Published online Oct 7, 2007. doi: 10.3748/wjg.v13.i37.4925
Revised: July 11, 2007
Accepted: July 26, 2007
Published online: October 7, 2007
Patients with alcoholic liver disease frequently exhibit increased body iron stores, as reflected by elevated serum iron indices (transferrin saturation, ferritin) and hepatic iron concentration. Even mild to moderate alcohol consumption has been shown to increase the prevalence of iron overload. Moreover, increased hepatic iron content is associated with greater mortality from alcoholic cirrhosis, suggesting a pathogenic role for iron in alcoholic liver disease. Alcohol increases the severity of disease in patients with genetic hemochromatosis, an iron overload disorder common in the Caucasian population. Both iron and alcohol individually cause oxidative stress and lipid peroxidation, which culminates in liver injury. Despite these observations, the underlying mechanisms of iron accumulation and the source of the excess iron observed in alcoholic liver disease remain unclear. Over the last decade, several novel iron-regulatory proteins have been identified and these have greatly enhanced our understanding of iron metabolism. For example, hepcidin, a circulatory antimicrobial peptide synthesized by the hepatocytes of the liver is now known to play a central role in the regulation of iron homeostasis. This review attempts to describe the interaction of alcohol and iron-regulatory molecules. Understanding these molecular mechanisms is of considerable clinical importance because both alcoholic liver disease and genetic hemochromatosis are common diseases, in which alcohol and iron appear to act synergistically to cause liver injury.
- Citation: Harrison-Findik DD. Role of alcohol in the regulation of iron metabolism. World J Gastroenterol 2007; 13(37): 4925-4930
- URL: https://www.wjgnet.com/1007-9327/full/v13/i37/4925.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i37.4925
Iron is essential for an array of key biological processes including erythrocyte production, DNA synthesis and cellular respiration[1-3]. The normal iron content of the body in an adult male is 35 to 45 mg of iron per kilogram of body weight[2]. The majority of the iron is bound to hemoglobin in erythrocytes. The rest is incorporated into myoglobin in the muscle, the tissue enzymes and plasma transferrin[2]. Parenchymal cells of the liver and reticuloendothelial macrophages serve as depots for excess iron storage[2,4]. However, due to its potential to take part in the Fenton reaction as a transition metal, iron can also be toxic to the cell[5,6]. Hepatic iron overload is common in many liver diseases, where iron is a risk factor in disease progression[7-13].
Alcoholic liver disease (ALD) patients frequently display evidence of iron overload[14-18]. Recently, even mild to moderate alcohol consumption has been shown to elevate the indices of iron stores[19]. Suzuki et al[20] have demonstrated elevated expression of transferrin receptor-1 in ALD patients by immunohistochemical analysis of liver biopsy samples. Moreover, Kupffer cells isolated from experimental animal models of ALD also display increased iron content[21,22]. It is a well established fact, that both iron and alcohol individually cause oxidative stress and lipid peroxidation[6,23-26]. Hence, alcohol induced iron overload enhances the production of free radicals and proinflammatory cytokines, thereby leading to liver injury[11,12,27]. Elegant studies with experimental animal models of ALD have demonstrated, that increased iron content in Kupffer cells leads to the activation of the transcription factor, nuclear factor-kappa (NF-κB), and increased expression of the proinflammatory cytokine, tumor necrosis factor-alpha (TNF-α)[21,22,28]. These effects were abolished by iron chelation[29], thereby indicating a role for iron-mediated cell signaling in the pathogenesis of experimental alcoholic liver disease.
The iron overload disorder, genetic hemochromatosis (GH), is one of the most prevalent genetic diseases in individuals of Caucasian origin[30-32]. The majority of GH patients are homozygous for a C282Y mutation in their Hfe gene[33,34]. Excessive alcohol consumption has been reported to exacerbate liver injury in GH patients homozygous for the C282Y mutation of Hfe gene[35]. However, despite all these findings, the underlying mechanisms of iron accumulation observed in alcoholic liver disease, and the source of the excess iron remain elusive. In vivo whole-body retention studies have demonstrated a two-fold increase in intestinal iron absorption in chronic alcoholics[36]. Changes in intestinal permeability are thought to be the underlying mechanism of enhanced intestinal iron absorption[36]. Of note, hepatocytes are the primary site of iron storage in the liver, and iron may also leak out of injured hepatocytes. However, it is also feasible that the iron stores in alcoholic patients are increased through recognized regulatory mechanism(s). Significant advances have been made with the discovery of novel iron-regulatory molecules in recent years, which have improved our understanding of iron metabolism. Studying the regulation of these molecules by alcohol is important for understanding the underlying mechanisms of iron overload in alcoholic liver disease.
Since there is no physiological pathway of excretion for excess iron in the body, the uptake, transport and storage of iron must be tightly regulated[37-41]. A series of novel iron-regulatory molecules including iron transporters and soluble mediators have recently been identified. Divalent metal transporter 1 (DMT1, also known as Nramp2) is a multi-transmembrane protein[42], responsible for importing dietary non-heme iron through the apical site of absorptive enterocytes in the duodenum[42]. Studies employing mice with the targeted deletion of DMT1 in the duodenum have confirmed the role of DMT1 in intestinal iron absorption[43]. Conversely, ferroportin (also known as MTP1, Ireg1) exports iron into the bloodstream[44-46]. As a transition metal, iron undergoes reduction and oxidation reactions during these cellular uptake and export processes. Understanding the mechanisms involved in these reactions and identifying the candidate enzymes will require further investigation[47-49]. Iron circulates in the plasma by binding to the glycoprotein, transferrin (Tf). Iron-laden Tf is taken up into the cell by forming complexes with transferrin receptor-1, TrfR1[2,50]. Recently, another homologous receptor, TrfR2 has been identified[51]. Unlike TrfR1, which is ubiquitously expressed, TrfR2 is mainly expressed in the liver[51].
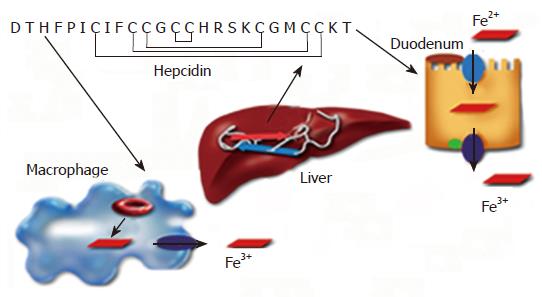
The regulation of iron metabolism involves multiple organs including the duodenum, liver and bone marrow. Hence, the presence of soluble mediators has long been suspected. The discovery of hepcidin peptide has not only confirmed these notions but also highlighted the importance of the liver in the regulation of iron homeostasis. Hepcidin is an antimicrobial peptide, which was first isolated from human urine and blood[52,53]. It is synthesized in the hepatocytes of the liver as an 84 amino acid precursor protein, which is subsequently cleaved into the 25 amino-acid disulphide-bridged active peptide form[54,55]. Mice express two copies of the hepcidin gene, Hepc 1 and Hepc 2, resulting from a tandem duplication of the hepcidin gene[54,56,57]. Transgenic mice studies have confirmed a role for hepcidin in the regulation of iron metabolism. Hepcidin knockout mice develop iron overload in the liver, pancreas and heart[58], whereas mice overexpressing hepcidin display severe iron deficiency and anemia[59]. Hepcidin synthesis in the liver is sensitive to body iron levels; increasing with iron overload and decreasing in the case of iron deficiency[54,60,61]. Interestingly, hepcidin is also regulated by inflammatory signals, and the inflammatory cytokines, IL-1 and IL-6[62-65]. However, the role of Kupffer cells in the regulation of hepcidin expression by inflammation is controversial[66,67].
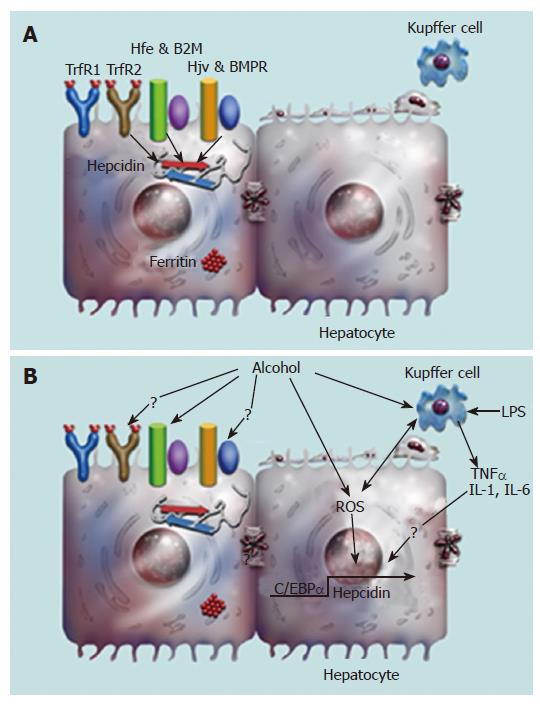
Hepcidin plays a central role in the regulation of iron metabolism by inhibiting intestinal iron transport and the release of iron from macrophages (Figure 1)[60,61]. Hepcidin achieves this by binding to the iron exporter ferroportin and inducing its internalization and degradation[68,69]. Studies with both hemochromatosis patients and transgenic mouse models have identified candidate proteins, which modulate hepcidin synthesis in the liver (Figure 2A)[70-74]. Hemochromatosis patients, homozygous for the expression of TrfR2 mutations display increased transferrin saturation but reduced urinary hepcidin levels[70]. Despite the iron overload phenotype, expression of hepcidin in the livers of TrfR2 knockout mice is similar to that of control littermates[72,75]. Similarly, both genetic hemochromatosis patients with Hfe mutations and Hfe transgenic mice display significantly reduced hepcidin expression in the liver[73,74]. Taken together, these studies demonstrate a role for both TrfR2 and Hfe in the regulation of hepcidin expression in the liver. However, the underlying mechanisms are unknown. Recently, the juvenile hemochromatosis gene hemojuvelin, has been shown to regulate hepcidin expression via the bone morphogenetic protein (BMP) signaling pathway in the liver (Figure 2A)[76,77].
The promoter of the hepcidin gene harbors consensus binding sites for several transcription factors including C/EBP-α, HNF4-α, Stat3 and Smad4[78-81]. CCAAT/enhancer-binding protein alpha (C/EBP-α) plays a role in the iron-mediated regulation of hepcidin gene transcription[78].
Alcohol consumption increases the transfer of both iron and endotoxin from the intestine into the circulation[36,82]. Hepcidin synthesis in the liver is regulated by iron and inflammation, with hepcidin playing a central role in iron homeostasis[54,58,59,62,63,69,83]. Hence, a role for alcohol is implicated in the regulation of hepcidin expression in the liver. Indeed, alcohol was found to down-regulate hepcidin expression both in vitro with alcohol metabolizing hepatoma cells, and in vivo with mice subjected to short-term alcohol exposure[84]. The effect of alcohol on hepcidin expression in hepatoma cells was abolished by 4-methylpyrazole, a specific inhibitor of the alcohol metabolizing enzymes[84]. Furthermore, alcohol did not alter the expression of transferrin receptor-1 and the iron storage protein, ferritin, or the activation of iron regulatory RNA-binding proteins, IRP1 and IRP2[84]. These findings demonstrate that alcohol does not regulate hepcidin expression by altering the iron status of the cell but rather acts on hepcidin directly. Short-term alcohol exposure down-regulated hepcidin 1, but not hepcidin 2 mRNA expression in mice[84]. Similarly, iron has also been shown to up-regulate hepcidin 1 gene expression in mice[57]. Furthermore, rats with chronic alcohol exposure also display reduced hepcidin expression[85,86]. Collectively, these studies demonstrate a role for alcohol in the regulation of hepcidin expression in the liver.
Alcohol-mediated down-regulation of hepcidin expression in the liver leads to elevated expression of the iron transporter proteins, DMT1 and ferroportin in the duodenum[84]. This effect is abolished by injecting mice with the hepcidin peptide confirming a role for hepcidin in the alcohol-mediated increase in duodenal iron transporter protein expression[84]. The increase in intestinal iron transporter expression leads to increased intestinal iron absorption, and hence to increased body iron indices. A recent study has also reported increased serum iron in mice exposed to alcohol[87]. We have observed an increased expression of the iron storage protein, ferritin in the livers of rats with chronic alcohol exposure[86]. Taken together, these findings suggest that iron overload observed in patients with alcoholic liver disease is mediated by regulatory mechanisms, and that the alcohol-mediated down-regulation of hepcidin synthesis in the liver may be one of the underlying mechanisms of iron overload.
Hepcidin synthesis in the liver responds to body iron levels, and is up-regulated by iron overload in vivo[54,61,83]. This raises the question of whether the alcohol-induced decrease in liver hepcidin expression would be sustained while the body iron levels progressively increase through continued alcohol consumption. It is feasible that the decrease in liver hepcidin expression may only be an initial response to alcohol, which may eventually be negated by elevated iron levels. On the other hand, it is also possible, that hepcidin expression in the liver is continuously suppressed by alcohol despite the iron overload state, which will eventually lead to liver injury. Thus, we investigated the combined effect of iron and alcohol in the regulation of hepcidin expression. Despite iron overload, alcohol down-regulated the expression of hepcidin in the liver[86] demonstrating that alcohol renders liver hepcidin synthesis insensitive to body iron levels. A further decrease in hepcidin expression was also observed in Hfe knockout mice exposed to alcohol[86]. It is worth noting that hepcidin protects the body from iron overload by inhibiting duodenal iron uptake and iron release from macrophages (Figure 1)[60,61,69]. These findings suggest that the mechanisms which protect the body from the harmful effects of iron overload (e.g. increased hepcidin expression and decreased iron uptake and storage) are compromised by alcohol[86].
Both iron and alcohol induce oxidative stress and oxidative stress plays an important role in alcoholic liver disease[23-25,88-90]. Treatment with antioxidants abolished the effect of alcohol on hepcidin expression in the liver and on duodenal iron transporter expression in the duodenum[84]. These findings strongly suggest a role for acute alcohol-induced oxidative stress in the regulation of hepcidin expression. Alcohol down-regulated both hepcidin promoter activity and the DNA-binding activity of the transcription factor, C/EBP alpha. This effect was abolished by treating mice with antioxidants[84]. Furthermore, alcohol also inhibited the iron-mediated up-regulation of C/EBP activity in the liver[86]. These findings demonstrate that redox changes and oxidative stress associated with alcohol metabolism regulate hepcidin gene transcription by altering C/EBP alpha activity. Oxidative stress is therefore one of the mechanisms by which moderate alcohol consumption regulates liver hepcidin expression and hence iron homeostasis, without causing steatosis or apparent liver injury (Figure 2B)[84].
Hepcidin is expressed in hepatocytes of the liver[54]. However, we observed a more prominent down-regulation of hepcidin expression in vivo compared to in vitro in hepatoma cells suggesting a role for non-parenchymal cells of the liver in the regulation of hepcidin expression[84]. Kupffer cells play an important role in the progression of alcoholic liver disease[13,91]. Since alcohol down-regulates, and inflammation up-regulates hepcidin expression[62,63,84,85], this raises the question of whether Kupffer cells and inflammation play a role in alcohol-mediated regulation of hepcidin expression. Recently, we have shown a role for oxidative stress in the regulation of hepcidin transcription by alcohol[84]. Alcohol metabolism induces oxidative stress in the hepatocytes and Kupffer cells of the liver[13,90,91]. Oxidative stress leads to the release of the pro-inflammatory cytokine, TNF-α from activated Kupffer cells[12,13,92,93]. TNF-α has been reported to down-regulate hepcidin expression in vitro[62]. Thus, alcohol-mediated regulation of hepcidin expression may involve both the hepatocytes and Kupffer cells of the liver (Figure 2B). The role of parenchymal and non-parenchymal cells of the liver, and the proinflammatory cytokines in the regulation of hepcidin expression by alcohol requires further investigation.
A role has been established for alcohol in the regulation of hepcidin expression in the liver. Alcohol-mediated oxidative stress inhibits C/EBP-α DNA-binding activity and down-regulates hepcidin transcription in the liver. Increased down-regulation of hepcidin expression by alcohol may play a role in the disease severity of genetic hemochromatosis patients in combination with alcohol intake. Down-regulation of hepcidin expression in the liver leads to increased intestinal iron transporter expression. Moreover, alcohol also abrogates the protective effect of hepcidin in iron overload by rendering the synthesis of hepcidin in the liver insensitive to body iron levels. Deregulation of hepcidin synthesis in the liver may be one of the underlying mechanisms by which alcohol consumption leads to iron overload. Ultimately, iron acts as a secondary risk factor in alcoholic liver disease. A better understanding of the molecular mechanisms underlying the regulation of iron homeostasis by alcohol may help us to develop therapeutic strategies or diagnostic tools to detect alcohol-induced liver injury at earlier stages before it develops into a chronic disease with irreversible liver damage.
| 1. | Aisen P, Wessling-Resnick M, Leibold EA. Iron metabolism. Curr Opin Chem Biol. 1999;3:200-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 312] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 2. | Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999;341:1986-1995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1329] [Cited by in RCA: 1289] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 3. | Kaplan J, O'Halloran TV. Iron metabolism in eukaryotes: Mars and Venus at it again. Science. 1996;271:1510-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 87] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Knutson M, Wessling-Resnick M. Iron metabolism in the reticuloendothelial system. Crit Rev Biochem Mol Biol. 2003;38:61-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 229] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Winterbourn CC. Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol Lett. 1995;82-83:969-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 813] [Cited by in RCA: 980] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 6. | McCord JM. Iron, free radicals, and oxidative injury. Semin Hematol. 1998;35:5-12. [PubMed] |
| 7. | Bulaj ZJ, Phillips JD, Ajioka RS, Franklin MR, Griffen LM, Guinee DJ, Edwards CQ, Kushner JP. Hemochromatosis genes and other factors contributing to the pathogenesis of porphyria cutanea tarda. Blood. 2000;95:1565-1571. [PubMed] |
| 8. | Bonkovsky HL, Lambrecht RW, Shan Y. Iron as a co-morbid factor in nonhemochromatotic liver disease. Alcohol. 2003;30:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Di Bisceglie AM, Axiotis CA, Hoofnagle JH, Bacon BR. Measurements of iron status in patients with chronic hepatitis. Gastroenterology. 1992;102:2108-2113. [PubMed] |
| 10. | Cederbaum AI. Iron and CYP2E1-dependent oxidative stress and toxicity. Alcohol. 2003;30:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Tsukamoto H, Horne W, Kamimura S, Niemelä O, Parkkila S, Ylä-Herttuala S, Brittenham GM. Experimental liver cirrhosis induced by alcohol and iron. J Clin Invest. 1995;96:620-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 369] [Article Influence: 11.9] [Reference Citation Analysis (1)] |
| 12. | Tsukamoto H, Lu SC. Current concepts in the pathogenesis of alcoholic liver injury. FASEB J. 2001;15:1335-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 257] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 13. | Tsukamoto H, Takei Y, McClain CJ, Joshi-Barve S, Hill D, Schmidt J, Deaciuc I, Barve S, Colell A, Garcia-Ruiz C. How is the liver primed or sensitized for alcoholic liver disease? Alcohol Clin Exp Res. 2001;25:171S-181S. [PubMed] |
| 14. | Chapman RW, Morgan MY, Laulicht M, Hoffbrand AV, Sherlock S. Hepatic iron stores and markers of iron overload in alcoholics and patients with idiopathic hemochromatosis. Dig Dis Sci. 1982;27:909-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 151] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Potter BJ, Chapman RW, Nunes RM, Sorrentino D, Sherlock S. Transferrin metabolism in alcoholic liver disease. Hepatology. 1985;5:714-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Irving MG, Halliday JW, Powell LW. Association between alcoholism and increased hepatic iron stores. Alcohol Clin Exp Res. 1988;12:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 60] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | De Feo TM, Fargion S, Duca L, Cesana BM, Boncinelli L, Lozza P, Cappellini MD, Fiorelli G. Non-transferrin-bound iron in alcohol abusers. Alcohol Clin Exp Res. 2001;25:1494-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Whitfield JB, Zhu G, Heath AC, Powell And LW, Martin NG. Effects of alcohol consumption on indices of iron stores and of iron stores on alcohol intake markers. Alcohol Clin Exp Res. 2001;25:1037-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Ioannou GN, Dominitz JA, Weiss NS, Heagerty PJ, Kowdley KV. The effect of alcohol consumption on the prevalence of iron overload, iron deficiency, and iron deficiency anemia. Gastroenterology. 2004;126:1293-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 156] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 20. | Suzuki Y, Saito H, Suzuki M, Hosoki Y, Sakurai S, Fujimoto Y, Kohgo Y. Up-regulation of transferrin receptor expression in hepatocytes by habitual alcohol drinking is implicated in hepatic iron overload in alcoholic liver disease. Alcohol Clin Exp Res. 2002;26:26S-31S. [PubMed] |
| 21. | Tsukamoto H, Lin M, Ohata M, Giulivi C, French SW, Brittenham G. Iron primes hepatic macrophages for NF-kappaB activation in alcoholic liver injury. Am J Physiol. 1999;277:G1240-G1250. [PubMed] |
| 22. | Xiong S, She H, Sung CK, Tsukamoto H. Iron-dependent activation of NF-kappaB in Kupffer cells: a priming mechanism for alcoholic liver disease. Alcohol. 2003;30:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Pietrangelo A. Iron, oxidative stress and liver fibrogenesis. J Hepatol. 1998;28 Suppl 1:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 107] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Bacon BR, Britton RS. The pathology of hepatic iron overload: a free radical--mediated process? Hepatology. 1990;11:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 269] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 25. | Cederbaum AI. Introduction-serial review: alcohol, oxidative stress and cell injury. Free Radic Biol Med. 2001;31:1524-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 145] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Feierman DE, Winston GW, Cederbaum AI. Ethanol oxidation by hydroxyl radicals: role of iron chelates, superoxide, and hydrogen peroxide. Alcohol Clin Exp Res. 1985;9:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Valerio LG, Parks T, Petersen DR. Alcohol mediates increases in hepatic and serum nonheme iron stores in a rat model for alcohol-induced liver injury. Alcohol Clin Exp Res. 1996;20:1352-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | She H, Xiong S, Lin M, Zandi E, Giulivi C, Tsukamoto H. Iron activates NF-kappaB in Kupffer cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:G719-G726. [PubMed] |
| 29. | Lin M, Rippe RA, Niemelä O, Brittenham G, Tsukamoto H. Role of iron in NF-kappa B activation and cytokine gene expression by rat hepatic macrophages. Am J Physiol. 1997;272:G1355-G1364. [PubMed] |
| 30. | Eijkelkamp EJ, Yapp TR, Powell LW. HFE-associated hereditary hemochromatosis. Can J Gastroenterol. 2000;14:121-125. [PubMed] |
| 31. | Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, Dormishian F, Domingo R, Ellis MC, Fullan A. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13:399-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2676] [Cited by in RCA: 2555] [Article Influence: 85.2] [Reference Citation Analysis (0)] |
| 32. | Feder JN, Tsuchihashi Z, Irrinki A, Lee VK, Mapa FA, Morikang E, Prass CE, Starnes SM, Wolff RK, Parkkila S. The hemochromatosis founder mutation in HLA-H disrupts beta2-microglobulin interaction and cell surface expression. J Biol Chem. 1997;272:14025-14028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 347] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 33. | Waheed A, Parkkila S, Zhou XY, Tomatsu S, Tsuchihashi Z, Feder JN, Schatzman RC, Britton RS, Bacon BR, Sly WS. Hereditary hemochromatosis: effects of C282Y and H63D mutations on association with beta2-microglobulin, intracellular processing, and cell surface expression of the HFE protein in COS-7 cells. Proc Natl Acad Sci U S A. 1997;94:12384-12389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 278] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 34. | Merryweather-Clarke AT, Pointon JJ, Shearman JD, Robson KJ. Global prevalence of putative haemochromatosis mutations. J Med Genet. 1997;34:275-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 543] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 35. | Fletcher LM, Dixon JL, Purdie DM, Powell LW, Crawford DH. Excess alcohol greatly increases the prevalence of cirrhosis in hereditary hemochromatosis. Gastroenterology. 2002;122:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 182] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 36. | Duane P, Raja KB, Simpson RJ, Peters TJ. Intestinal iron absorption in chronic alcoholics. Alcohol Alcohol. 1992;27:539-544. [PubMed] |
| 37. | Andrews NC, Fleming MD, Gunshin H. Iron transport across biologic membranes. Nutr Rev. 1999;57:114-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 56] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Kaplan J. Strategy and tactics in the evolution of iron acquisition. Semin Hematol. 2002;39:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Kaplan J. Mechanisms of cellular iron acquisition: another iron in the fire. Cell. 2002;111:603-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 40. | Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117:285-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1263] [Cited by in RCA: 1346] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 41. | Frazer DM, Anderson GJ. The orchestration of body iron intake: how and where do enterocytes receive their cues? Blood Cells Mol Dis. 2003;30:288-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 142] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 42. | Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2383] [Cited by in RCA: 2331] [Article Influence: 80.4] [Reference Citation Analysis (7)] |
| 43. | Gunshin H, Fujiwara Y, Custodio AO, Direnzo C, Robine S, Andrews NC. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J Clin Invest. 2005;115:1258-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 313] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 44. | Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, Paw BH, Drejer A, Barut B, Zapata A. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1226] [Cited by in RCA: 1227] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 45. | McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, Miret S, Bomford A, Peters TJ, Farzaneh F. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell. 2000;5:299-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 1035] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 46. | Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem. 2000;275:19906-19912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 943] [Cited by in RCA: 936] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 47. | McKie AT, Barrow D, Latunde-Dada GO, Rolfs A, Sager G, Mudaly E, Mudaly M, Richardson C, Barlow D, Bomford A. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science. 2001;291:1755-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 734] [Cited by in RCA: 742] [Article Influence: 29.7] [Reference Citation Analysis (1)] |
| 48. | Gunshin H, Starr CN, Direnzo C, Fleming MD, Jin J, Greer EL, Sellers VM, Galica SM, Andrews NC. Cybrd1 (duodenal cytochrome b) is not necessary for dietary iron absorption in mice. Blood. 2005;106:2879-2883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 119] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 49. | Vulpe CD, Kuo YM, Murphy TL, Cowley L, Askwith C, Libina N, Gitschier J, Anderson GJ. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat Genet. 1999;21:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 749] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 50. | Ponka P, Lok CN. The transferrin receptor: role in health and disease. Int J Biochem Cell Biol. 1999;31:1111-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 417] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 51. | Kawabata H, Yang R, Hirama T, Vuong PT, Kawano S, Gombart AF, Koeffler HP. Molecular cloning of transferrin receptor 2. A new member of the transferrin receptor-like family. J Biol Chem. 1999;274:20826-20832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 455] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 52. | Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276:7806-7810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1500] [Cited by in RCA: 1529] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 53. | Krause A, Neitz S, Mägert HJ, Schulz A, Forssmann WG, Schulz-Knappe P, Adermann K. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480:147-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 890] [Cited by in RCA: 897] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 54. | Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, Loréal O. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276:7811-7819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1204] [Cited by in RCA: 1200] [Article Influence: 48.0] [Reference Citation Analysis (1)] |
| 55. | Kulaksiz H, Gehrke SG, Janetzko A, Rost D, Bruckner T, Kallinowski B, Stremmel W. Pro-hepcidin: expression and cell specific localisation in the liver and its regulation in hereditary haemochromatosis, chronic renal insufficiency, and renal anaemia. Gut. 2004;53:735-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 169] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 56. | Courselaud B, Troadec MB, Fruchon S, Ilyin G, Borot N, Leroyer P, Coppin H, Brissot P, Roth MP, Loréal O. Strain and gender modulate hepatic hepcidin 1 and 2 mRNA expression in mice. Blood Cells Mol Dis. 2004;32:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 57. | Lou DQ, Nicolas G, Lesbordes JC, Viatte L, Grimber G, Szajnert MF, Kahn A, Vaulont S. Functional differences between hepcidin 1 and 2 in transgenic mice. Blood. 2004;103:2816-2821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 58. | Nicolas G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, Kahn A, Vaulont S. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci USA. 2001;98:8780-8785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 917] [Cited by in RCA: 920] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 59. | Nicolas G, Bennoun M, Porteu A, Mativet S, Beaumont C, Grandchamp B, Sirito M, Sawadogo M, Kahn A, Vaulont S. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci USA. 2002;99:4596-4601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 617] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 60. | Ganz T. Hepcidin in iron metabolism. Curr Opin Hematol. 2004;11:251-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 61. | Nicolas G, Viatte L, Bennoun M, Beaumont C, Kahn A, Vaulont S. Hepcidin, a new iron regulatory peptide. Blood Cells Mol Dis. 2002;29:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 234] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 62. | Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1638] [Cited by in RCA: 1698] [Article Influence: 77.2] [Reference Citation Analysis (0)] |
| 63. | Lee P, Peng H, Gelbart T, Wang L, Beutler E. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc Natl Acad Sci USA. 2005;102:1906-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 398] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 64. | Andrews NC. Anemia of inflammation: the cytokine-hepcidin link. J Clin Invest. 2004;113:1251-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 226] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 65. | Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, Beaumont C, Kahn A, Vaulont S. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 994] [Cited by in RCA: 968] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 66. | Lou DQ, Lesbordes JC, Nicolas G, Viatte L, Bennoun M, Van Rooijen N, Kahn A, Renia L, Vaulont S. Iron- and inflammation-induced hepcidin gene expression in mice is not mediated by Kupffer cells in vivo. Hepatology. 2005;41:1056-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 67. | Montosi G, Corradini E, Garuti C, Barelli S, Recalcati S, Cairo G, Valli L, Pignatti E, Vecchi C, Ferrara F. Kupffer cells and macrophages are not required for hepatic hepcidin activation during iron overload. Hepatology. 2005;41:545-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 54] [Reference Citation Analysis (0)] |
| 68. | Nemeth E, Preza GC, Jung CL, Kaplan J, Waring AJ, Ganz T. The N-terminus of hepcidin is essential for its interaction with ferroportin: structure-function study. Blood. 2006;107:328-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 192] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 69. | Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090-2093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3282] [Cited by in RCA: 3623] [Article Influence: 164.7] [Reference Citation Analysis (1)] |
| 70. | Nemeth E, Roetto A, Garozzo G, Ganz T, Camaschella C. Hepcidin is decreased in TFR2 hemochromatosis. Blood. 2005;105:1803-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 288] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 71. | Fleming RE, Ahmann JR, Migas MC, Waheed A, Koeffler HP, Kawabata H, Britton RS, Bacon BR, Sly WS. Targeted mutagenesis of the murine transferrin receptor-2 gene produces hemochromatosis. Proc Natl Acad Sci USA. 2002;99:10653-10658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 179] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 72. | Wallace DF, Summerville L, Subramaniam VN. Targeted disruption of the hepatic transferrin receptor 2 gene in mice leads to iron overload. Gastroenterology. 2007;132:301-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 73. | Bridle KR, Frazer DM, Wilkins SJ, Dixon JL, Purdie DM, Crawford DH, Subramaniam VN, Powell LW, Anderson GJ, Ramm GA. Disrupted hepcidin regulation in HFE-associated haemochromatosis and the liver as a regulator of body iron homoeostasis. Lancet. 2003;361:669-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 444] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 74. | Muckenthaler M, Roy CN, Custodio AO, Miñana B, deGraaf J, Montross LK, Andrews NC, Hentze MW. Regulatory defects in liver and intestine implicate abnormal hepcidin and Cybrd1 expression in mouse hemochromatosis. Nat Genet. 2003;34:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 213] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 75. | Wallace DF, Summerville L, Lusby PE, Subramaniam VN. First phenotypic description of transferrin receptor 2 knockout mouse, and the role of hepcidin. Gut. 2005;54:980-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 76. | Babitt JL, Huang FW, Wrighting DM, Xia Y, Sidis Y, Samad TA, Campagna JA, Chung RT, Schneyer AL, Woolf CJ. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006;38:531-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 823] [Cited by in RCA: 787] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 77. | Papanikolaou G, Samuels ME, Ludwig EH, MacDonald ML, Franchini PL, Dubé MP, Andres L, MacFarlane J, Sakellaropoulos N, Politou M. Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Nat Genet. 2004;36:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 716] [Cited by in RCA: 665] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 78. | Courselaud B, Pigeon C, Inoue Y, Inoue J, Gonzalez FJ, Leroyer P, Gilot D, Boudjema K, Guguen-Guillouzo C, Brissot P. C/EBPalpha regulates hepatic transcription of hepcidin, an antimicrobial peptide and regulator of iron metabolism. Cross-talk between C/EBP pathway and iron metabolism. J Biol Chem. 2002;277:41163-41170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 193] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 79. | Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108:3204-3209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 733] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 80. | Verga Falzacappa MV, Vujic Spasic M, Kessler R, Stolte J, Hentze MW, Muckenthaler MU. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 2007;109:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 444] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 81. | Wang RH, Li C, Xu X, Zheng Y, Xiao C, Zerfas P, Cooperman S, Eckhaus M, Rouault T, Mishra L. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005;2:399-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 480] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 82. | Rao RK, Seth A, Sheth P. Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G881-G884. [PubMed] |
| 83. | Détivaud L, Nemeth E, Boudjema K, Turlin B, Troadec MB, Leroyer P, Ropert M, Jacquelinet S, Courselaud B, Ganz T. Hepcidin levels in humans are correlated with hepatic iron stores, hemoglobin levels, and hepatic function. Blood. 2005;106:746-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 135] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 84. | Harrison-Findik DD, Schafer D, Klein E, Timchenko NA, Kulaksiz H, Clemens D, Fein E, Andriopoulos B, Pantopoulos K, Gollan J. Alcohol metabolism-mediated oxidative stress down-regulates hepcidin transcription and leads to increased duodenal iron transporter expression. J Biol Chem. 2006;281:22974-22982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 227] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 85. | Bridle K, Cheung TK, Murphy T, Walters M, Anderson G, Crawford DG, Fletcher LM. Hepcidin is down-regulated in alcoholic liver injury: implications for the pathogenesis of alcoholic liver disease. Alcohol Clin Exp Res. 2006;30:106-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 86. | Harrison-Findik D, Klein E, Crist C, Evans J, Timchenko N, Gollan J. Iron-mediated regulation of liver hepcidin expression rats and mice is abolished by alcohol. Hepatology. 2007;in press. |
| 87. | Flanagan JM, Peng H, Beutler E. Effects of alcohol consumption on iron metabolism in mice with hemochromatosis mutations. Alcohol Clin Exp Res. 2007;31:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 88. | Arteel GE. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology. 2003;124:778-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 362] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 89. | Cahill A, Wang X, Hoek JB. Increased oxidative damage to mitochondrial DNA following chronic ethanol consumption. Biochem Biophys Res Commun. 1997;235:286-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 89] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 90. | Sun AY, Ingelman-Sundberg M, Neve E, Matsumoto H, Nishitani Y, Minowa Y, Fukui Y, Bailey SM, Patel VB, Cunningham CC. Ethanol and oxidative stress. Alcohol Clin Exp Res. 2001;25:237S-243S. [PubMed] |
| 91. | Koop DR, Klopfenstein B, Iimuro Y, Thurman RG. Gadolinium chloride blocks alcohol-dependent liver toxicity in rats treated chronically with intragastric alcohol despite the induction of CYP2E1. Mol Pharmacol. 1997;51:944-950. [PubMed] |
| 92. | Wheeler MD, Kono H, Yin M, Nakagami M, Uesugi T, Arteel GE, Gäbele E, Rusyn I, Yamashina S, Froh M. The role of Kupffer cell oxidant production in early ethanol-induced liver disease. Free Radic Biol Med. 2001;31:1544-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 202] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 93. | Molina PE, Hoek JB, Nelson S, Guidot DM, Lang CH, Wands JR, Crawford JM. Mechanisms of alcohol-induced tissue injury. Alcohol Clin Exp Res. 2003;27:563-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
S- Editor Ma N L- Editor Alpini GD E- Editor Li JL