Published online Sep 28, 2007. doi: 10.3748/wjg.v13.i36.4873
Revised: July 2, 2007
Accepted: July 12, 2007
Published online: September 28, 2007
AIM: To investigate the effects of electroacupuncture (EA) at neiguan (PC6) on gastric distention-induced transient lower esophageal sphincter relaxations (TLESRs) and discuss the mechanisms of this treatment.
METHODS: ProtocolI: Twelve healthy cats underwent gastric distention for 60 min on the first day. Electrical acupoint stimulation was applied at the neiguan or a sham point on the hip in randomized order before gastric distention, on the third day and fifth day. Those cats that underwent EA at neiguan on the fifth day were named “Neiguan Group” and the cats that underwent EA at a sham acupoint on the fifth day were named “Sham Group” (control group). During the experiment the frequency of TLESRs and lower esophageal sphincter (LES) pressure were observed by a perfused sleeve assembly. Plasma levels of gastrin (GAS) and motilin (MTL) were determined by radioimmunoassay. Nitrite/nitrate concentration in plasma and tissues were measured by Griess reagent. The nuclei in the brain stem were observed by immunohistochemistry method of c-Fos and NADPH-d dyeing. Protocol II: Thirty six healthy cats were divided into 6 groups randomly. We gave saline (2 mL iv. control group), phaclofen (5 mg/kg iv. GABA-B antagonist), cholecystokinin octapeptide (CCK-8) (1 μg/kg per hour iv.), L-Arginine (200 mg/kg iv.), naloxone (2.5 μmol/kg iv.) and tacrine (5.6 mg/kg ip. cholinesterase inhibitor) respectively before EA at Neiguan and gastric distention. And the frequencies of TLESRs in experimental groups were compared with the control group.
RESULTS: ProtocolI: Not only the frequency of gastric distention-induced TLESR in 60 min but also the rate of common cavity during TLESRs were significantly decreased by EA at neiguan compared to that of sham acupoint stimulation. C-Fos immunoreactivity and NOS reactivity in the solitarius (NTS) and dorsal motor nucleus of the vagus (DMV) were significantly decreased by EA at neiguan compared to that of the sham group. However, the positive nuclei of C-Fos and NOS in reticular formation of the medulla (RFM) were increased by EA at neiguan. Protocol II: The inhibited effect of EA at neiguan on TLESR’s frequency was completely restored by pretreatment with CCK (23.5/h vs 4.5/h, P < 0.05), L-arginine (17.5/h vs 4.5/h, P < 0.05) and naloxone(12/h vs 4.5/h, P < 0.05). On the contrary, phaclofen (6/h vs 4.5/h, P > 0.05) and tacrine (9.5/h vs 4.5/h, P > 0.05) did not influence it.
CONCLUSION: Electric acupoint stimulation at Neiguan significantly inhibits the frequency of TLESR and the rate of common cavity during TLESR in cats. This effect appears to act on the brain stem, and may be mediated through nitric oxide (NO), CCK-A receptor and mu-opioid receptors. But the GABAB receptor and acetylcholine may not be involved in it.
- Citation: Wang C, Zhou DF, Shuai XW, Liu JX, Xie PY. Effects and mechanisms of electroacupuncture at PC6 on frequency of transient lower esophageal sphincter relaxation in cats. World J Gastroenterol 2007; 13(36): 4873-4880
- URL: https://www.wjgnet.com/1007-9327/full/v13/i36/4873.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i36.4873
Transient lower esophageal sphincter relaxations (TLESRs) is the most important mechanism of gastroesophageal reflux (GER) either in the patients of gastroesophageal reflux disease (GERD)[1] or in normal subjects[2]. Distention of the proximal stomach is a major stimulus for triggering TLESRs[3]. The stimulus passes through vago-vagal reflex and is integrated in the brain stem. There is ongoing interest in developing drugs that can decrease GER by interfering with TLESRs, including GABA-B receptor agonist[4], Cholecystokinin-A receptor antagonist[5], nitric oxide synthase (NOS) inhibitor[5,6], morphine[7] and atropine (act through central cholinergic blockade)[8-10]. The aim of our study is to explore new approaches (EA at Neiguan acupoint) to decrease the rate of TLESRs during gastric distention, and discuss the mechanisms of this treatment.
Acupuncture has been used to treat functional gastrointestinal disorders in eastern countries for centuries. It can modulate visceral sensation as well as function through stimulation at selected acupoints along the meridians (channels of acupoints)[11]. EA at zusanli (ST-36) can increase the basal LES pressure[12]. Transcutaneous electric nerve stimulation (TENS) at Hukou acupoint increased the degree of LES relaxation in volunteers[13] and reduced basal LES pressure in patients with achalasia[14,15].Previous studies have suggested that TENS at neiguan may inhibit the rate of TLESRs triggered by gastric distention[16] and reduce the perception to gastric distention[17] in human beings. But the precise mechanisms for this phenomenon have not been extensively investigated and are not fully understood.
ProtocolIwas performed on 12 adult cats weighing 3.7 ± 0.2 kg (M/F: 8/4), and 36 adult cats weighing 3.6 ± 0.5 kg (M/F: 25/11) were studied for protocol II. Cats were provided by the Animal Center of the First Hospital of Peking University. They were kept in individual cages in a controlled environment with a temperature of 22-26°C, 12/12-h light/dark cycles, and fed with standard cat diet. The animals were deprived of food 10 h before each experiment. All procedures were approved by the Committee for Animal Care and Usage for Research and Education of the Peking University. Anesthesia was initially induced with katamine (30 mg/kg im). Supplementary doses of katamine (15 mg/kg ip) were given whenever necessary to maintain an appropriate depth of anesthesia, as assessed they remained motionless yet still had cornea reflex. They were euthanized with pentobarbital sodium (0.5 mL/kg ip) at the end of the protocol.
Phaclofen, CCK octapeptide and tacrine were obtained from Sigma Chemical Co. (St. Louis, Missouri, USA). Naloxone was provided from Beijing Shuanghe Chemical Company. L-arginine was offered by Beijing Dingguo Chemical Company.
The GAS Radioimmunoassay kit was purchased from the China Institute of Atomic Energy, Beijing, China. The MTL Radioimmunoassay kit was purchased from the Neurobiological Technique Center of the Second Military Medical University, Shanghai, China. The Griess reagent for testing nitrite/nitrate concentration was purchased from Promega Corporation, USA. The reduced form nicotinamide adenine dinucleotide phosphate (NADPH) of and NBT (nitro blue tetrazolium) were obtained from Biomol Corporation, London, UK. The antibody of C-Fos was obtained from Santa Cruz Biotechnology, California, USA.
The manometry catheter (outer diameter 0.5 cm) consisted of a multilumen silicone tube with five side holes located at 9, 6, 3, 0 and -6 cm from the upper margin of the 6 cm-long Dent sleeve sensor (Dentsleeve, Belair, Australia).The catheter was continuously perfused with distilled water by a low compliance pneumohydraulic capillary infusion system (UPS-2020, Holland) at a rate of 0.2 mL/min. The external pressures transducers were connected via an analog/digital converter to a personal computer system. The data were displayed continuously on a monitor and stored on the personal computer system (MMS B.V. the Netherlands).
After anesthesia the cat was set in a supine position. A manometry catheter was placed through the mouth into the esophagus and positioned so that the sleeve sensor straddled the LES to register LES pressure. The distal side hole was used as a reference point for intragastric pressure. And the upper LES side holes were used to measure esophageal body pressure.
A mylohyoid electromyography (MMS B.V. the Netherlands) was used to record swallowing[1,18]. The pinhead electrode was inserted in the mylohyoid muscle, and the reference electrode was fixed to the interscapular region of the back.
Two acupuncture needles of 0.22 mm in diameter (Suzhou global acupuncture instrument Co. Ltd, Suzhou, China) were inserted perpendicularly at the bilateral Neiguan acupoint (PC6,located 1.5-2.0 cm above the wrist between the ligaments of the flexor carpi radialis and the palmaris longus[19]) overlying the median nerve to a depth of 5 mm. An electrical stimulator (Model LH202H Hans, Beijing Huawei Medical Instrument Co. Ltd, Beijing, China) provided current to the needles. Wave patterns were sparse with dense pulse intervals ranging from 2 to 100 Hz (2/100 Hz), with constant amplitude and current flow (3-4 mA). The duration was 60 min. Correct positioning was confirmed by observing slight repetitive paw flexion during stimulation[20].
Control stimulation on a sham acupoint was conducted at the hip, a point away from the traditional meridians and dermatomes.
Air was insufflated (at a rate of 15 mL/s) into the stomach through a 2.0-mm diameter tube intubated through mouth to stomach. It’s depth equal to 5 cm plus the esophageal body length. 30 mL air into the stomach every 6 min amount to 300 mL were insufflated in the 1 h period of gastric distention.
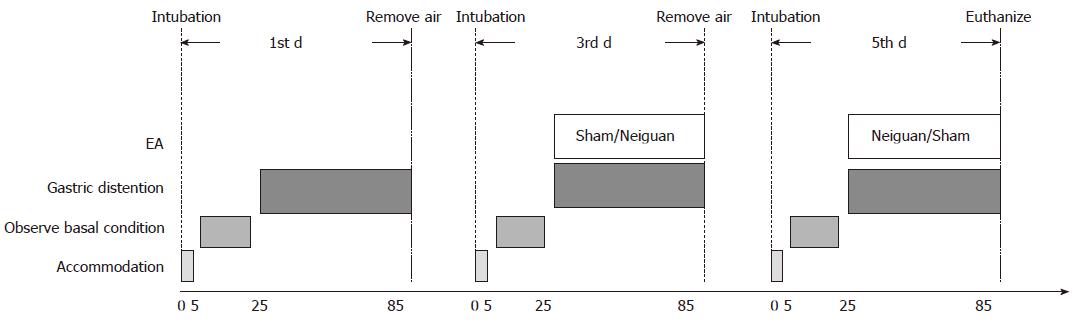
Twelve adult cats were divided into Neiguan group and Sham group randomly. Each cat was studied for three sessions, and an interval of each session is 2 d (Figure 1). In the first session, the basal rate of TLESRs triggered by gastric distention was observed for one hour. In session 2 and session 3, the influence of EA at neiguan or sham acupoint to the rate of gastric distention induced TLESRs was observed in a random order.
After 5min of accommodation, the basal conditions were observed for 20 min (including basal rate of TLESRs and basal LES pressure). And then the gastric distention was applied in session 1. In session 2 and 3, gastric distention and electroacupuncture were applied at the same time. The remaining air was removed at the end of each session. At the end of session 3, the cats were euthanized and the blood, gastric fundus and brain stem were obtained for further research.
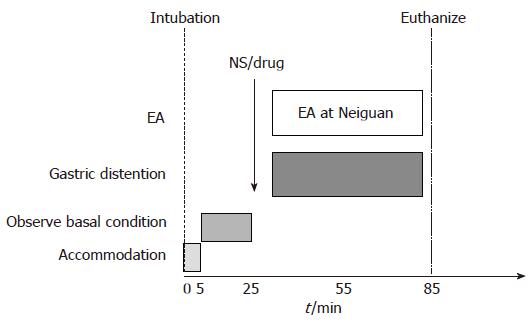
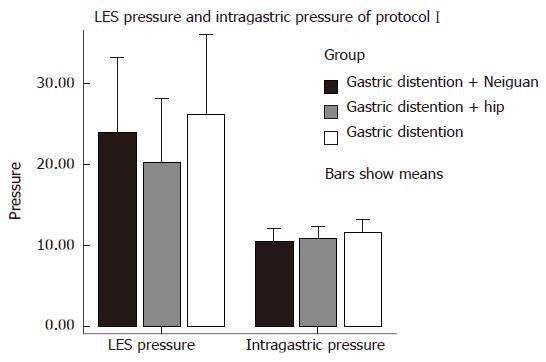
Thirty six healthy cats were divided into 6 groups randomly. We gave normal saline(NS) (2 mL iv.), GABA-B antagonist phaclofen[21] (5 mg/kg iv.), CCK octapeptide[5] (1 μg/kg per hour iv.), L-arginine[5] (200 mg/kg iv.), naloxone[22] (2.5 μmol/kg iv.) and tacrine[23] (5.6 mg/kg ip. cholinesterase inhibitor in central nerve system) respectively before EA at Neiguan and gastric distention, and observed the frequency of TLESR and LES pressure (Figure 2). At the end of this study, the cats were euthanized and the blood, gastric fundus and brain stem were obtained for further research.
TLESRs were defined according to established methods[9]. Basal LES pressure was measured at the end of expiration relative to gastric pressure. The LES pressure during gastric distention was measured for 1 min every 6 min, and an overall mean for each period of the study was calculated. Common cavities were defined as abrupt simultaneous and sustained rises of basal esophageal pressure to intragastric pressure in at least the two lower esophageal body manometry recording sites[24]. Common cavities are considered as markers of gas or liquid reflux from the stomach into the esophagus.
When the cats were euthanized, 15 mL venous blood was collected into a test tube containing 400 μL of 10% EDTA-Na2 (ananticoagulant) and 200 μL of trasylol. The blood samples were centrifuged at 4°C at 3500 r/min for 20 min. The serum was separated and stored at -70°C before analysis. The tissue of gastric fundus is about 2 cm× 2 cm. After weighting, tissues were added to 1 mL of 1 mol/L acetic acid and mixed evenly in a homogenizer to obtain a homogenate and were refrigerated at 4°C for 100 min. The extracted homogenates were added to 1 mL of 1 mmol/l NaOH and centrifuged at 3500 r/min 4°C for 20 min. The supernatant fluid was collected and stored at -70°C.
Gastrin (GAS) and motilin (MTL) levels were measured with commercial radioimmunoassay kits. Nitrite/nitrate levels were tested by Griess reagent.
After 60 min of gastric distention on the fifth day, the animal was transcardially perfused with 9 g/L saline followed by 40 g/L paraformaldehyde in 0.1 mol/L phosphate buffer saline (PBS, pH 7.3). The brain stem was removed and postfixed in the same fixative overnight and cryoprotected by immersion in 200 g/L sucrose for 72 h.
Coronal sections (40 μm) of the brain were cut in a cryostat. Every fourth section was used to reveal c-Fos immunoreactivity and the second set of sections was used to reveal NADPH-diaphorase (NADPH-d) staining.
The first set of sections were placed into a 50 g/L goat serum for 30 min at room temperature (RT), and incubated overnight at RT in primary antibody c-Fos (1:200). After washing for 15 min with PBST, the sections were incubated in biotinylated anti-rabbit IgG (Zymed, South San Francisco, Canada) diluted 1:300 in PBST at RT for 2 h, and then incubated in peroxidase-conjugated streptavidin (1:300 dilution, Zymed) for 2 h at RT. The immunoreactivity was visualized by incubating with 0.05 mol/L Tris-HCl buffer containing 0.1 g/L 3, 3’-diaminobenzidine, and 0.3 mL/L H2O2 for 10-20 min at RT. The stained sections were mounted on APES-coated glass slides, dehydrated and coverslipped.
The second set of sections were incubated at 37°C for 2 h in a solution containing 1 mmol/L NADPH, 0.5 mmol/L NBT, Tris-HCl 50 mmol/L, and Triton X-100 2 g/L. After a rinse in PBST, sections were mounted on APES-coated glass slides, dehydrated and coverslipped.
The distribution of c-Fos and NADPH-d positive cells was detected under a microscope (Olympus, Tokyo, Japan), and the cells were counted on LEICA Q550CW system (Leica Microsystems Imaging Solutions Ltd, Wetzlar, Germany), 10 sections for NTS/DMV, 8 sections for RFM. The average number of c-Fos or NADPH-d positive neurons per section for each cat was calculated, respectively.
The number of TLESRs was compared using Wilcoxon signedrank test and expressed as median (interquartile range). Basal LES pressure and intragastric pressure were presented as means ± SD and were compared using repeated-measures. The rate of common cavity is compared using paired sample χ2. Nitrate concentrations, plasma hormone levels and the average number of c-Fos or NADPH-d positive nucleus of respective brain areas per section were expressed as mean ± SD and was compared using independent t.
SPSS 11.0 was used for statistical analysis, and P < 0.05 was considered statistically significant.
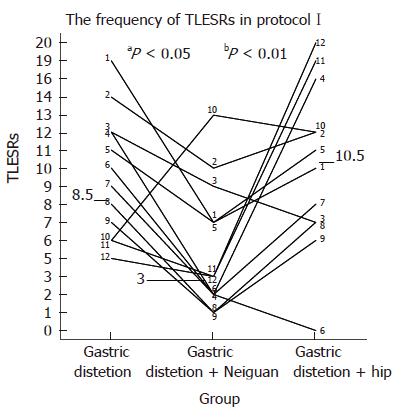
Transient LES relaxations: The frequency of TLESRs during acupoint stimulation at neiguan [3 per hour (range, 1-13)] was significantly lower than that during both the baseline period without any stimulation [8.5 per hour (range, 5-19), P < 0.05] and the period of sham stimulation at the hip [10.5 per hour (range, 0-20), P < 0.01] (Figure 3).
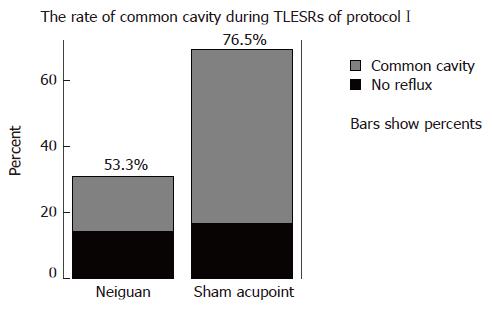
Common cavities during TLESRs: During EA at neiguan, a total of 60 TLESRs induced by gastric distention were observed in one hour, from it 32 were associated with common cavity, and its rate was 53.3%. During EA at sham acupoint, there are 136 gastric distention induced TLESRs in all, and 104 were associated with common cavity. The rate was 76.5%. Between-group comparisons showed that the rate of common cavity during EA at Neriguan was significantly lower than that occurring during EA at Sham acupoint (P < 0.05) (Figure 4).
LES pressure: EA at neiguan (PC6) had no effect on LES pressure. Overall mean LES pressure during electrical acupoint stimulation at neiguan (34.33 ± 18.16 mmHg) was similar to that during stimulation at the sham acupoint (30.97 ± 15.72 mmHg, P > 0.05) and during the baseline period without any acupoint stimulation (37.74 ± 18.69 mmHg, P > 0.05) (Figure 5) .
Intragastric pressure: EA at neiguan (PC6) had no effect on gastric pressure during gastric distention. Overall mean gastric pressure during EA at neiguan (10.37 ± 3.17 mmHg) was similar to that during stimulation at the sham acupoint (10.81 ± 2.89 mmHg, P > 0.05) and during the baseline period without any acupoint stimulation (11.58 ± 3.16 mmHg, P > 0.05) (Figure 5).
Nitrite concentration: EA at neiguan (PC6) did not influence the nitrite levels in plasma and gastric fundus tissues compared with Sham group (P > 0.05) (Table 1).
| Group | Serum (mmol/mL) | Gastric fundus (mmol/mg) |
| Neiguan | 0.85 ± 0.62 | 3.50 ± 0.87 |
| Sham | 0.66 ± 0.59 | 4.18 ± 1.09 |
Gastrin (GAS) and motilin (MTL) levels: EA at neiguan (PC6) did not influence the GAS and MTL levels in plasma (Table 2) .
| Group | GAS (pg/mL) | MTL (pg/mL) |
| Neiguan group | 79.43 ± 28.84 | 82.23 ± 43.79 |
| Sham group | 156.30 ± 72.53 | 103.6 ± 68.16 |
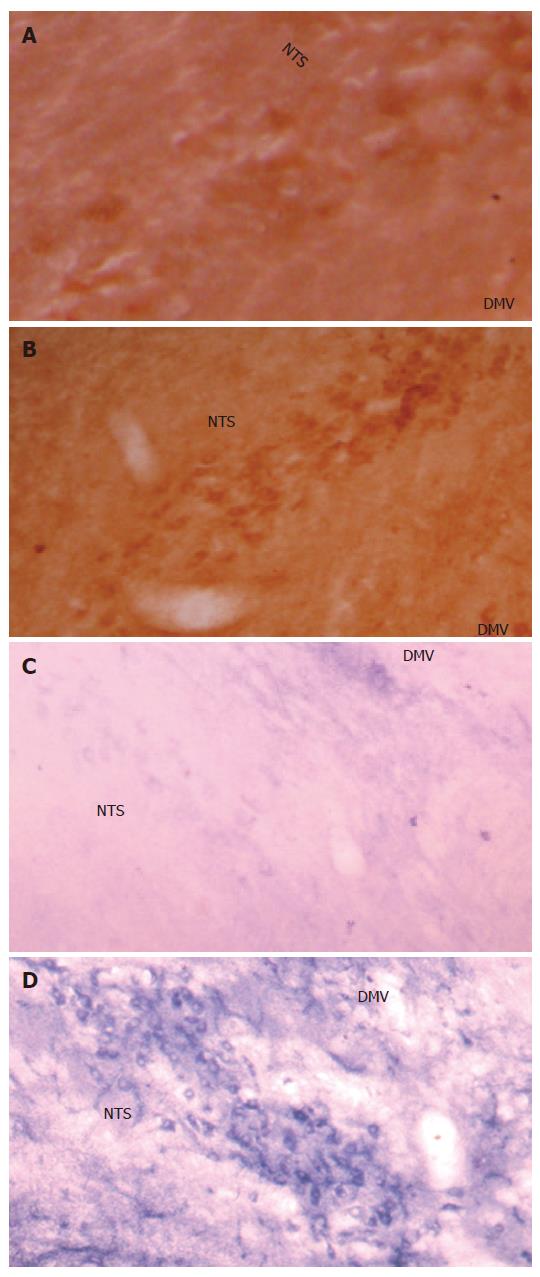
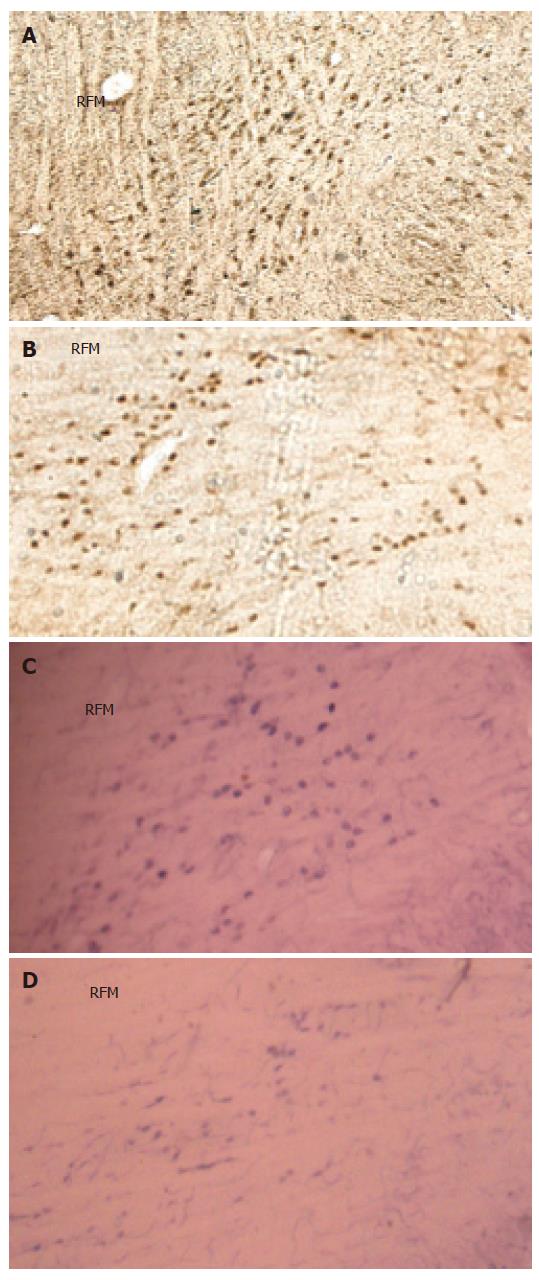
The c-Fos positive cell nuclei of activated cells showed the characteristic dark brown staining of oxidized DAB. Compared with the Sham group, electroacupuncture at neiguan significantly inhibited the number of C-Fos-labeled neurons in nucleus tractus solitarius/dorsal motor nucleus of vagus (NTS/DMV) (Figure 6A and B). However, it stimulated a significantly greater number of C-Fos positive nuclei in areas of reticular formation of the medulla (RFM) (Figure 7A and B).
NADPH-d activity was visualized as a vibrant blue color within perikarya, dendrites and axons. Electroacu-puncture at neiguan significantly decreased the number of NADPH-d stained cells in NTS/DMV (Figure 6C and D), but increased the number of positive cells in RFM (Figure 7C and D ).
The number of positive nuclei in each group is listed in Table 3.
Transient LES relaxations: After saline infusion, the frequency of TLESRs during acupoint stimulation at neiguan [4.5 per hour (range, 1-11)] was still significantly lower than that during sham stimulation at the hip in protocolI[10.5 per hour (range, 0-20), P < 0.05].
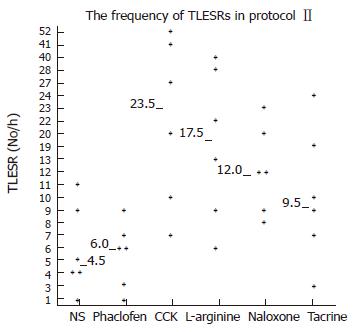
Infusion of Phaclofen and tacrine did not influence the rate of TLESRs during acupoint stimulation at neiguan. They were [6 per hour (range 1-9) vs 4.5 per hours (range 1-11), P > 0.05] and [9.5 per hour (range 3-24) vs 4.5 per hour (range, 1-11), P > 0.05] respectively. However, during infusion of CCK octapeptide, L-arginine and naloxone, the inhibited effect of EA at neigtuan had been completely restored. The rate of TLESRs were CCK [23.5 per hour (range 7-52) vs 4.5 per hours (range 1-11), P < 0.05], L-arginine [17.5 per hour (range 6-40) vs 4.5 per hours (range 1-11), P < 0.05] and naloxone [12 per hour (range 8-23) vs 4.5 per hours (range 1-11), P < 0.05] respectively (Figure 8) .
Acupuncture has been used to treat functional gastro-intestinal disorders in the eastern countries for centuries. A large amount of clinical evidence supports the effectiveness of acupuncture for treating functional disorders of the gastrointestinal tract, and the most commonly used acupoints in treating gastrointestinal symptoms are Neiguan (PC6) and Zusanli (ST-36). In the present study, we found electric acupoint stimulation at the neiguan acupoint resulted in a significant reduction of the rate of TLESRs induced by gastric distention. This result was consistent with previous investigations on human beings [12]. Furthermore, the present work also demonstrated the action site and neurotransmitters of this effect.
We postulate that the site at which electric acupuncture stimulation acts to inhibit the occurrence of TLESRs may be as follows: First, it may increase the proximal gastric motility or increase the gastric fundus tone, so that to decrease the volume of gastric fundus, and inhibit the stretch receptors localized in the gastric fundus (As we know, the stretch receptors were the major receptor in triggering TLESRs[25,26]) and then reduce the sensory input from gastric distention. Second, it may inhibit the integration of TLESRs in some area of the brain stem, such as NTS (nucleus tractus solitarius) and DMV (dorsomotor nucleus of the vagus nerve). Third, electric acupoint stimulation at Neiguan may exert its action primarily on the efferent motor pathway.
With present research, electric stimulation at Neiguan did not change the intragastric pressure and nitrite levels in the tissue of gastric fundus compared with EA at sham acupoint. Nitric oxide is well accepted as an inhibitory neurotransmitter in the gastrointestinal tract, and may exert a tonic inhibition on the proximal stomach[27]. So the result of our study suggested that EA at neiguan cannot change the tone of gastric fundus. And it is consistent with previous research[12,28].
In the current research, electric stimulation at neiguan had no effect on the residual LES pressure, so it is unlikely that it exerts action primarily on the efferent motor pathway.
The dorsal vagal complex (DVC) comprising nucleus tractus solitarius (NTS) and dorsomotor nucleus of the vagus nerve (DMV) is the center of the integration of TLESRs[9]. The brain stem dyeing shows that when compared with sham group EA at neiguan significantly decreases the C-Fos and NOS positive nucleus in NTS/DMV. However, it stimulated a significantly greater number of C-Fos and NOS positive nucleus in areas of reticular formation of the medulla (RFM). And RFM may be one of the acupuncture action sites[29].
Consequently, the action site of EA at neiguan may be localized within the brain stem. It may increase NOS in the nucleus of RFM, so that it inhibits NOS in NTS/DMV, and than decreases the frequency of TLESRs.
In the second part of this research, the inhibited effect of EA at neiguan on TLESR’s rate was completely restored by pretreatment with CCK, L-arginine and naloxone. On the contrary, phaclofen and tacrine did not influence it. So this effect appeared to be mediated through nitric oxide (NO), CCK-A receptor and mu-opioid receptors. But the GABAB receptor and acetylcholine may not be involved in it.
In our study, electric stimulation at Neiguan also did not influence the gastrin and motilitin levels in plasma. It suggested that the inhibited effects of electric stimulation were not through these two neuropeptides.
Endogenous opioid peptides (EOPs) are considered as major candidates for a role in acupuncture action because numerous investigations have clearly demonstrated that electroacupuncture effect is antagonized by the opioid receptor antagonist naloxone[30]. And in our research, the naloxone can reverse the inhibited effect of electroacupuncture on TLESRs. In contrast to our results in cats, Zou et al[12] found the inhibited effect of acupoint stimulation was not inhibited by naloxone. In that study, the frequency of electrical stimulation is 100 Hz. However, our wave patterns were sparse and dense pulse intervals ranging from 2 Hz to 100 Hz (2/100 Hz). Previous studies have demonstrated that low-frequency(2 Hz) electroacupuncture analgesia (EAA) is induced by the activation of μ- and δ-opioid receptors via the release of enkephalin, β-endorphin, and endomorphin; and high-frequency (100 Hz) EAA is caused by activation of κ opioid receptors via release of dynorphin[31]. So it may be the reason of the variance between our findings and Zou’s research.
In conclusion, electric acupoint stimulation at the Neiguan result in a significant reduction of the rate of TLESRs induced by gastric distention. This effect appears to act at the brain stem, and may be mediated through NO, CCK-A receptor and mu-opioid receptor.
Gastroesophageal reflux disease (GERD) is a disorder characterized by an increased exposure of the esophagus to the intragastric contents. Recent studies have suggested that transient lower esophageal sphincter relaxation is the main mechanism underlying gastroesophageal reflux. It involves a prolonged relaxation of the lower esophageal sphincter, mediated by a vago-vagal neural pathway, synapsing in the brainstem. Acupuncture has been used to treat functional gastrointestinal disorders in the eastern countries for centuries. It can modulate visceral sensation as well as function through stimulation at selected acupoints along the meridians (channels of acupoints).
Transient lower esophageal sphincter relaxation (TLESR) is the most important mechanism of gastroesophageal reflux (GER) in the patients of GERD. So it had become an important target in dealing with gastroesophageal reflux disease.
Althrough there have one article indicated that electroacupuncture at neiguan (PC6) may decrease the frequency of TLESRs, our research is the first paper to observe the mechanisms carefully.
Our research observed the relationship between electroacupuncture at neiguan (PC6) and TLESRs. And there may be a significant clinical impact in the future.
TLESR: It is a spontaneous relaxation of LES without swallow induced. Its definition included: (1) absence of swallowing for 4 s before to 2 s after the onset of LES relaxation. (2) relaxation rate of > or = 1 mmHg/s. (3) time from onset to complete relaxation of < or = 10 s. (4) nadir pressure of < or = 2 mmHg. Exception: a markedly prolonged LES relaxation > or = 10 s,and nadir pressure < or = 2 mmHg can also be classified as TLESR, irrespective of the relation with swallow; Common Cavity: It was defined as abrupt simultaneous and sustained rises of basal esophageal pressure to intragastric pressure in at least the two lower esophageal body manometry recording sites.
This manuscript it is well written and the experimental design is sound. In addition, there may be a significant clinical impact if these results will be confirmed in the future.
| 1. | Mittal RK, McCallum RW. Characteristics and frequency of transient relaxations of the lower esophageal sphincter in patients with reflux esophagitis. Gastroenterology. 1988;95:593-599. [PubMed] |
| 2. | Dent J, Dodds WJ, Friedman RH, Sekiguchi T, Hogan WJ, Arndorfer RC, Petrie DJ. Mechanism of gastroesophageal reflux in recumbent asymptomatic human subjects. J Clin Invest. 1980;65:256-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 700] [Cited by in RCA: 632] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 3. | Holloway RH, Hongo M, Berger K, McCallum RW. Gastric distention: a mechanism for postprandial gastroesophageal reflux. Gastroenterology. 1985;89:779-784. [PubMed] |
| 4. | Lee KJ, Vos R, Janssens J, Tack J. Differential effects of baclofen on lower oesophageal sphincter pressure and proximal gastric motility in humans. Aliment Pharmacol Ther. 2003;18:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Boulant J, Fioramonti J, Dapoigny M, Bommelaer G, Bueno L. Cholecystokinin and nitric oxide in transient lower esophageal sphincter relaxation to gastric distention in dogs. Gastroenterology. 1994;107:1059-1066. [PubMed] |
| 6. | Hirsch DP, Tiel-Van Buul MM, Tytgat GN, Boeckxstaens GE. Effect of L-NMMA on postprandial transient lower esophageal sphincter relaxations in healthy volunteers. Dig Dis Sci. 2000;45:2069-2075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Penagini R, Allocca M, Cantù P, Mangano M, Savojardo D, Carmagnola S, Bianchi PA. Relationship between motor function of the proximal stomach and transient lower oesophageal sphincter relaxation after morphine. Gut. 2004;53:1227-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Fang JC, Sarosiek I, Yamamoto Y, Liu J, Mittal RK. Cholinergic blockade inhibits gastro-oesophageal reflux and transient lower oesophageal sphincter relaxation through a central mechanism. Gut. 1999;44:603-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Mittal RK, Holloway RH, Penagini R, Blackshaw LA, Dent J. Transient lower esophageal sphincter relaxation. Gastroenterology. 1995;109:601-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 469] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 10. | Lidums I, Hebbard GS, Holloway RH. Effect of atropine on proximal gastric motor and sensory function in normal subjects. Gut. 2000;47:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Li Y, Tougas G, Chiverton SG, Hunt RH. The effect of acupuncture on gastrointestinal function and disorders. Am J Gastroenterol. 1992;87:1372-1381. [PubMed] |
| 12. | Mu XD, Xie PY, Liu JX, Shuai XW, Li J. Effect of Electro-acupuncture at Zusanli acupoint on LESP, plasma gastrin and motilin in rats. Shijie Huaren Xiaohua Zazhi. 2005;13:1069-1073. |
| 13. | Chang FY, Chey WY, Ouyang A. Effect of transcutaneous nerve stimulation on esophageal function in normal subjects--evidence for a somatovisceral reflex. Am J Chin Med. 1996;24:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Guelrud M, Rossiter A, Souney PF, Sulbaran M. Transcutaneous electrical nerve stimulation decreases lower esophageal sphincter pressure in patients with achalasia. Dig Dis Sci. 1991;36:1029-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Kaada B. Successful treatment of esophageal dysmotility and Raynaud's phenomenon in systemic sclerosis and achalasia by transcutaneous nerve stimulation. Increase in plasma VIP concentration. Scand J Gastroenterol. 1987;22:1137-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Zou D, Chen WH, Iwakiri K, Rigda R, Tippett M, Holloway RH. Inhibition of transient lower esophageal sphincter relaxations by electrical acupoint stimulation. Am J Physiol Gastrointest Liver Physiol. 2005;289:G197-G201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Coffin B, Azpiroz F, Malagelada JR. Somatic stimulation reduces perception of gut distention in humans. Gastroenterology. 1994;107:1636-1642. [PubMed] |
| 18. | Mittal RK, McCallum RW. Characteristics of transient lower esophageal sphincter relaxation in humans. Am J Physiol. 1987;252:G636-G641. [PubMed] |
| 19. | Tjen-A-Looi SC, Li P, Longhurst JC. Prolonged inhibition of rostral ventral lateral medullary premotor sympathetic neurons by electroacupuncture in cats. Auton Neurosci. 2003;106:119-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Grossi L, Ciccaglione AF, Travaglini N, Marzio L. Transient lower esophageal sphincter relaxations and gastroesophageal reflux episodes in healthy subjects and GERD patients during 24 hours. Dig Dis Sci. 2001;46:815-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Han Y, Qing J, Bu DF. Gamma-aminobutyric acid B receptor regulates the expression of hydrogen sulfide/cystathionine-β-synthase system in recurrent febrile seizures. Zhonghua Dangdai Erke Zazhi. 2006;4:141-143. |
| 22. | Gustafsson BI, Delbro DS. Vagal influence on the motility of the feline jejunum. J Physiol. 1994;480:587-595. [PubMed] |
| 23. | Nielsen JA, Mena EE, Williams IH, Nocerini MR, Liston D. Correlation of brain levels of 9-amino-1,2,3,4-tetrahydroacridine (THA) with neurochemical and behavioral changes. Eur J Pharmacol. 1989;173:53-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Holloway RH, Wyman JB, Dent J. Failure of transient lower oesophageal sphincter relaxation in response to gastric distension in patients with achalasia: evidence for neural mediation of transient lower oesophageal sphincter relaxations. Gut. 1989;30:762-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Penagini R, Carmagnola S, Cantù P, Allocca M, Bianchi PA. Mechanoreceptors of the proximal stomach: Role in triggering transient lower esophageal sphincter relaxation. Gastroenterology. 2004;126:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Tack J, Sifrim D. A little rest and relaxation. Gut. 2000;47:11-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Shen GM, Zhou MQ, Xu GS, Xu Y, Yin G. Role of vasoactive intestinal peptide and nitric oxide in the modulation of electroacupucture on gastric motility in stressed rats. World J Gastroenterol. 2006;12:6156-6160. [PubMed] |
| 28. | Li P, Rowshan K, Crisostomo M, Tjen-A-Looi SC, Longhurst JC. Effect of electroacupuncture on pressor reflex during gastric distension. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1335-R1345. [PubMed] |
| 29. | Miller AD. Central mechanisms of vomiting. Dig Dis Sci. 1999;44:39S-43S. [PubMed] |
| 30. | Sodipo JO, Gilly H, Pauser G. Endorphins: mechanism of acupuncture analgesia. Am J Chin Med. 1981;9:249-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Han JS. Acupuncture and endorphins. Neurosci Lett. 2004;361:258-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 534] [Article Influence: 24.3] [Reference Citation Analysis (1)] |
S- Editor Zhu LH L- Editor Li M E- Editor Ma WH