Published online Oct 7, 2007. doi: 10.3748/wjg.v13.i37.5003
Revised: July 12, 2007
Accepted: July 26, 2007
Published online: October 7, 2007
AIM: To investigate the effects of ursodeoxycholic acid (UDCA) on chenodeoxycholic acid (CDCA)-induced liver injury in hamsters, and to elucidate a correlation between liver injury and bile acid profiles in the liver.
METHODS: Liver injury was induced in hamsters by administration of 0.5% (w/w) CDCA in their feed for 7 d. UDCA (50 mg/kg and 150 mg/kg) was administered for the last 3 d of the experiment.
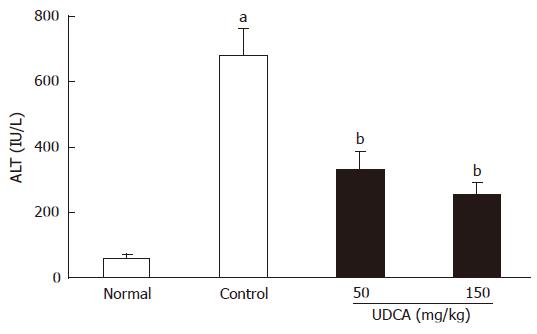
RESULTS: At the end of the experiment, serum alanine aminotransferase (ALT) increased more than 10 times and the presence of liver injury was confirmed histologically. Marked increase in bile acids was observed in the liver. The amount of total bile acids increased approximately three-fold and was accompanied by the increase in hydrophobic bile acids, CDCA and lithocholic acid (LCA). UDCA (50 mg/kg and 150 mg/kg) improved liver histology, with a significant decrease (679.3 ± 77.5 U/L vs 333.6 ± 50.4 U/L and 254.3 ± 35.5 U/L, respectively, P < 0.01) in serum ALT level. UDCA decreased the concentrations of the hydrophobic bile acids, and as a result, a decrease in the total bile acid level in the liver was achieved.
CONCLUSION: The results show that UDCA improves oral CDCA-induced liver damage in hamsters. The protective effects of UDCA appear to result from a decrease in the concentration of hydrophobic bile acids, CDCA and LCA, which accumulate and show the cytotoxicity in the liver.
- Citation: Iwaki T, Ishizaki K, Kinoshita S, Tanaka H, Fukunari A, Tsurufuji M, Imada T. Protective effects of ursodeoxycholic acid on chenodeoxycholic acid-induced liver injury in hamsters. World J Gastroenterol 2007; 13(37): 5003-5008
- URL: https://www.wjgnet.com/1007-9327/full/v13/i37/5003.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i37.5003
It is generally accepted that ursodeoxycholic acid (UDCA) can improve values for clinical and biochemical indices in patients with cholestatic liver disease, such as primary biliary cirrhosis (PBC)[1-4], primary sclerosing cholangitis (PSC)[5,6] and viral hepatitis[7,8]. UDCA exerts its choleretic action on the cholestatic liver and consequently decreases the values for serum indices of hepatotoxicity, such as alanine aminotransferase (ALT).
Cholestasis, defined as impairment of bile flow in hepatobiliary circulation, is accompanied by the retention of bile acids in the liver[9]. As mentioned above, cholestasis is observed widely not only in cholestatic liver diseases such as PBC and PSC, but also in viral hepatitis such as hepatitis C[10]. It is necessary to determine the changes in the bile acid amounts in the liver in liver diseases and how UDCA affects the bile acid pool changes. Such studies are limited clinically and interpretation of the data from experimental animals is required. In studies using mice and rats, however, care should be taken in interpreting the data because bile acids have different metabolic profiles[11,12]. For example, β-muricholic acid is one of the major bile acids, which is not produced in humans. On the other hand, hamsters have the advantage that their profile of the bile acid metabolism resembles that in humans[13,14].
In this study, we fed CDCA, a hydrophobic bile acid, to hamsters to induce liver injury, and to investigate the efficacy of UDCA administration using the model for the first time. We also evaluated the correlation between bile acid concentration in the liver and the protective effects of UDCA in the liver.
Ursodeoxycholic acid (UDCA) was synthesized at Mitsubishi Pharma Corporation (Osaka, Japan), and its purity was confirmed to be higher than 99%. Chenodeoxycholic acid was purchased from CALBIOCHEM (San Diego, California).
Six-week-old Syrian golden hamsters (weighing 89-117 g) were purchased from Japan SLC Inc. (Hamamatsu, Japan). The animals were maintained in a 12-h day/night cycle, with standard powder chow (MF, Oriental Yeast Co., Ltd. Tokyo, Japan) and tap water given ad libitum. All animal experiments were approved by the Animal Ethical Committee of Mitsubishi Pharma Corporation and performed in accordance with guidelines of the Japanese Pharmacological Society.
After acclimation for 5 d, the hamsters were randomly allocated to four groups, 10 animals each. For 7 d, the animals in each group were fed a standard powder chow (MF) with or without 0.5% (w/w) CDCA as follows: chow without 0.5% (w/w) CDCA (normal group); chow with 0.5% (w/w) CDCA (control group and UDCA-treated groups). UDCA was orally administered (50 or 150 mg/kg) to the CDCA-fed hamsters at a volume of 5 mL/kg between 3 pm and 5 pm, once a day for the last 3 d of the experiment. Purified water was administered to hamsters in the normal group and control groups.
All animals were fasted for 18 h before sacrifice. The animals were weighed, and then anesthetized with diethyl ether. Blood was collected by cardiac puncture for the determination of serum ALT which was analyzed with an autoanalyzer (FUJI DRI-CHEM 7000, FUJIFILM, Tokyo). Then the liver was rapidly removed, weighed and rinsed with saline. The liver was processed for histopathological examination and for bile acid analysis (other than the UDCA 50 mg/kg group).
Part of the rinsed liver tissue was fixed in 100 mL/L formalin and embedded in a paraffin block. The paraffin section was stained with hematoxylin-eosin (HE) and examined microscopically. Histological findings were assessed blindly and independently by two pathologists and graded from (-) to (+++) for the severity of vacuolation, cell infiltration and focal necrosis as follows: -: no change, ±: slight change, +: mild change, ++: marked change, and +++: severe change.
Bile acids in the liver were analyzed according to a method described elsewhere[15]. Briefly, 100 μL each of methanol and 23-nordeoxycholic acid (the internal standard), and 1 mL of 0.2 mol/L NaOH (maintained at 80°C) were added to about 50 mg of liver tissue homogenate. The mixture was immediately heated at 80°C for 20 min to dissolve the liver tissue, then mixed with 3 mL of water, and left to cool to room temperature. A 3-mL n-hexane extraction was performed three times to remove neutral lipids. Bile acids were then extracted from the remaining aqueous phase with a BondElut C18 cartridge. The methanol eluate was evaporated and the bile acids were analyzed by HPLC (Inertsil ODS-2 column). Examined bile acids were as follows: cholic acid (CA), CDCA, deoxycholic acid (DCA), LCA and UDCA. Concentrations of these 5 bile acids were calculated with summations of the 3 different types of conjugates (i.e., unconjugated, glycine and taurine conjugated bile acid) and expressed per gram of tissue weight. They were also expressed as composition percentages of total bile acids detected.
During the experiment, data on one out of every ten animals of each group were removed due to experimental treatment failures. Data on 9 animals of each group were processed as results in this study. Results were expressed as mean ± SE except the case for the bile acid analyses, which were expressed as mean ± SD. Differences among the group means were tested using Student’s t-test or Dunnett’s multiple comparison test. P < 0.05 was considered statistically significant.
The results of body weight and food intake are shown in Table 1. No significant differences were found in those parameters among the hamsters in the four groups during the experiment.
| Group | Body weight (g) | Weight gain | Food intake | |
| Before theexperiment | End of theexperiment | (g) | (g/d) | |
| Normal | 104.8 ± 2.5 | 110.6 ± 3.3 | 5.8 ± 1.8 | 7.1 ± 0.2 |
| Control | 103.7 ± 2.5 | 106.9 ± 1.9 | 3.2 ± 1.2 | 6.5 ± 0.3 |
| UDCA 50 mg/kg | 103.1 ± 2.9 | 104.7 ± 3.1 | 1.6 ± 1.3 | 6.2 ± 0.4 |
| UDCA 150 mg/kg | 104.7 ± 2.1 | 107.2 ± 1.9 | 2.5 ± 1.6 | 6.2 ± 0.2 |
As shown in Figure 1, feeding of CDCA for 7 d induced liver injury, and the serum ALT level (679.3 ± 77.5 U/L) in the CDCA-fed group (control group) was significantly (P < 0.01) elevated as compared with that of the normal group (61.4 ± 8.7 U/L). UDCA administration for the last 3 d of the experiment significantly (P < 0.01) reduced the serum ALT values (50 mg/kg: 333.6 ± 50.4 U/L, 150 mg/kg: 254.3 ± 35.5 U/L) as compared to the control group (679.3 ± 77.5 U/L).
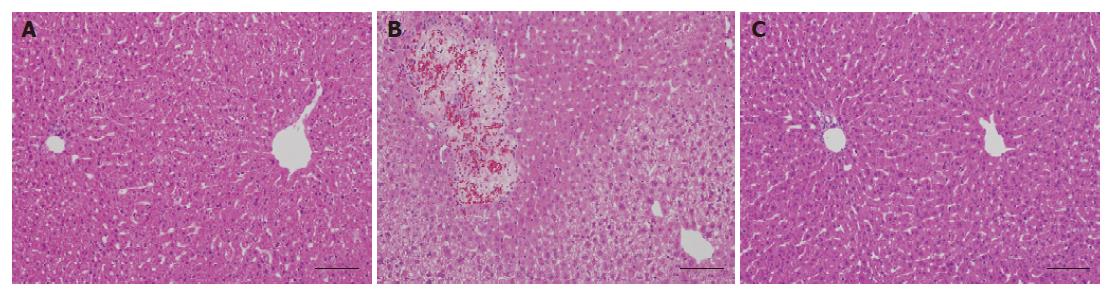
Induction of hepatic injury was confirmed histopath-ologically. Slight to mild vacuolation in a broad area was observed in the livers in the CDCA-fed group (control group), which was not observed in the normal group (Table 2, Figure 2A and B). UDCA treatment (150 mg/kg) led to a significant reduction in the grades of vacuolation observed in the control group (Table 2, Figure 2C). No changes were observed in cell infiltration and focal necrosis by CDCA feeding.
| Grade | ||||
| Findings | - | ± | + | |
| Normal | Vacuolation (Hydropic swelling) | 9 | 0 | 0 |
| Control | 0 | 2 | 7 | |
| UDCA 150 mg/kg | 9 | 0 | 0 | |
| Normal | 8 | 1 | 0 | |
| Control | Cell infiltration | 8 | 1 | 0 |
| UDCA 150 mg/kg | 7 | 2 | 0 | |
| Normal | 8 | 1 | 0 | |
| Control | Focal necrosis | 8 | 1 | 0 |
| UDCA 150 mg/kg | 7 | 2 | 0 | |
Concentrations and compositions of 5 major bile acids in the liver in each group are shown in Table 3. Livers from the normal group contained CA 102.0 ± 41.7 nmol/g (68.2% ± 3.2% of total bile acids), CDCA 36.0 ± 17.1 nmol/g (23.8% ± 3.6%), DCA 10.3 ± 4.4 nmol/g (6.8% ± 1.3%), and UDCA 1.9 ± 2.2 nmol/g (1.2% ± 1.3%). The concentration of LCA was less than 1.0 nmol/g. In CDCA-fed hamsters, the total bile acids level was increased more than three-fold (484.7 ± 189.7 nmol/g). Analysis of each bile acid concentration revealed that CDCA concentration increased ten-fold (364.9 ± 141.5 nmol/g), and LCA concentration also markedly increased (89.4 ± 32.4 nmol/g). Compositions of the two hydrophobic bile acids were increased to 75.3% ± 2.7% and 18.7% ± 2.0%, respectively. The concentration of UDCA was increased to 4.0 ± 0.9 nmol/g, whereas its composition did not change (0.9% ± 0.3%) by CDCA feeding. On the other hand, concentrations and compositions of CA and DCA were decreased significantly (CA: 20.9 ± 13.8 nmol/g and 4.0% ± 1.1%; DCA: 5.6 ± 3.2 nmol/g and 1.1% ± 0.3%).
| Normal | Control | UDCA 150 mg/kg | ||||
| nmol/g liver | % | nmol/g liver | % | nmol/g liver | % | |
| UDCA | 1.9 ± 2.2 | 1.2 ± 1.3 | 4.0 ± 0.9a | 0.9 ± 0.3 | 27.4 ± 12.8d | 9.5 ± 4.9d |
| CA | 102.0 ± 41.7 | 68.2 ± 3.2 | 20.9 ± 13.8b | 4.0 ± 1.1b | 12.8 ± 3.8 | 4.4 ± 1.2 |
| CDCA | 36.0 ± 17.1 | 23.8 ± 3.6 | 364.9 ± 141.5b | 75.3 ± 2.7b | 196.4 ± 65.3d | 66.0 ± 4.7d |
| DCA | 10.3 ± 4.4 | 6.8 ± 1.3 | 5.6 ± 3.2a | 1.1 ± 0.3b | 3.3 ± 2.2 | 1.1 ± 0.7 |
| LCA | < 1 | 0.0 | 89.4 ± 32.4b | 18.7 ± 2.0b | 56.0 ± 15.7c | 19.1 ± 1.9 |
| Total | 150.2 ± 62.9 | 484.7 ± 189.7b | 296.0 ± 88.0c | |||
When 150 mg/kg of UDCA was administered, the total bile acids level was significantly decreased (P < 0.01) to 296.0 ± 88.0 nmol/g, which suggests the improvement of cholestasis. Increase of UDCA (27.4 ± 12.8 nmol/g) level was observed accompanied by decrease of CDCA (196.4 ± 65.3 nmol/g) and LCA (56.0 ± 15.7 nmol/g) level. Concentrations of CA (12.8 ± 3.8 nmol/g) and DCA (3.3 ± 2.2 nmol/g) did not change statistically from those of the control group by the administration of UDCA.
The liver injury model was produced by a 7-d diet of 0.5% (w/w) CDCA administered to hamsters, which have a similar metabolic profile of bile acids as in humans. An increase in serum ALT was observed and the appearance of liver injury was confirmed histopathologically, which was assessed (Figures 1 and 2, Table 2). These phenomena were in accordance with the results of previous studies[16,17] which showed the toxicity of CDCA in hamsters. First of all, we determined the change in concentrations of major bile acids in liver of the hamsters to clarify the correlation of those bile acids and the liver injuries. We also administered UDCA in the hamsters to examine the improvement of liver damage caused by CDCA feeding, using the changes in the concentrations of bile acids.
In terms of bile acid concentration, CDCA feeding increased the amounts of CDCA and LCA in the hamster liver; on the other hand, it decreased the amounts of CA and DCA (Table 3). This might be explained as follows: At first, CDCA feeding itself increases CDCA in the liver. The increased CDCA is transported from the liver to the digestive tract through the bile duct. CDCA is metabolized to LCA by the enterobacteria there. LCA, which is a potent hydrophobic bile acid[18], migrates to the liver by enterohepatic circulation. In contrast, a decrease in CA and DCA would be explained as follows: Oda et al[16] showed that CDCA was a potent inhibitor of cholesterol-7α-hydroxylase. Based on this inhibition of the enzyme activity, feeding of CDCA inhibited biosynthesis of CA which is synthesized from cholesterol, and DCA which is formed by the bacterial 7α-hydroxylation of CA in the intestine and migrates to the liver by enterohepatic circulation, resulting in decreased proportions of these bile acids in the liver.
It is reported that cholestasis is believed to be an impairment of bile transport or the molecular mechanism from the liver to intestine, consequently leading to intrahepatic accumulation of hydrophobic bile acids, such as CDCA, DCA and LCA. Increase in the hydrophobic bile acids is reported to induce cytotoxicity in hepatocytes[16,19]. The mechanism of hepatic damage by these bile acids has not been clarified completely; however, several possibilities have been presented. It has been reported that some bile acids induce mitochondrial perturbation[20,21]. More recently hydrophobic bile acids have been shown to regulate the expression of several genes by acting as ligands of some nuclear receptors in the liver. Through these mechanisms, they affect bile salt synthesis[22,23], detoxification[24,25], and transporting molecules[26,27].
UDCA is widely used for the treatment of liver dysfunction in patients with primary biliary cirrhosis and acute and chronic intrahepatic cholestatic disorders[1-8]. The powerful choleretic effect of the drug was also confirmed in this hamster model. Improvement of liver histology associated with a significant decrease in serum ALT level was observed. The 150 mg/kg UDCA-administered group showed a significant decrease in the concentrations of total bile acids in the liver as comparison with the control group. The concentration of UDCA in the liver became 27.4 ± 12.8 nmol/g liver by the administration of 150 mg/kg UDCA. Setchell et al[28] have shown that concentration of UDCA became 40.1 ± 9.0 nmol/g liver when clinical dosage of the drug (600 mg/d) was administered for 4 d in patients suffering chronic hepatitis C. These findings suggested that liver UDCA concentration after UDCA administration in hamster was not extremely different from that in human.
In the liver of this group, the concentration of UDCA was raised, whereas that of the hydrophobic bile acids, CDCA and LCA was significantly decreased. UDCA is more hydrophilic compared to CDCA and LCA[12]. It was confirmed that the reduction in the concentration of hydrophobic bile acids and the replacement with hydrophilic bile acids in liver might be the mechanism of UDCA.
In recent years, several studies have been conducted to determine the mechanisms of action for UDCA at the molecular level. Schuetz et al[29] showed that UDCA activated the pregnane X receptor (PXR), and the reversal of cholestasis in humans by UDCA might include PXR-mediated activation of CYP3A4 and perhaps drug transporter targets that lead to enhanced metabolism and efflux of hepatotoxic bile acids. Rost et al[30] showed that UDCA might prevent impairment of hepatic function by restoring the expression of the hepatic transporter. Our next study will be the simultaneous investigation of the transporter expression and the bile acid concentration in this hamster model.
In conclusion, this study showed that the liver injury model was successfully produced by a 7-d diet of 0.5% (w/w) CDCA administered to hamsters. In this model, liver accumulations of two hydrophobic bile acids, CDCA and LCA, were observed. UDCA improved liver damages, which was confirmed with the decrease in serum ALT, and improvement of the liver histology. The protective effects of UDCA seem to result from a decrease in the concentration of the hydrophobic bile acids which accumulate in the liver.
The authors thank Mr. Akihiro Fujii for helpful advice and discussion and Ms. Hiroko Sato and Ms. Yoshie Anabuki for excellent technical assistance.
Ursodeoxycholic acid (UDCA) is widely used for the therapy of liver dysfunction in many types of liver disease. The mechanisms of this drug, however, have not been fully clarified yet. Abnormal retention of bile acids in the liver is a common finding when hepatic function is impaired. In this study we investigated the hepatoprotective effects of UDCA, using chenodeoxycholic acid (CDCA)-induced liver injury in hamsters to elucidate a correlation between liver injury and bile acid profiles in the liver.
It has been reported that the profile of bile acid metabolism in hamsters resembles that in humans. Administration of CDCA is known to cause liver injury in hamsters. Thus, we considered that it was possible to evaluate the relationship between hepatoprotective action of UDCA and liver bile acid profiles in hamsters.
There are some reports that the rate of UDCA increased and those of CDCA and DCA decreased in serum and bile by UDCA treatment. One of the mechanisms of action of UDCA for improvement of liver dysfunction is replacement hydrophobic bile acids [CDCA, deoxycholic acid (DCA) and lithocholic acid (LCA)] with hydrophilic bile acids including UDCA. Because the liver is thought to be the major target organ of UDCA, changes of bile acid concentrations in the liver must be very important. There have been, however, few reports to study the correlation between liver bile acids concentrations and the hepatoprotective effects of UDCA.
UDCA is widely used for the therapy of liver dysfunction in many types of liver disease, especially those with the accumulation of bile acids. Cholestasis is one of the typical diseases accompanied by the phenomenon. The results obtained in this study have shown that decrease of hydrophobic bile acids in the liver are correrate with the hepatoprotective action of UDCA and it may be one of the mechanisms of the drug.
Individual bile acids differ in hydrophobicity and hepatotoxicity. CDCA, DCA and LCA are more hydrophobic and hepatotoxic than UDCA.
The manuscript written by Iwaki et al describes the protective effect of UDCA on liver injury induced by CDCA. Although UDCA is clinically used for many liver diseases, the mechanisms for actions have not been fully understood. Therefore, their study is important, and the results are interesting.
| 1. | Poupon R, Chrétien Y, Poupon RE, Ballet F, Calmus Y, Darnis F. Is ursodeoxycholic acid an effective treatment for primary biliary cirrhosis? Lancet. 1987;1:834-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 370] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 2. | Leuschner U, Fischer H, Kurtz W, Güldütuna S, Hübner K, Hellstern A, Gatzen M, Leuschner M. Ursodeoxycholic acid in primary biliary cirrhosis: results of a controlled double-blind trial. Gastroenterology. 1989;97:1268-1274. [PubMed] |
| 3. | Oka H, Toda G, Ikeda Y, Hashimoto N, Hasumura Y, Kamimura T, Ohta Y, Tsuji T, Hattori N, Namihisa T. A multi-center double-blind controlled trial of ursodeoxycholic acid for primary biliary cirrhosis. Gastroenterol Jpn. 1990;25:774-780. [PubMed] |
| 4. | Poupon RE, Balkau B, Eschwège E, Poupon R. A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. UDCA-PBC Study Group. N Engl J Med. 1991;324:1548-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 560] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 5. | van de Meeberg PC, Wolfhagen FH, Van Berge-Henegouwen GP, Salemans JM, Tangerman A, van Buuren HR, van Hattum J, van Erpecum KJ. Single or multiple dose ursodeoxycholic acid for cholestatic liver disease: biliary enrichment and biochemical response. J Hepatol. 1996;25:887-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Mitchell SA, Bansi DS, Hunt N, Von Bergmann K, Fleming KA, Chapman RW. A preliminary trial of high-dose ursodeoxycholic acid in primary sclerosing cholangitis. Gastroenterology. 2001;121:900-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 202] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 7. | Takano S, Ito Y, Yokosuka O, Ohto M, Uchiumi K, Hirota K, Omata M. A multicenter randomized controlled dose study of ursodeoxycholic acid for chronic hepatitis C. Hepatology. 1994;20:558-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Lirussi F, Beccarello A, Bortolato L, Morselli-Labate AM, Crovatto M, Ceselli S, Santini G, Crepaldi G. Long-term treatment of chronic hepatitis C with ursodeoxycholic acid: influence of HCV genotypes and severity of liver disease. Liver. 1999;19:381-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Elferink RO. Cholestasis. Gut. 2003;52 Suppl 2:ii42-ii48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Fabris P, Tositti G, Mazzella G, Zanetti AR, Nicolin R, Pellizzer G, Benedetti P, de Lalla F. Effect of ursodeoxycholic acid administration in patients with acute viral hepatitis: a pilot study. Aliment Pharmacol Ther. 1999;13:1187-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Greim H, Trülzsch D, Roboz J, Dressler K, Czygan P, Hutterer F, Schaffner F, Popper H. Mechanism of cholestasis. 5. Bile acids in normal rat livers and in those after bile duct ligation. Gastroenterology. 1972;63:837-845. [PubMed] |
| 12. | Heuman DM. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J Lipid Res. 1989;30:719-730. [PubMed] |
| 13. | Khallou J, Riottot M, Parquet M, Verneau C, Lutton C. Biodynamics of cholesterol and bile acids in the lithiasic hamster. Br J Nutr. 1991;66:479-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Kasbo J, Saleem M, Perwaiz S, Mignault D, Lamireau T, Tuchweber B, Yousef I. Biliary, fecal and plasma deoxycholic acid in rabbit, hamster, guinea pig, and rat: comparative study and implication in colon cancer. Biol Pharm Bull. 2002;25:1381-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Sakakura H, Suzuki M, Kimura N, Takeda H, Nagata S, Maeda M. Simultaneous determination of bile acids in rat bile and serum by high-performance liquid chromatography. J Chromatogr. 1993;621:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Oda H, Kuroki S, Yamashita H, Nakayama F. Effects of bile acid feeding on hepatic deoxycholate 7 alpha-hydroxylase activity in the hamster. Lipids. 1990;25:706-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Kuroki S, Hoshita T. Effect of bile acid feeding on hepatic steroid 12 alpha-hydroxylase activity in hamsters. Lipids. 1983;18:789-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Delzenne NM, Calderon PB, Taper HS, Roberfroid MB. Comparative hepatotoxicity of cholic acid, deoxycholic acid and lithocholic acid in the rat: in vivo and in vitro studies. Toxicol Lett. 1992;61:291-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 89] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Spivey JR, Bronk SF, Gores GJ. Glycochenodeoxycholate-induced lethal hepatocellular injury in rat hepatocytes. Role of ATP depletion and cytosolic free calcium. J Clin Invest. 1993;92:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 191] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Sokol RJ, McKim JM, Goff MC, Ruyle SZ, Devereaux MW, Han D, Packer L, Everson G. Vitamin E reduces oxidant injury to mitochondria and the hepatotoxicity of taurochenodeoxycholic acid in the rat. Gastroenterology. 1998;114:164-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 135] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Rolo AP, Oliveira PJ, Moreno AJ, Palmeira CM. Chenodeoxycholate is a potent inducer of the permeability transition pore in rat liver mitochondria. Biosci Rep. 2001;21:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Palmer RH. Bile acids, liver injury, and liver disease. Arch Intern Med. 1972;130:606-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 137] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Greim H, Trülzsch D, Czygan P, Rudick J, Hutterer F, Schaffner F, Popper H. Mechanism of cholestasis. 6. Bile acids in human livers with or without biliary obstruction. Gastroenterology. 1972;63:846-850. [PubMed] |
| 24. | Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2012] [Cited by in RCA: 2139] [Article Influence: 79.2] [Reference Citation Analysis (6)] |
| 25. | Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1717] [Cited by in RCA: 1793] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 26. | Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci USA. 2001;98:3369-3374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1020] [Cited by in RCA: 1073] [Article Influence: 42.9] [Reference Citation Analysis (18)] |
| 27. | Xie W, Radominska-Pandya A, Shi Y, Simon CM, Nelson MC, Ong ES, Waxman DJ, Evans RM. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc Natl Acad Sci USA. 2001;98:3375-3380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 579] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 28. | Setchell KD, Rodrigues CM, Clerici C, Solinas A, Morelli A, Gartung C, Boyer J. Bile acid concentrations in human and rat liver tissue and in hepatocyte nuclei. Gastroenterology. 1997;112:226-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 148] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Schuetz EG, Strom S, Yasuda K, Lecureur V, Assem M, Brimer C, Lamba J, Kim RB, Ramachandran V, Komoroski BJ. Disrupted bile acid homeostasis reveals an unexpected interaction among nuclear hormone receptors, transporters, and cytochrome P450. J Biol Chem. 2001;276:39411-39418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 283] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 30. | Rost D, Herrmann T, Sauer P, Schmidts HL, Stieger B, Meier PJ, Stremmel W, Stiehl A. Regulation of rat organic anion transporters in bile salt-induced cholestatic hepatitis: effect of ursodeoxycholate. Hepatology. 2003;38:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
S- Editor Zhu LH L- Editor Kumar M E- Editor Lu W