 in the angiogenesis of hepatitis C virus-positive cases. Clin Cancer Res. 2001;7:1325-1332. [PubMed] |
| 32. | Rahman MA, Kohno H, Nagasue N. COX-2 - a target for preventing hepatic carcinoma? Expert Opin Ther Targets. 2002;6:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Hu KQ. Rationale and feasibility of chemoprovention of hepatocellular carcinoma by cyclooxygenase-2 inhibitors. J Lab Clin Med. 2002;139:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Ishikawa H, Nakao K, Matsumoto K, Ichikawa T, Hamasaki K, Nakata K, Eguchi K. Antiangiogenic gene therapy for hepatocellular carcinoma using angiostatin gene. Hepatology. 2003;37:696-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
S- Editor Ma N L- Editor Rampone B E- Editor Wang HF