ETHANOL METABOLISM AND FATTY LIVER
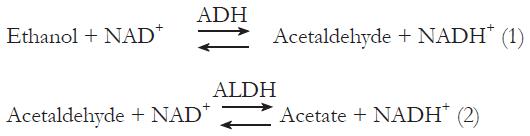
The liver is the primary site of ethanol metabolism, as hepatocytes express the major ethanol metabolizing enzymes, alcohol dehydrogenase (ADH), and cytochrome P450 2E1 (CYP2E1) at higher levels than any other tissue[4-6]. ADH, with a Michaelis constant of 1 to 2 mmol/L. catalyzes ethanol oxidation, using NAD as a coenzyme in a reaction that forms acetaldehyde and reduced NAD (i.e. NADH+) in reaction (1) below. This and the subsequent oxidation of acetaldehyde to acetate with the formation of another reducing equivalent of NADH+ in a reaction catalyzed by aldehyde dehydrogenase (ALDH) in reaction (2) below were shown to cause significant changes in cellular redox potential and provide more substrate availability for fatty acid synthesis.
Math 1
Enhanced generation of reduced NAD also disrupts mitochondrial β-oxidation of fatty acids thereby diminishing the rate of lipid oxidation. This metabolic explanation for fatty liver was the prevailing concept for a number of years, but it was not sufficient to explain the rapid formation of fatty liver after acute ethanol administration. In addition, the degree of change in liver redox potential that occurs in vivo after chronic ethanol administration to rodents was significant but rather modest. Thus, the metabolic explanation for alcohol-induced fatty liver became insufficient to account for all changes that occurred in hepatic lipids after ethanol consumption. Later discoveries in cell signaling and characterization of specific transcription factors prompted investigations of specific factors that are operative in the pathophysiology of ethanol-induced steatosis.
UPDATED MECHANISMS OF ETHANOL-INDUCED FATTY LIVER
Enhancement of lipid synthesis by ethanol consumption
Hepatic fatty acid triglyceride and phospholipid synthesis are accelerated after acute and chronic ethanol consumption[7]. Enhanced lipogenesis is a reflection of higher expression of lipogenic enzymes including fatty acid synthase, acyl CoA carboxylase (ACC), ATP citrate lyase (ACL), stearoyl CoA desasturase and malic enzyme.[8,9]. These enzymes are encoded by genes regulated by the transcription factor, sterol regulatory element binding protein-1 or SREBP-1.
Activation of SREBP-1 and enhanced expression of lipogenic enzymes
By activating SREBP, a member of a family of tran-scription factors that target genes encoding enzymes involved in cholesterol metabolism, ethanol consumption can short circuit normal hepatic lipid metabolism, converting the liver from a lipid burning to a lipid storing organ. There are three isoforms of SREBP: SREBP-1a, 1c and 2. SREBP 1a and 1c are alternative forms of the protein encoded by the same exon. Forms 1c and 2 are principally involved in regulating fatty acid and cholesterol synthesis, respectively. All SREBP proteins reside in the endoplasmic reticulum. To function as a transcription factor, the amino terminal domain of SREBP is proteolytically cleaved from the carboxy terminal domain in the Golgi apparatus and the fragment translocates to the cell nucleus. This is accomplished by proteolytic cleavage through the action of two proteases; site-1 protease (S1P) and site-2 protease (S2P), both located in the Golgi apparatus. SREBP is initially translocated from its site in the ER to the Golgi by SREBP cleavage activating protein (SCAP). SCAP activity is sensitive to intracellular cholesterol levels and possesses a cholesterol sensing domain in its primary structure. When cholesterol levels are low, SCAP translocates SREBP from the ER to the Golgi where S1P cleaves the precursor form of SREBP, producing a membrane-bound intermediate. The intermediate is then cleaved by S2P to release the active transcription factor domain from the membrane, enabling it to bind to specific promoter elements on the DNA. Under normal circumstances, when intracellular cholesterol levels rise, SCAP activity is suppressed, thereby preventing transport and activation of SREBP by S1P and S2P. Ethanol metabolism, on the other hand, disturbs the regulatory loop of SBEBP activation and causes increased cleavage of SREBP-1c, even when intracellular levels of cholesterol and/or fatty acids are high. Active SREBP-1c is detected experimentally in nuclear fractions as a -65 kDa protein, derived originally from a -125 kDa precursor. Thus, SREBP-1c has an essential function in the development of ethanol-elicited fatty liver. Nuclear fractions from livers of ethanol-fed rodents or from cultured cells that are capable of ethanol metabolism have elevated levels of the active form of SREBP-1c. A requirement for ethanol metabolism in SREBP induction has been reported using cultured cells and there is considerable evidence that the activating factor is acetaldehyde[10] The mechanism of ethanol-elicited SREBP-1 activation is also partly due to inhibition of the AMP-activated protein kinase (AMPK), described in greater detail below. AMPK catalyzes the phosphorylation of target enzymes, some of which are important rate-limiting enzymes in hepatic lipid metabolism. By inhibiting the activity of AMPK, ethanol relieves suppression of SREBP-1, causing its activation and increasing its levels in the nucleus.
Ethanol-elicited decline in fatty acid oxidation: Regulation of the peroxisome proliferator activated receptor alpha
Ethanol-elicited decline in fatty acid oxidation: Regulation of the peroxisome proliferator activated receptor alpha is a nuclear receptor that regulates expression of genes that possess peroxisome proliferator response elements in their promoter regions. Several such genes are those that encode fatty acid transporters, including carntitine palmitoyl transferase I, proteins involved in export, such as apolipoprotein B, the microsomal triglyceride transfer protein, fatty acid binding protein, and acyl CoA dehydrogenase. PPAR-α interacts with the retinoid X receptor (RXR). The protein complex is activated by binding to fatty acids, thereby enhancing expression of proteins involved in the fatty acid transport and oxidation.
Ethanol administration to rodents or ethanol exposure to ethanol-metabolizing cultured cells causes a general down-regulation of PPAR-α, as reflected by reduced binding by the RXR-PPAR-α complex to a PPAR-α- specific promoter sequence[11]. This effect of ethanol is enhanced by exposure of ethanol-treated hepatoma cells to the aldehyde dehydrogenase inhibitor, cyanamide and is abolished by the alcohol dehydrogenase inhibitor, 4-methylpyrazole. Further, RXR-PPAR-α extracted from hepatoma cells previously exposed to ethanol or acetaldehyde binds poorly to an oligonucleotide containing peroxisome proliferator response elements, indicating that acetaldehyde is responsible for the action of ethanol. It is possible that acetaldehyde, because of its ability to covalently bind proteins[12] can form adducts with the PPAR-α transcription complex, thereby preventing its ability to bind the promoter element(s). It is noteworthy; however, that the effect at the level of transcription does not universally down-regulate all PPAR-α- regulated enzymes. In vivo studies have shown that the steady state level of mRNA encoding medium chain acyl CoA dehydrogenase is decreased by ethanol, but mRNAs encoding other PPAR-α- sensitive enzymes, including those mentioned above are minimally affected, unaffected, and in the case of fatty acid binding protein, actually induced by ethanol. An explanation for this, as described by You and Crabb[13] is that the regulation of fatty acid oxidation is not solely controlled by the activities of the fatty acid oxidative enzymes. There is additional allosteric regulation of fatty acid transport into the mitochondrion by the intramitochondrial levels of malonyl CoA, which inhibits the carnitine palmitoyltransferaseI(CPTI), thereby preventing transport of fatty acyl CoA derivatives into this organelle. With the concomitant up-regulation of fatty acid biosynthesis by ethanol, this is an important step in the down-regulation of fatty acid oxidation. An additional natural regulatory factor is the adipocyte hormone adiponectin. Chronic ethanol administration causes a significant reduction in circulating adiponectin, which is associated with the ethanol-elicited reduction in fatty acid oxidation[14]. Restoration of adiponectin to ethanol-fed animals by treatment with the recombinant form of the hormone restores fatty acid oxidation to normal. Moreover, such treatment decreases the levels of TNF-α in these animals, as the hormone and the cytokine are known to regulate each other’s production[13].
It is also noteworthy that ethanol-elicited inhibition of fatty acid oxidation can be reversed, as demonstrated by experiments in which ethanol-fed animals were co-treated with the PPAR-α agonist, WY14, 643. Fatty liver was blocked in these animals due to induction of PPAR-α, which, in turn, accelerated fatty acid oxidation thereby preventing ethanol-elicited fatty liver[15]. These findings underscore the metabolic importance of fatty acid oxidation in the elimination of steatosis and emphasize the therapeutic potential of this agent and other PPAR-α agonists as adjuncts to the natural hormone, adiponectin.
Adenosine monophosphate -activated protein kinase and alcohol-induced fatty liver
Hepatic lipid metabolism has been shown to be tightly regulated by adenosine monophosphate -activated protein kinase (AMPK). This enzyme, a heterotrimeric protein, is, itself, activated by AMP as well as by phosphorylation by another kinase, LKB-1. Elevated AMP levels are indicators of low intracellular energy charge. Therefore, when AMP activates the AMPK it down-regulates energy requiring pathways, presumably as a means of energy (ATP) conservation. Such ATP-utilizing pathways are generally biosynthetic and include lipid, RNA and protein synthesis. Conversely, AMPK activates ATP-generating catabolic pathways, such as fatty acid oxidation, the TCA cycle and glycolysis. The rate-limiting enzyme in lipid biosynthesis is the acetyl CoA carboxylase (ACC), the activity of which is down-regulated via phosphorylation by AMPK[16]. ACC catalyzes the carboxylation of acetyl CoA to malonyl CoA. The latter is a potent inhibitor of lipid oxidation and its declining levels potentiate mitochondrial transport and fatty acid oxidation.
When ethanol is added to cultured hepatoma cells that oxidize ethanol, it induces transcription of an SREBP-regulated promoter and increases the levels of the mature form of SREBP-1. Both these effects are blocked by the inclusion in the culture medium of 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) or metformin, both AMPK activators[17]. Furthermore, overexpression of AMPK in these cells blocks the ethanol-induced increase in promoter activity and SREBP protein. Exposure of cells to ethanol reduces the activity of AMPK. In vivo studies confirm these findings. Feeding mice an ethanol diet significantly reduces hepatic AMPK activity, while increasing the activity of the rate-limiting enzyme, ACC[17]. At the same time, fatty acid oxidation is reduced. These findings demonstrate that AMPK regulates the ethanol-elicited SREBP-1 activation and the development of steatosis. They also demonstrate the therapeutic value of AICAR and of metformin in preventing and/or reversing fatty liver caused not only by ethanol but also by dietary induction. The latter has been demonstrated in metformin-treated leptin-deficient experimental animals[18]. Thus the central role of AMPK in regulating these activities is well illustrated by ethanol’s effect in the genesis of fatty liver.
Role of Egr-1 in ethanol-induced steatosis
Another transcription factor, early growth response-1 (Egr-1) also appears to have a role in ethanol-elicited steatosis. Egr-1, also known as nerve growth factor 1-A (NGF1-A), is an immediate early gene and transcription factor that regulates genes involved in response to cellular stress. Early work demonstrated that Egr-1 is induced in response to growth factors[19,20]. Several genes relevant to alcohol-induced liver injury have promoter regions that bind Egr-1. Among these genes are those that encode platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-β), and intercellular adhesion molecule-1 (ICAM-1)[21]. Specifically, with regard to ethanol-induced fatty liver, Egr-1 also binds the gene encoding tumor necrosis factor alpha (TNF-α)[22]. This latter finding is noteworthy as this cytokine is considered lipogenic, causing the activation of SREBP-1, thereby enhancing lipid biosynthesis[23]. Perhaps the most compelling evidence for the essentiality of Egr-1 in ethanol-elicited steatosis is that reported by McMullen et al[24], who showed that, in contrast to wild type mice, which develop fatty liver in response to chronic ethanol administration, that Egr-1 null mice exhibit neither hepatic steatosis nor elevated TNF-α, after four weeks of chronic ethanol feeding. These workers also reported enhanced production of Egr-1 in ethanol-fed mice treated with lipopolysaccharide (LPS), compared with similarly-treated pair-fed control mice. The data suggest that the absence of Egr-1 prevents ethanol-elicited fatty liver and reduces sensitivity to LPS. Studies by Zhou et al[1] demonstrated that 90 min after an acute dose of ethanol (6 g/kg body wt), hepatic TNF-α levels rise three-fold, followed by elevated levels of hepatic triglycerides. While these investigators did not measure Egr-1, they showed a close association between elevated levels of plasma endotoxin, oxidative stress, and TNF-α in ethanol-fed mice, suggesting that endotoxin, via oxidative stress, stimulates the production of TNF-α. This latter may result from enhanced transcription by Egr-1.
While Egr-1 is rapidly synthesized, its levels are also regulated through degradation by the proteasome[25]. It has been postulated that inhibition of proteasome activity by ethanol administration has a distinct role in the increase in SREBP-1 protein, which is also a proteasome substrate[17]. Similarly, PPAR-α and RXR levels are controlled by the ubiquitin-proteasome pathway[26,27]. So, too proteasome inhibition may also contribute to the ethanol-induced rise in Egr-1. Recent experiments in our laboratory have demonstrated that acute ethanol administration to wild type mice causes enhancement of both Egr-1 mRNA and protein levels in livers of these animals one hr after ethanol administration. These increases precede the ethanol-elicited rise in triglycerides, detected after 3 h. In cultured hepatoma cells that metabolize ethanol, overexpression of Egr-1 following transfection with an adenoviral Egr-1 expression vector, enhances triglyceride accumulation in these cells over those of control cells. Furthermore, exposure of transfected cells to ethanol causes a further rise in the level of Egr-1 with a concomitant rise in cellular triglycerides (Donohue, unpublished). These findings provide further evidence for an essential role of Egr-1 in ethanol-induced fatty liver and the possibility that it may initiate downstream events. They also suggest that in cultured cells, there may be post-translational regulation of Egr-1 via ethanol-elicited suppression of proteasome activity. While Egr-1 regulates the expression of TNF-α, it also binds to promoter regions of other genes, including that encoding transforming growth factor-β (TGF-β), which has a major role in hepatic fibrosis[28]. Thus, Egr-1 may not only be a major factor in steatosis, but also in the more advanced stages of alcohol-induced liver injury, as suggested by Pritchard and Nagy[20].
Alcohol-elicited steatosis: What is the actual toxin?
The preceding sections have described the molecular mechanisms by which ethanol consumption disrupts hepatic lipid metabolism, resulting in fatty liver. As stated earlier, an evolving concept that is gaining acceptance is that certain accumulated fatty acids are toxic to the liver, but that their toxicity is blunted by esterification with glycerol to form triacylglycerols (triglycerides). Ironically, triglyceride accumulation is the principal means by which fatty liver is quantified. Thus the question of whether there is a generalized toxicity of accumulated fatty acids or that specific fatty acids are hepatotoxic is important in determining whether alcoholics are at risk for more advanced disease. Recent work by Yamaguchi et al[30,31] used antisense oligonucleotide (ASO) to block the synthesis of diacylglycerol acyltransferase (DAG), thereby inhibiting triglyceride synthesis. Blockade of DAG, which catalyzes the final step in triglyceride synthesis, reduced hepatic steatosis in these animals when they were fed a methionine-choline-deficient diet to induce NASH. However, such treatment with the ASO exacerbated liver injury to cause fibrosis, despite the fact that TNF-α levels were decreased, adiponectin levels were increased, and insulin sensitivity was improved. The explanation for these findings is that the prevention of triglyceride synthesis enhanced the intracellular levels of free fatty acids in the liver. While the specific fatty acids were not identified, the fact that enhanced lipid peroxidation occurred in liver-injured animals suggests that accumulation of unsaturated fatty acids would most likely be incriminated in causing further liver damage. With regard to ethanol-elicited steatosis, there is a substantial body of evidence that unsaturated fatty acids from corn oil or fish oil cause greater liver damage than saturated fatty acids when either of these are fed in combination with alcohol.
CONCLUSION
The foregoing review has summarized both the formerly and currently accepted models of alcohol-induced fatty liver. In summary, ethanol consumption causes activation of SREBP-1, which induces genes involved in lipid biosynthesis. Conversely, ethanol consumption down-regulates the PPAR-α transcription factor, which regulates enzymes involved in fatty acid oxidation, and simultaneously prevents the import of fatty acids into the mitochondrion for oxidation. The regulation of Egr-1 by ethanol is a less well characterized but an important area of investigation, as its rapid induction may well be upstream of the latter two factors or it may exert coordinate regulation along with SREBP-1 and PPAR-α. Finally, it should be emphasized that in sections of this review, comparisons of ASH were made to other fatty liver diseases, including NASH. The latter was used simply as an analogous disorder, but there was no intent to imply that the two (ASH and NASH) are the same. Clearly, further investigations are warranted into the pathophysiology of ethanol-induced steatosis, but recent investigations have demonstrated considerable progress toward therapeutic measures. These latter discoveries will most certainly prompt further ones in preventing or abating liver damage caused by alcohol abuse.