Published online Sep 28, 2007. doi: 10.3748/wjg.v13.i36.4839
Revised: July 2, 2007
Accepted: July 9, 2007
Published online: September 28, 2007
Virus-specific CD8+ T cells are thought to be the major anti-viral effector cells in hepatitis C virus (HCV) infection. Indeed, viral clearance is associated with vigorous CD8+ T cell responses targeting multiple epitopes. In the chronic phase of infection, HCV-specific CD8+ T cell responses are usually weak, narrowly focused and display often functional defects regarding cytotoxicity, cytokine production, and proliferative capacity. In the last few years, different mechanisms which might contribute to the failure of HCV-specific CD8+ T cells in chronic infection have been identified, including insufficient CD4+ help, deficient CD8+ T cell differentiation, viral escape mutations, suppression by viral factors, inhibitory cytokines, inhibitory ligands, and regulatory T cells. In addition, host genetic factors such as the host’s human leukocyte antigen (HLA) background may play an important role in the efficiency of the HCV-specific CD8+ T cell response and thus outcome of infection. The growing understanding of the mechanisms contributing to T cell failure and persistence of HCV infection will contribute to the development of successful immunotherapeutical and -prophylactical strategies.
- Citation: Neumann-Haefelin C, Spangenberg HC, Blum HE, Thimme R. Host and viral factors contributing to CD8+ T cell failure in hepatitis C virus infection. World J Gastroenterol 2007; 13(36): 4839-4847
- URL: https://www.wjgnet.com/1007-9327/full/v13/i36/4839.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i36.4839
The host immune response to pathogens involves various components of the immune system, including innate, humoral, and cellular immunity, the latter consisting of CD4+ and CD8+ T cells. All components of the immune response might have distinct roles in the outcome and pathogenesis of HCV infection and will be discussed in separate reviews in this issue of WJG. In this review, we will focus on the CD8+ T cell response to HCV infection. CD8+ T cells recognize viral antigen presented by HLA class I molecules on professional antigen presenting cells (CD8+ T cell priming) and on infected target cells (e.g. hepatocytes). Their antiviral activity includes cytotoxicity as well as the secretion of antiviral cytokines such as interferon-gamma (IFN-γ). In the following, successful virus-specific CD8+ T cell responses associated with viral clearance as well as ineffective CD8+ T cell responses present in persistent HCV infection will be described. The main focus of this review, however, is the multiple mechanisms that contribute to CD8+ T cell failure and viral persistence.
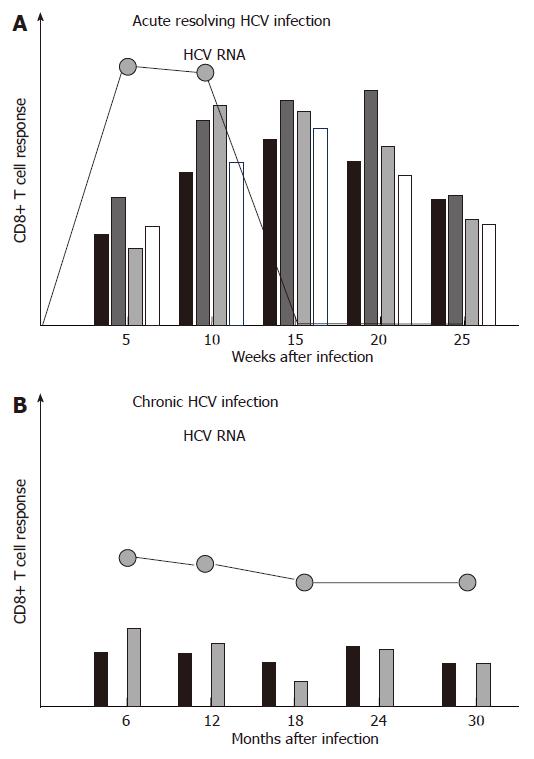
During acute resolving HCV infection, vigorous virus-specific CD8+ T cell responses that target multiple epitopes can be detected approximately 4-8 wk after infection, and their emergence is temporally associated with the onset of liver disease[1-4] (Figure 1A). However, the virus-specific CD8+ T cells are not able to secrete antiviral cytokines such as IFN-γ in this early phase of infection, a status referred to as ‘stunned phenotype’[2,3]. In a later phase of infection, virus-specific CD8+ T cells regain their ability to secrete antiviral cytokines, and this is temporally associated with a rapid decline of viremia and finally viral clearance. Knowledge about the intrahepatic virus-specific CD8+ T cell response during acute HCV infection was obtained from experimentally infected chimpanzees. Responses accumulate in the liver 8-14 wk after infection and coincide with liver disease as well as viral clearance[5,6]. After resolution of infection, virus-specific CD4+ and CD8+ T cell responses persist for decades and can even outlast humoral responses[7]. Virus-specific CD8+ T cells also play a role in mediating protective immunity. Indeed, evidence for protective immunity comes from both, epidemiological studies as well as experimental studies[8]. Chimpanzees re-challenged by HCV showed a shorter period and lower level of viremia than naïve animals[9-11]. Sterilizing immunity against HCV, however, may not exist, since multiple episodes of heterologous or homologous re-infection have been observed in both, humans and chimpanzees.
In contrast to acute resolving HCV infection, the CD8+ T cell response in acute persisting HCV infection has been less defined. Previous reports comparing the CD8+ T cell response in acute resolving versus acute persisting HCV infection in chimpanzees and men found significantly weaker and more narrowly focused virus-specific CD8+ T cell responses in those subjects developing persistent infection[1,2,5,6]. More recent studies, however, could not confirm this finding[4,12,13]. For example, Cox et al performed a prospective longitudinal study in young iv drug users and analyzed the T cell response in 4 individuals with resolution of acute HCV infection and 15 individuals who progressed to chronic infection. Although all 4 individuals with resolving infection mounted virus-specific CD8+ T cell responses and those 4 individuals who lacked CD8+ T cell responses developed chronic infection, the CD8+ T cell response did not differ significantly between resolvers and persistently infected individuals[4]. Urbani et al studied 6 patients with acute resolving and 11 patients with acute persisting HCV infection and found an association between strong and multispecific CD4+, but not CD8+ T cells with viral clearance. However, patients developing chronic infection displayed prolonged CD8+ T cell dysfunctions and maturational defects[12]. This discordant role of CD4+ and CD8+ T cells was confirmed by Kaplan et al, albeit their analysis was limited to two HLA-A2 restricted CD8+ T cell epitopes[13].
In sum, acute resolving HCV infection is associated with strong, broadly directed and sustained CD8+ T cell responses, while a universal picture of the CD8+ T cell response in acute persistent HCV infection has not yet been defined.
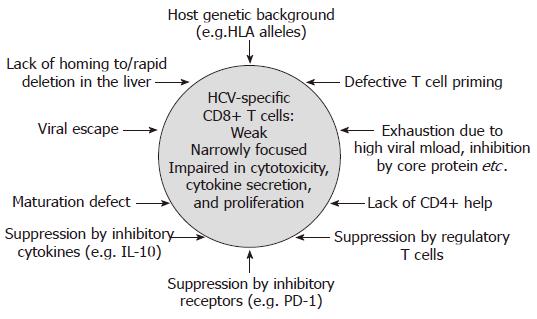
In contrast to acute resolving infection, CD8+ T cell responses are usually weak or even absent in chronic HCV infection, targeting only few epitopes[14-22] (Figure 1B). In this context, it is important to point out that at least in some chronically infected patients, the CD8+ T cell response targets several epitopes[17,18,22]. Importantly, however, these HCV-specific CD8+ T cells display functional impairments, including reduced cytotoxicity, reduced secretion of antiviral cytokines such as IFN-γ, and a reduced proliferative capacity[23-25]. In addition, many CD8+ T cell responses do not target a present antigen, but rather a historical antigen due to viral escape (see below). Different mechanisms which might be involved in the failure of the HCV-specific CD8+ T cell response in persistent infection will be discussed in the following (Figure 2).
As discussed above, some patients with chronic HCV infection lack strong and multispecific CD8+ T cell responses, however, it is difficult to distinguish if virus-specific CD8+ T cell responses were not primed initially (primary CD8+ T cell failure) or responses were primed, but vanished quickly (CD8+ T cell exhaustion). Results obtained from the early phase of acute HCV infection in chimpanzees and in health care workers infected through needle stick exposure support the hypothesis that CD8+ T cells are not primed at least in some patients with acute persisting HCV infection[2,5,6]. In a prospective longitudinal study of young iv drug users, however, CD8+ T cell exhaustion was indeed demonstrated for at least one targeted epitope in each subject developing chronic infection[4].
An impaired priming of HCV-specific CD8+ T cells might be mediated by numeric and functional impairments of antigen-presenting cells, e.g. macrophages and dendritic cells; however, this topic remains controversial[26-35].
CD8+ T cell exhaustion might be explained by general as well as HCV-specific mechanisms. Of note, it has been demonstrated in the lymphocytic choriomeningitis virus (LCMV) mouse model that high viral loads lead to an unresponsive state of virus-specific CD8+ T cells, downregulation of T cell receptors, and finally physical deletion of virus-specific CD8+ T cells[36-39]. Recent data indicate that the inhibitory receptor PD-1 might be involved in this process (see below), and it has been postulated that the detrimental effect of high viral load may not only apply in LCMV infection, but in different viral infections including HCV infection. Regarding HCV-specific mechanisms of CD8+ T cell exhaustion, the core protein has been reported to impair CD8+ T cell activation, e.g. through interaction with membrane-bound complement receptor gC1qR[40-42].
While CD8+ T cells are considered the major effector cells against viral pathogens, the successful elimination of HCV might be highly dependent on sufficient CD4+ T cell help. Indeed, it has been demonstrated in the LCMV mouse model that CD4+ T cell help is needed to sustain cytotoxic CD8+ T cell responses during chronic viral infections[43]. In chronic HCV infection, however, CD4+ T cell responses are very weak or even absent and functionally impaired, e.g. secrete low amounts of IL-2[44,45]. Findings in the chimpanzee model support the central role of CD4+ help in CD8+ T cell mediated viral clearance. When CD4+ T cells were depleted by neutralizing antibodies prior to viral re-challenge, HCV viremia was prolonged, CD8+ escape variants were selected and HCV finally persisted[46]. Consistent with this concept, HCV-specific CD8+ T cell responses were seen almost exclusively in the face of a strong CD4+ T cell response in a study of acutely HCV infected patients[1]. A recent study demonstrated that the outcome of acute HCV infection was associated with efficient virus-specific CD4+ T cell responses. In this study, however, HCV-specific CD8+ T cell responses were induced irrespective of virological outcome or HCV-specific CD4+ T cell responses[13].
In the last few years, the concept of regulatory T cells has undergone a comeback and different types of regulatory T cells have been characterized in different clinical settings. In HCV infection, the role of CD4+CD25+ Foxp3+ regulatory T cells as well as IL-10 producing CD8+ T cells has been defined. In chronically HCV infected patients, CD4+CD25+ T cells have been found in a higher frequency compared to individuals with resolved HCV infection or healthy controls[47-49]. These regulatory T cells suppress the proliferation as well as interferon-gamma secretion of virus-specific CD8+ T cells in vitro. The suppression by CD4+CD25+ T cells was cell-cell contact dependent[47,48], it was independent of suppressive cytokines such as IL-10 and TGF-β in some but not all studies[47,48,50]. Interestingly, the suppression was not restricted to HCV-specific CD8+ T cells, but also included CD8+ T cells specific for other viruses, such as EBV or influenza[47,50]. However, specificity in vivo might be mediated by the enrichment of CD4+CD25+ T cells in the liver[51]. While CD4+CD25+ T cells might limit immunopathology in the chronic phase of HCV infection[52], it has been suggested that they may facilitate viral persistence in the acute phase of infection. However, studies in larger cohorts of patients with acute HCV infection have not yet been reported. The induction of CD4+CD25+ regulatory T cells is still little characterized, however, they could be induced by certain HCV peptides from peripheral blood mononuclear cells (PBMCs) from HCV-infected, but not healthy individuals in vitro[53].
Another type of regulatory T cells in HCV infection is virus-specific regulatory CD8+ T cells that express high levels of IL-10. These regulatory T cells have been detected in the liver of HCV-infected individuals; they could be expanded upon stimulation with HCV epitope peptides and their suppression of virus-specific CD8+ effector T cells could be blocked by neutralizing IL-10 antibodies[54]. This virus-specific regulatory T cell population might have an important role in the prevention of liver damage during chronic HCV infection[55].
The spectrum of regulatory T cells involved in HCV infection may further expand, since we recently described the induction of regulatory CD8+ T cells from the PBMC of HCV-infected patients which also expressed high levels of FoxP3 and CD25[56]. A comprehensive review on the different types of regulatory T cells in HCV and HBV infection by Billerbeck et al is also included in this issue of WJG.
The inhibitory receptor PD-1 (“programmed cell death 1”) has been demonstrated to be a strong marker for exhausted virus-specific CD8+ T cells in the LCMV mouse model. The antibody-mediated blockade of the interaction between PD-1 and its ligand PD-L1 led to the restoration of cytokine secretion, proliferation, and cytotoxicity by the exhausted virus-specific CD8+ T cells and a substantial reduction in viral load[57]. Similar roles of PD-1 have been shown in human chronic viral infections[58] such as HIV[59], HBV[60,61], and HCV. In the acute phase of HCV infection, similar to LCMV infection, PD-1 is up-regulated on HCV-specific CD8+ T cells independent of outcome. However, in individuals with resolving infection PD-1 expression decreases soon, while in patients with a chronic course of infection, HCV-specific CD8+ T cells remain PD-1 positive[62]. This finding is in parallel with the “stunned” phenotype of HCV-specific CD8+ T cells in the early acute phase of infection, which is restored in resolving infection but remains in persisting infection[2,3,24]. In chronic HCV infection, HCV-specific CD8+ T cells in the peripheral blood[63] as well as liver[64] have been shown to express high levels of PD-1. Blockade of PD-1/ PD-L1 interaction by antibodies restored cytokine production and proliferation of the exhausted CD8+ T cells from acute and chronic infection in vitro.
It is important to note, however, that the antibody-mediated blockade of the PD-1/PD-L1 pathway in chronically LCMV-infected mice did not result in viral clearance although a significant reduction of viral load was achieved. Even more importantly, PD-L1-/- mice died due to immunopathologic damage after infection with a LCMV strain usually establishing persistent infection[57]. These findings indicate that a subtle balance in the blockade of the PD-1/PD-L1 pathway must be granted before it can be applied in the clinics.
Two recent reports on the role of IL-10 in the dysfunction of virus-specific T cells and viral persistence gained much attention in the field. These reports showed that in mice with persistent LCMV infection, IL-10 was highly up-regulated early in infection, which was associated with the dysfunction of virus-specific CD4+ and CD8+ T cells. The blockade of the IL-10/IL-10 receptor (IL-10R) pathway by a genetic approach or by an anti-IL-10R antibody early in infection, however, led to the restoration of T cell function and to clearance of infection[65-67].
Although these reports definitely point towards an important general mechanism of T cell dysfunction, a role of IL-10 in HCV infection has been postulated before, and IL-10 therapy has even been tested in clinical trials in HCV infected patients. Indeed, many reports showed an association of IL-10 polymorphisms and outcome, disease progression, or response to antiviral therapy of HCV infection[68-74], while other studies failed to confirm these data[75-79]. Clinical trials with administration of recombinant IL-10 to patients with chronic HCV infection who had failed antiviral therapy with interferon-alpha led to a decrease in transaminases and histological disease progression; however, viral titers strongly increased in some IL-10 treated patients[80,81]. This indicates that IL-10 might not only mediate viral dysfunction and thus facilitate viral persistence in acute infection, but may also reduce immunopathology in the chronic phase of infection. In this context, it is important to point out, however, that IL-10/ IL-10R blockade in the LCMV mouse model did not result in severe immunopathology[65,66].
The exact mechanism of HCV-induced up-regulation of IL-10 remains elusive. Some groups have reported induction of IL-10 production by monocytes[82] or natural killer (NK) cells[83] through core[74,84], non-structural protein 3[84], or 4[82]. More intriguingly, HCV-specific CD8+ T cells with regulatory properties which produce IL-10 have been described in the liver of chronically HCV infected patients[54]. These IL-10 producing intrahepatic CD8+ T cells were associated with mild inflammation and low progression of fibrosis in liver histology[55], once more suggesting that IL-10 may protect from immunopathology in chronic HCV infection. Blockade of the IL-10 pathway by anti-IL10R antibodies in vitro led to increased HCV-specific CD4+ T cell responses[85]. In addition, antiviral therapy led to reduced production of IL-10 by virus-specific T cells in patients with chronic HCV infection[86]. A direct inhibition of the IL-10 pathway, however, needs further careful evaluation in additional animal models before it can be transferred to men.
HCV is a RNA virus with an enormous replication rate (approximately 1012 virions per day) with a RNA-dependent RNA polymerase that lacks a proofreading function. Therefore, multiple viral variants, called quasispecies, circulate in a single individual. It has been suggested that the selection of viral variants escaping from CD8+ T cell responses might facilitate the persistence of HCV infection. Indeed, the first evidence for viral escape in HCV infection came from chronically infected patients[87] and experimentally infected chimpanzees[88,89]. Chronically infected patients harbored variant viral sequences in targeted epitopes which were non-immuno-genic and not cross-reactive with the prototype peptides. These viral escape mutations remained fixed over a follow-up time of up to four years, indicating that escape mutations occur early in infection[87]. In the chimpanzee model, it could further be demonstrated that viral escape mutations occurred during the first 16 wk of infection and were associated with a chronic course of infection[89].
Important additional information came from studies in acutely infected patients[90-92] as well as population-based approaches[90,93]. In these studies, viral escape from CD8+ T cell responses was demonstrated in patients developing persistent infection[90-92], but not in individuals with resolving infection[91,92]. Interestingly, many mutations outside of targeted CD8+ T cell epitopes represented conversion to consensus[91], and the transmission of an HLA-B8 associated escape mutation to an HLA-B8 negative subject resulted in rapid reversion of the mutation[90]. These results were supported by a study in a well-defined cohort of Irish women accidentally infected with HCV from a single source more than 20 years ago. In this unique cohort, amino acid substitutions in known epitopes were directed away from consensus in women having the HLA allele associated with that epitope, and toward consensus in those lacking the allele[93]. These findings are in agreement with the concept of viral fitness cost, indicating that viral escape mutations are often associated with a reduced replicative capacity of the virus[94]. In the absence of the T cell pressure, e.g. upon transmission to an individual negative for the restricting HLA allele, the virus reverts to consensus and thus regains its full replicative capacity. This phenomenon has been analyzed in more detail in the background of an immunodominant HLA-A2 restricted epitope, identifying that certain amino acid residue substitutions abolish HLA binding without strongly influencing viral replication, while some substitutions lead to a strong reduction of viral fitness[95]. Importantly, there might be some CD8+ T cell epitopes which are not affected by viral escape due to high functional constraints. For example, we have recently identified an HLA-A26 restricted epitope located at the NS5A/5B cleavage site which was targeted in all studied HLA-A26+ patients (3/3) with acute HCV infection and a significant number of patients with chronic HCV infection (3/15). However, the epitope sequence was highly conserved in HLA-A26 positive and negative patients, indicating that viral escape did not occur in this functionally constrained region[96].
Based on the finding that immunodominant CD8+ T cell epitopes leave their footprint in viral sequences in chronic HCV infection[90], viral genome sequencing studies were performed in order to identify footprints of additional potential CD8+ T cell epitopes[97,98]. In addition to previously defined epitopes, these studies identified HLA allele dependent polymorphisms and thus candidate CD8+ T cell epitopes. Importantly, the strongest association with any HLA allele in the study by Timm et al was found for HLA-B27 in a region that was shown to contain an immunodominant HLA-B27 restricted CD8+ T cell epitope by an independent study in another patient cohort[99].
There are different molecular mechanisms by which a certain mutation escapes from the CD8+ T cell response. Especially those mutations located at the HLA binding anchors, usually P2 and the C-terminal amino acid, lead to the interruption of the peptide binding to the HLA molecule. Mutations in the center of the epitope, in contrast, are more likely to interfere with T cell receptor (TCR) recognition[95]. Mutations in the flanking region, however, prevent proteasomal epitope processing[90,100,101].
The determinants of viral escape are less understood. In the chimpanzee model of HCV, it has been shown that upon depletion of CD4+ T cells in the acute phase of infection viral escape from the CD8+ T cell response occurs and is associated with a persistent course of infection[46]. This finding has led to the hypothesis that viral escape is caused by insufficient CD4+ help. Other studies indicate that a limited T cell receptor (TCR) diversity might be responsible for viral escape[102]. Of note, viral escape does not occur in the context of dysfunctional CD8+ T cell responses[103]. The strong association between HLA-B27 and viral escape within an immunodominant HLA-B27 restricted epitope as well as the suggestion that escape variant epitopes might preferentially be restricted by HLA-B alleles indicates that the restricting HLA allele background also plays an important role in determining viral escape[97-99].
Experimentally HCV infected chimpanzees which progressed to viral persistence without temporary viral control lacked virus-specific CD8+ T cell responses in the liver despite of detectable responses in the peripheral blood[2]. This finding led to the tempting hypothesis that the failure of the virus-specific CD8+ T cell response might be caused by an insufficient homing to the primary location of infection, the liver. However, in chronically HCV infected patients virus-specific CD8+ T cells are detectable and even enriched in the liver[22,25,104-108]. In a comprehensive study comparing the overall breath and vigor of CD8+ T cell responses in the peripheral blood and liver of chronically HCV infected patient’s, we found that virus-specific CD8+ T cell responses were strongly enriched in the liver. Many responses were only detectable in the liver; however, few responses were limited to the peripheral blood (Neumann-Haefelin et al, unpublished results). Therefore, it is possible that a defective homing of HCV-specific CD8+ T cells or their rapid deletion in the liver also contributes to T cell failure and viral persistence in a subset of patients.
CD8+ T cells recognize antigens presented by human leukocyte antigen (HLA) class I molecules. It has, therefore, been suggested that different HLA class I alleles are associated with differential outcomes of HCV infection, e.g. viral clearance versus persistence[109]. Analysis of the role of HLA alleles in viral infections are hindered by multiple factors, including the wide polymorphism of HLA alleles, their association with other genetic characteristics e.g. in certain racial backgrounds (founder effect)[110], and the variability of viral strains (genotypes, quasispecies etc.). However, an Irish cohort of women accidentally infected with HCV (genotype 1b) from a single source more then 20 years ago, represents a homogeneous group in which the role of HLA alleles in the outcome of HCV infection could be studied[111]. Importantly, the HLA class I alleles A3, B27 and Cw*01 were significantly associated with viral clearance, while B8 was associated with viral persistence. Interestingly, the strongest protective effect was observed for HLA-B27: 80% (12/15) of B27 positive women were able to clear the infection spontaneously, while only a minority developed chronic infection. We recently identified an immunodominant HLA-B27 restricted HCV-specific CD8+ T cell epitope, which was targeted in the vast majority (5/6) of B27 positive Irish women who had cleared the infection[99]. Of note, such a clear dominance of a single epitope-specific CD8+ T cell response has not been described for any other HLA allele in HCV infection. In chronically infected patients, still a remarkable percentage of patients (3/8) recognized the epitope. However, most B27 positive chronically infected patients had evidence of viral escape within the otherwise conserved viral region containing this epitope. Thus, a single immunodominant HLA-B27 restricted CD8+ T cell epitopes might mediate both, clearance of HCV infection in the majority of B27 positive individuals, and viral evolution associated with viral persistence in a minority of individuals.
Strikingly, a very similar frequency of viral escape variation was demonstrated within an immunodominant HLA-B8 restricted CD8+ T cell epitopes[90]. This indicates that in the background of both, a protective HLA allele (B27) as well as a detrimental HLA allele (B8) the principle mechanisms of CD8+ T cell failure might be the same. More precise details such as viral fitness cost associated with the respective escape variation[94,95], T cell receptor (TCR) diversity[102] or heterologous immunity[112,113] may play an additional critical role in the definition of a protective HLA allele.
Two other population studies in more heterogeneous cohorts showed an association between HLA-B57 and HCV clearance in Caucasian as well as African Americans and West Africans[114,115]. Interestingly, HLA-B27 and HLA-B57 have also been shown to be protective in HIV infection, being strongly associated with low viral titers, low CD4+ T cell decline, and long-term non-progression of the disease[116]. Thus, a picture emerges that the same HLA alleles may confer protection in different clinical infections, indicating that similar mechanisms of viral control and disease progression apply in these infections. A better understanding of the host-virus interactions leading to different clinical outcomes of HCV infection will be important not only to understand the mechanisms of viral clearance and persistence, but also for the development of new antiviral vaccine strategies.
CD8+ T cells are generally thought to be the major effectors in viral infections; however, multiple host and viral mechanisms contribute to the failure of antiviral CD8+ T cell responses and viral persistence in the majority of HCV infected patients. In the last few years compelling progress has been achieved in the understanding of these mechanisms (compare with[117]). These findings are not only important for the development of successful immunoprophylactic approaches, but may also be more directly adopted for immunotherapeutic interventions.
| 1. | Grüner NH, Gerlach TJ, Jung MC, Diepolder HM, Schirren CA, Schraut WW, Hoffmann R, Zachoval R, Santantonio T, Cucchiarini M. Association of hepatitis C virus-specific CD8+ T cells with viral clearance in acute hepatitis C. J Infect Dis. 2000;181:1528-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 278] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 2. | Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395-1406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 925] [Cited by in RCA: 906] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 3. | Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, Robbins G, Phillips R, Klenerman P, Walker BD. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499-1512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1003] [Cited by in RCA: 1004] [Article Influence: 38.6] [Reference Citation Analysis (1)] |
| 4. | Cox AL, Mosbruger T, Lauer GM, Pardoll D, Thomas DL, Ray SC. Comprehensive analyses of CD8+ T cell responses during longitudinal study of acute human hepatitis C. Hepatology. 2005;42:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 186] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Thimme R, Bukh J, Spangenberg HC, Wieland S, Pemberton J, Steiger C, Govindarajan S, Purcell RH, Chisari FV. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc Natl Acad Sci USA. 2002;99:15661-15668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 479] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 6. | Cooper S, Erickson AL, Adams EJ, Kansopon J, Weiner AJ, Chien DY, Houghton M, Parham P, Walker CM. Analysis of a successful immune response against hepatitis C virus. Immunity. 1999;10:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 591] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 7. | Takaki A, Wiese M, Maertens G, Depla E, Seifert U, Liebetrau A, Miller JL, Manns MP, Rehermann B. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat Med. 2000;6:578-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 560] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 8. | Mehta SH, Cox A, Hoover DR, Wang XH, Mao Q, Ray S, Strathdee SA, Vlahov D, Thomas DL. Protection against persistence of hepatitis C. Lancet. 2002;359:1478-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 329] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 9. | Bassett SE, Guerra B, Brasky K, Miskovsky E, Houghton M, Klimpel GR, Lanford RE. Protective immune response to hepatitis C virus in chimpanzees rechallenged following clearance of primary infection. Hepatology. 2001;33:1479-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 174] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Weiner AJ, Paliard X, Selby MJ, Medina-Selby A, Coit D, Nguyen S, Kansopon J, Arian CL, Ng P, Tucker J. Intrahepatic genetic inoculation of hepatitis C virus RNA confers cross-protective immunity. J Virol. 2001;75:7142-7148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Major ME, Mihalik K, Puig M, Rehermann B, Nascimbeni M, Rice CM, Feinstone SM. Previously infected and recovered chimpanzees exhibit rapid responses that control hepatitis C virus replication upon rechallenge. J Virol. 2002;76:6586-6595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 142] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Urbani S, Amadei B, Fisicaro P, Tola D, Orlandini A, Sacchelli L, Mori C, Missale G, Ferrari C. Outcome of acute hepatitis C is related to virus-specific CD4 function and maturation of antiviral memory CD8 responses. Hepatology. 2006;44:126-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 149] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Kaplan DE, Sugimoto K, Newton K, Valiga ME, Ikeda F, Aytaman A, Nunes FA, Lucey MR, Vance BA, Vonderheide RH. Discordant role of CD4 T-cell response relative to neutralizing antibody and CD8 T-cell responses in acute hepatitis C. Gastroenterology. 2007;132:654-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Battegay M, Fikes J, Di Bisceglie AM, Wentworth PA, Sette A, Celis E, Ching WM, Grakoui A, Rice CM, Kurokohchi K. Patients with chronic hepatitis C have circulating cytotoxic T cells which recognize hepatitis C virus-encoded peptides binding to HLA-A2.1 molecules. J Virol. 1995;69:2462-2470. [PubMed] |
| 15. | Cerny A, McHutchison JG, Pasquinelli C, Brown ME, Brothers MA, Grabscheid B, Fowler P, Houghton M, Chisari FV. Cytotoxic T lymphocyte response to hepatitis C virus-derived peptides containing the HLA A2.1 binding motif. J Clin Invest. 1995;95:521-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 255] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 16. | Chang KM, Thimme R, Melpolder JJ, Oldach D, Pemberton J, Moorhead-Loudis J, McHutchison JG, Alter HJ, Chisari FV. Differential CD4(+) and CD8(+) T-cell responsiveness in hepatitis C virus infection. Hepatology. 2001;33:267-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 267] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 17. | Lauer GM, Barnes E, Lucas M, Timm J, Ouchi K, Kim AY, Day CL, Robbins GK, Casson DR, Reiser M. High resolution analysis of cellular immune responses in resolved and persistent hepatitis C virus infection. Gastroenterology. 2004;127:924-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 229] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 18. | Lauer GM, Ouchi K, Chung RT, Nguyen TN, Day CL, Purkis DR, Reiser M, Kim AY, Lucas M, Klenerman P. Comprehensive analysis of CD8(+)-T-cell responses against hepatitis C virus reveals multiple unpredicted specificities. J Virol. 2002;76:6104-6113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 148] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Rehermann B, Chang KM, McHutchison JG, Kokka R, Houghton M, Chisari FV. Quantitative analysis of the peripheral blood cytotoxic T lymphocyte response in patients with chronic hepatitis C virus infection. J Clin Invest. 1996;98:1432-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 230] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 20. | Wong DK, Dudley DD, Dohrenwend PB, Lauer GM, Chung RT, Thomas DL, Walker BD. Detection of diverse hepatitis C virus (HCV)-specific cytotoxic T lymphocytes in peripheral blood of infected persons by screening for responses to all translated proteins of HCV. J Virol. 2001;75:1229-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Koziel MJ, Dudley D, Afdhal N, Choo QL, Houghton M, Ralston R, Walker BD. Hepatitis C virus (HCV)-specific cytotoxic T lymphocytes recognize epitopes in the core and envelope proteins of HCV. J Virol. 1993;67:7522-7532. [PubMed] |
| 22. | Koziel MJ, Dudley D, Afdhal N, Grakoui A, Rice CM, Choo QL, Houghton M, Walker BD. HLA class I-restricted cytotoxic T lymphocytes specific for hepatitis C virus. Identification of multiple epitopes and characterization of patterns of cytokine release. J Clin Invest. 1995;96:2311-2321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 231] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 23. | Wedemeyer H, He XS, Nascimbeni M, Davis AR, Greenberg HB, Hoofnagle JH, Liang TJ, Alter H, Rehermann B. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J Immunol. 2002;169:3447-3458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 494] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 24. | Urbani S, Boni C, Missale G, Elia G, Cavallo C, Massari M, Raimondo G, Ferrari C. Virus-specific CD8+ lymphocytes share the same effector-memory phenotype but exhibit functional differences in acute hepatitis B and C. J Virol. 2002;76:12423-12434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 144] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Spangenberg HC, Viazov S, Kersting N, Neumann-Haefelin C, McKinney D, Roggendorf M, von Weizsäcker F, Blum HE, Thimme R. Intrahepatic CD8+ T-cell failure during chronic hepatitis C virus infection. Hepatology. 2005;42:828-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Bain C, Fatmi A, Zoulim F, Zarski JP, Trépo C, Inchauspé G. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology. 2001;120:512-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 308] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 27. | Lee CH, Choi YH, Yang SH, Lee CW, Ha SJ, Sung YC. Hepatitis C virus core protein inhibits interleukin 12 and nitric oxide production from activated macrophages. Virology. 2001;279:271-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Sarobe P, Lasarte JJ, Casares N, López-Díaz de Cerio A, Baixeras E, Labarga P, García N, Borrás-Cuesta F, Prieto J. Abnormal priming of CD4(+) T cells by dendritic cells expressing hepatitis C virus core and E1 proteins. J Virol. 2002;76:5062-5070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 117] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 29. | Anthony DD, Yonkers NL, Post AB, Asaad R, Heinzel FP, Lederman MM, Lehmann PV, Valdez H. Selective impairments in dendritic cell-associated function distinguish hepatitis C virus and HIV infection. J Immunol. 2004;172:4907-4916. [RCA] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Kanto T, Hayashi N, Takehara T, Tatsumi T, Kuzushita N, Ito A, Sasaki Y, Kasahara A, Hori M. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J Immunol. 1999;162:5584-5591. [PubMed] |
| 31. | Della Bella S, Crosignani A, Riva A, Presicce P, Benetti A, Longhi R, Podda M, Villa ML. Decrease and dysfunction of dendritic cells correlate with impaired hepatitis C virus-specific CD4+ T-cell proliferation in patients with hepatitis C virus infection. Immunology. 2007;121:283-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Longman RS, Talal AH, Jacobson IM, Albert ML, Rice CM. Presence of functional dendritic cells in patients chronically infected with hepatitis C virus. Blood. 2004;103:1026-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Rollier C, Drexhage JA, Verstrepen BE, Verschoor EJ, Bontrop RE, Koopman G, Heeney JL. Chronic hepatitis C virus infection established and maintained in chimpanzees independent of dendritic cell impairment. Hepatology. 2003;38:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Larsson M, Babcock E, Grakoui A, Shoukry N, Lauer G, Rice C, Walker C, Bhardwaj N. Lack of phenotypic and functional impairment in dendritic cells from chimpanzees chronically infected with hepatitis C virus. J Virol. 2004;78:6151-6161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Piccioli D, Tavarini S, Nuti S, Colombatto P, Brunetto M, Bonino F, Ciccorossi P, Zorat F, Pozzato G, Comar C. Comparable functions of plasmacytoid and monocyte-derived dendritic cells in chronic hepatitis C patients and healthy donors. J Hepatol. 2005;42:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 912] [Cited by in RCA: 980] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 37. | Gallimore A, Glithero A, Godkin A, Tissot AC, Plückthun A, Elliott T, Hengartner H, Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med. 1998;187:1383-1393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 594] [Cited by in RCA: 644] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 38. | Ou R, Zhou S, Huang L, Moskophidis D. Critical role for alpha/beta and gamma interferons in persistence of lymphocytic choriomeningitis virus by clonal exhaustion of cytotoxic T cells. J Virol. 2001;75:8407-8423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 152] [Article Influence: 6.1] [Reference Citation Analysis (8)] |
| 39. | Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205-2213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1578] [Cited by in RCA: 1613] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 40. | Kittlesen DJ, Chianese-Bullock KA, Yao ZQ, Braciale TJ, Hahn YS. Interaction between complement receptor gC1qR and hepatitis C virus core protein inhibits T-lymphocyte proliferation. J Clin Invest. 2000;106:1239-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 238] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 41. | Yao ZQ, Nguyen DT, Hiotellis AI, Hahn YS. Hepatitis C virus core protein inhibits human T lymphocyte responses by a complement-dependent regulatory pathway. J Immunol. 2001;167:5264-5272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 118] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 42. | Yao ZQ, Shata MT, Tricoche N, Shan MM, Brotman B, Pfahler W, Hahn YS, Prince AM. gC1qR expression in chimpanzees with resolved and chronic infection: potential role of HCV core/gC1qR-mediated T cell suppression in the outcome of HCV infection. Virology. 2006;346:324-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056-8063. [PubMed] |
| 44. | Day CL, Seth NP, Lucas M, Appel H, Gauthier L, Lauer GM, Robbins GK, Szczepiorkowski ZM, Casson DR, Chung RT. Ex vivo analysis of human memory CD4 T cells specific for hepatitis C virus using MHC class II tetramers. J Clin Invest. 2003;112:831-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 220] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 45. | Semmo N, Day CL, Ward SM, Lucas M, Harcourt G, Loughry A, Klenerman P. Preferential loss of IL-2-secreting CD4+ T helper cells in chronic HCV infection. Hepatology. 2005;41:1019-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 46. | Grakoui A, Shoukry NH, Woollard DJ, Han JH, Hanson HL, Ghrayeb J, Murthy KK, Rice CM, Walker CM. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 632] [Cited by in RCA: 645] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 47. | Boettler T, Spangenberg HC, Neumann-Haefelin C, Panther E, Urbani S, Ferrari C, Blum HE, von Weizsäcker F, Thimme R. T cells with a CD4+CD25+ regulatory phenotype suppress in vitro proliferation of virus-specific CD8+ T cells during chronic hepatitis C virus infection. J Virol. 2005;79:7860-7867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 331] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 48. | Cabrera R, Tu Z, Xu Y, Firpi RJ, Rosen HR, Liu C, Nelson DR. An immunomodulatory role for CD4(+)CD25(+) regulatory T lymphocytes in hepatitis C virus infection. Hepatology. 2004;40:1062-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 434] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 49. | Sugimoto K, Ikeda F, Stadanlick J, Nunes FA, Alter HJ, Chang KM. Suppression of HCV-specific T cells without differential hierarchy demonstrated ex vivo in persistent HCV infection. Hepatology. 2003;38:1437-1448. [PubMed] |
| 50. | Rushbrook SM, Ward SM, Unitt E, Vowler SL, Lucas M, Klenerman P, Alexander GJ. Regulatory T cells suppress in vitro proliferation of virus-specific CD8+ T cells during persistent hepatitis C virus infection. J Virol. 2005;79:7852-7859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 227] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 51. | Ward SM, Fox BC, Brown PJ, Worthington J, Fox SB, Chapman RW, Fleming KA, Banham AH, Klenerman P. Quantification and localisation of FOXP3+ T lymphocytes and relation to hepatic inflammation during chronic HCV infection. J Hepatol. 2007;47:316-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 52. | Bolacchi F, Sinistro A, Ciaprini C, Demin F, Capozzi M, Carducci FC, Drapeau CM, Rocchi G, Bergamini A. Increased hepatitis C virus (HCV)-specific CD4+CD25+ regulatory T lymphocytes and reduced HCV-specific CD4+ T cell response in HCV-infected patients with normal versus abnormal alanine aminotransferase levels. Clin Exp Immunol. 2006;144:188-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 53. | Li S, Jones KL, Woollard DJ, Dromey J, Paukovics G, Plebanski M, Gowans EJ. Defining target antigens for CD25+ FOXP3 + IFN-gamma- regulatory T cells in chronic hepatitis C virus infection. Immunol Cell Biol. 2007;85:197-204. [PubMed] |
| 54. | Accapezzato D, Francavilla V, Paroli M, Casciaro M, Chircu LV, Cividini A, Abrignani S, Mondelli MU, Barnaba V. Hepatic expansion of a virus-specific regulatory CD8(+) T cell population in chronic hepatitis C virus infection. J Clin Invest. 2004;113:963-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 228] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 55. | Abel M, Sène D, Pol S, Bourlière M, Poynard T, Charlotte F, Cacoub P, Caillat-Zucman S. Intrahepatic virus-specific IL-10-producing CD8 T cells prevent liver damage during chronic hepatitis C virus infection. Hepatology. 2006;44:1607-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 56. | Billerbeck E, Blum HE, Thimme R. Parallel expansion of human virus-specific FoxP3- effector memory and de novo-generated FoxP3+ regulatory CD8+ T cells upon antigen recognition in vitro. J Immunol. 2007;179:1039-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 57. | Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2815] [Cited by in RCA: 3244] [Article Influence: 154.5] [Reference Citation Analysis (0)] |
| 58. | Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1019] [Cited by in RCA: 1237] [Article Influence: 65.1] [Reference Citation Analysis (0)] |
| 59. | Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1976] [Cited by in RCA: 2189] [Article Influence: 109.5] [Reference Citation Analysis (0)] |
| 60. | Isogawa M, Furuichi Y, Chisari FV. Oscillating CD8(+) T cell effector functions after antigen recognition in the liver. Immunity. 2005;23:53-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 156] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 61. | Boettler T, Panther E, Bengsch B, Nazarova N, Spangenberg HC, Blum HE, Thimme R. Expression of the interleukin-7 receptor alpha chain (CD127) on virus-specific CD8+ T cells identifies functionally and phenotypically defined memory T cells during acute resolving hepatitis B virus infection. J Virol. 2006;80:3532-3540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 164] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 62. | Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398-11403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 460] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 63. | Penna A, Pilli M, Zerbini A, Orlandini A, Mezzadri S, Sacchelli L, Missale G, Ferrari C. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology. 2007;45:588-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 232] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 64. | Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, Hanson HL, Steinberg JP, Masopust D, Wherry EJ. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007;81:2545-2553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 386] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 65. | Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301-1309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 766] [Cited by in RCA: 762] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 66. | Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, von Herrath MG. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med. 2006;203:2461-2472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 421] [Cited by in RCA: 452] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 67. | Blackburn SD, Wherry EJ. IL-10, T cell exhaustion and viral persistence. Trends Microbiol. 2007;15:143-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 68. | Yee LJ, Tang J, Gibson AW, Kimberly R, Van Leeuwen DJ, Kaslow RA. Interleukin 10 polymorphisms as predictors of sustained response in antiviral therapy for chronic hepatitis C infection. Hepatology. 2001;33:708-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 135] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 69. | Lio D, Caruso C, Di Stefano R, Colonna Romano G, Ferraro D, Scola L, Crivello A, Licata A, Valenza LM, Candore G. IL-10 and TNF-alpha polymorphisms and the recovery from HCV infection. Hum Immunol. 2003;64:674-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 70. | Knapp S, Hennig BJ, Frodsham AJ, Zhang L, Hellier S, Wright M, Goldin R, Hill AV, Thomas HC, Thursz MR. Interleukin-10 promoter polymorphisms and the outcome of hepatitis C virus infection. Immunogenetics. 2003;55:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 119] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 71. | Mangia A, Santoro R, Piattelli M, Pazienza V, Grifa G, Iacobellis A, Andriulli A. IL-10 haplotypes as possible predictors of spontaneous clearance of HCV infection. Cytokine. 2004;25:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 72. | Oleksyk TK, Thio CL, Truelove AL, Goedert JJ, Donfield SM, Kirk GD, Thomas DL, O'Brien SJ, Smith MW. Single nucleotide polymorphisms and haplotypes in the IL10 region associated with HCV clearance. Genes Immun. 2005;6:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 73. | Paladino N, Fainboim H, Theiler G, Schroder T, Muñoz AE, Flores AC, Galdame O, Fainboim L. Gender susceptibility to chronic hepatitis C virus infection associated with interleukin 10 promoter polymorphism. J Virol. 2006;80:9144-9150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 74. | Aborsangaya KB, Dembinski I, Khatkar S, Alphonse MP, Nickerson P, Rempel JD. Impact of aboriginal ethnicity on HCV core-induced IL-10 synthesis: interaction with IL-10 gene polymorphisms. Hepatology. 2007;45:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 75. | Constantini PK, Wawrzynowicz-Syczewska M, Clare M, Boron-Kaczmarska A, McFarlane IG, Cramp ME, Donaldson PT. Interleukin-1, interleukin-10 and tumour necrosis factor-alpha gene polymorphisms in hepatitis C virus infection: an investigation of the relationships with spontaneous viral clearance and response to alpha-interferon therapy. Liver. 2002;22:404-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 76. | Barrett S, Collins M, Kenny C, Ryan E, Keane CO, Crowe J. Polymorphisms in tumour necrosis factor-alpha, transforming growth factor-beta, interleukin-10, interleukin-6, interferon-gamma, and outcome of hepatitis C virus infection. J Med Virol. 2003;71:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 77. | Minton EJ, Smillie D, Smith P, Shipley S, McKendrick MW, Gleeson DC, Underwood JC, Cannings C, Wilson AG. Clearance of hepatitis C virus is not associated with single nucleotide polymorphisms in the IL-1, -6, or -10 genes. Hum Immunol. 2005;66:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 78. | Abbott WG, Rigopoulou E, Haigh P, Cooksley H, Mullerova I, Novelli M, Winstanley A, Williams R, Naoumov NV. Single nucleotide polymorphisms in the interferon-gamma and interleukin-10 genes do not influence chronic hepatitis C severity or T-cell reactivity to hepatitis C virus. Liver Int. 2004;24:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 79. | Kusumoto K, Uto H, Hayashi K, Takahama Y, Nakao H, Suruki R, Stuver SO, Ido A, Tsubouchi H. Interleukin-10 or tumor necrosis factor-alpha polymorphisms and the natural course of hepatitis C virus infection in a hyperendemic area of Japan. Cytokine. 2006;34:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 80. | Nelson DR, Lauwers GY, Lau JY, Davis GL. Interleukin 10 treatment reduces fibrosis in patients with chronic hepatitis C: a pilot trial of interferon nonresponders. Gastroenterology. 2000;118:655-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 245] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 81. | Nelson DR, Tu Z, Soldevila-Pico C, Abdelmalek M, Zhu H, Xu YL, Cabrera R, Liu C, Davis GL. Long-term interleukin 10 therapy in chronic hepatitis C patients has a proviral and anti-inflammatory effect. Hepatology. 2003;38:859-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 98] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 82. | Brady MT, MacDonald AJ, Rowan AG, Mills KH. Hepatitis C virus non-structural protein 4 suppresses Th1 responses by stimulating IL-10 production from monocytes. Eur J Immunol. 2003;33:3448-3457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 118] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 83. | De Maria A, Fogli M, Mazza S, Basso M, Picciotto A, Costa P, Congia S, Mingari MC, Moretta L. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur J Immunol. 2007;37:445-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 84. | Dolganiuc A, Kodys K, Kopasz A, Marshall C, Do T, Romics L, Mandrekar P, Zapp M, Szabo G. Hepatitis C virus core and nonstructural protein 3 proteins induce pro- and anti-inflammatory cytokines and inhibit dendritic cell differentiation. J Immunol. 2003;170:5615-5624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 192] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 85. | Rigopoulou EI, Abbott WG, Haigh P, Naoumov NV. Blocking of interleukin-10 receptor--a novel approach to stimulate T-helper cell type 1 responses to hepatitis C virus. Clin Immunol. 2005;117:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 86. | Cramp ME, Rossol S, Chokshi S, Carucci P, Williams R, Naoumov NV. Hepatitis C virus-specific T-cell reactivity during interferon and ribavirin treatment in chronic hepatitis C. Gastroenterology. 2000;118:346-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 234] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 87. | Chang KM, Rehermann B, McHutchison JG, Pasquinelli C, Southwood S, Sette A, Chisari FV. Immunological significance of cytotoxic T lymphocyte epitope variants in patients chronically infected by the hepatitis C virus. J Clin Invest. 1997;100:2376-2385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 241] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 88. | Weiner A, Erickson AL, Kansopon J, Crawford K, Muchmore E, Hughes AL, Houghton M, Walker CM. Persistent hepatitis C virus infection in a chimpanzee is associated with emergence of a cytotoxic T lymphocyte escape variant. Proc Natl Acad Sci USA. 1995;92:2755-2759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 238] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 89. | Erickson AL, Kimura Y, Igarashi S, Eichelberger J, Houghton M, Sidney J, McKinney D, Sette A, Hughes AL, Walker CM. The outcome of hepatitis C virus infection is predicted by escape mutations in epitopes targeted by cytotoxic T lymphocytes. Immunity. 2001;15:883-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 307] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 90. | Timm J, Lauer GM, Kavanagh DG, Sheridan I, Kim AY, Lucas M, Pillay T, Ouchi K, Reyor LL, Schulze zur Wiesch J. CD8 epitope escape and reversion in acute HCV infection. J Exp Med. 2004;200:1593-1604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 242] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 91. | Cox AL, Mosbruger T, Mao Q, Liu Z, Wang XH, Yang HC, Sidney J, Sette A, Pardoll D, Thomas DL. Cellular immune selection with hepatitis C virus persistence in humans. J Exp Med. 2005;201:1741-1752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 234] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 92. | Tester I, Smyk-Pearson S, Wang P, Wertheimer A, Yao E, Lewinsohn DM, Tavis JE, Rosen HR. Immune evasion versus recovery after acute hepatitis C virus infection from a shared source. J Exp Med. 2005;201:1725-1731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 138] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 93. | Ray SC, Fanning L, Wang XH, Netski DM, Kenny-Walsh E, Thomas DL. Divergent and convergent evolution after a common-source outbreak of hepatitis C virus. J Exp Med. 2005;201:1753-1759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 149] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 94. | Altman JD, Feinberg MB. HIV escape: there and back again. Nat Med. 2004;10:229-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 95. | Söderholm J, Ahlén G, Kaul A, Frelin L, Alheim M, Barnfield C, Liljeström P, Weiland O, Milich DR, Bartenschlager R. Relation between viral fitness and immune escape within the hepatitis C virus protease. Gut. 2006;55:266-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 96. | Neumann-Haefelin C, Killinger T, Timm J, Southwood S, McKinney D, Blum HE, Thimme R. Absence of viral escape within a frequently recognized HLA-A26-restricted CD8+ T-cell epitope targeting the functionally constrained hepatitis C virus NS5A/5B cleavage site. J Gen Virol. 2007;88:1986-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 97. | Gaudieri S, Rauch A, Park LP, Freitas E, Herrmann S, Jeffrey G, Cheng W, Pfafferott K, Naidoo K, Chapman R. Evidence of viral adaptation to HLA class I-restricted immune pressure in chronic hepatitis C virus infection. J Virol. 2006;80:11094-11104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 98. | Timm J, Li B, Daniels MG, Bhattacharya T, Reyor LL, Allgaier R, Kuntzen T, Fischer W, Nolan BE, Duncan J. Human leukocyte antigen-associated sequence polymorphisms in hepatitis C virus reveal reproducible immune responses and constraints on viral evolution. Hepatology. 2007;46:339-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 99. | Neumann-Haefelin C, McKiernan S, Ward S, Viazov S, Spangenberg HC, Killinger T, Baumert TF, Nazarova N, Sheridan I, Pybus O. Dominant influence of an HLA-B27 restricted CD8+ T cell response in mediating HCV clearance and evolution. Hepatology. 2006;43:563-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 162] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 100. | Seifert U, Liermann H, Racanelli V, Halenius A, Wiese M, Wedemeyer H, Ruppert T, Rispeter K, Henklein P, Sijts A. Hepatitis C virus mutation affects proteasomal epitope processing. J Clin Invest. 2004;114:250-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 108] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 101. | Kimura Y, Gushima T, Rawale S, Kaumaya P, Walker CM. Escape mutations alter proteasome processing of major histocompatibility complex class I-restricted epitopes in persistent hepatitis C virus infection. J Virol. 2005;79:4870-4876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 102. | Meyer-Olson D, Shoukry NH, Brady KW, Kim H, Olson DP, Hartman K, Shintani AK, Walker CM, Kalams SA. Limited T cell receptor diversity of HCV-specific T cell responses is associated with CTL escape. J Exp Med. 2004;200:307-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 143] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 103. | Urbani S, Amadei B, Cariani E, Fisicaro P, Orlandini A, Missale G, Ferrari C. The impairment of CD8 responses limits the selection of escape mutations in acute hepatitis C virus infection. J Immunol. 2005;175:7519-7529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 104. | Grabowska AM, Lechner F, Klenerman P, Tighe PJ, Ryder S, Ball JK, Thomson BJ, Irving WL, Robins RA. Direct ex vivo comparison of the breadth and specificity of the T cells in the liver and peripheral blood of patients with chronic HCV infection. Eur J Immunol. 2001;31:2388-2394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 105. | He XS, Rehermann B, López-Labrador FX, Boisvert J, Cheung R, Mumm J, Wedemeyer H, Berenguer M, Wright TL, Davis MM. Quantitative analysis of hepatitis C virus-specific CD8(+) T cells in peripheral blood and liver using peptide-MHC tetramers. Proc Natl Acad Sci USA. 1999;96:5692-5697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 308] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 106. | Koziel MJ, Dudley D, Wong JT, Dienstag J, Houghton M, Ralston R, Walker BD. Intrahepatic cytotoxic T lymphocytes specific for hepatitis C virus in persons with chronic hepatitis. J Immunol. 1992;149:3339-3344. [PubMed] |
| 107. | Penna A, Missale G, Lamonaca V, Pilli M, Mori C, Zanelli P, Cavalli A, Elia G, Ferrari C. Intrahepatic and circulating HLA class II-restricted, hepatitis C virus-specific T cells: functional characterization in patients with chronic hepatitis C. Hepatology. 2002;35:1225-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 108. | Wong DK, Dudley DD, Afdhal NH, Dienstag J, Rice CM, Wang L, Houghton M, Walker BD, Koziel MJ. Liver-derived CTL in hepatitis C virus infection: breadth and specificity of responses in a cohort of persons with chronic infection. J Immunol. 1998;160:1479-1488. [PubMed] |
| 109. | Neumann-Haefelin C, Thimme R. Impact of the genetic restriction of virus-specific T-cell responses in hepatitis C virus infection. Genes Immun. 2007;8:181-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 110. | Bhattacharya T, Daniels M, Heckerman D, Foley B, Frahm N, Kadie C, Carlson J, Yusim K, McMahon B, Gaschen B. Founder effects in the assessment of HIV polymorphisms and HLA allele associations. Science. 2007;315:1583-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 214] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 111. | McKiernan SM, Hagan R, Curry M, McDonald GS, Kelly A, Nolan N, Walsh A, Hegarty J, Lawlor E, Kelleher D. Distinct MHC class I and II alleles are associated with hepatitis C viral clearance, originating from a single source. Hepatology. 2004;40:108-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 185] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 112. | Wedemeyer H, Mizukoshi E, Davis AR, Bennink JR, Rehermann B. Cross-reactivity between hepatitis C virus and Influenza A virus determinant-specific cytotoxic T cells. J Virol. 2001;75:11392-11400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 193] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 113. | Kennedy PT, Urbani S, Moses RA, Amadei B, Fisicaro P, Lloyd J, Maini MK, Dusheiko G, Ferrari C, Bertoletti A. The influence of T cell cross-reactivity on HCV-peptide specific human T cell response. Hepatology. 2006;43:602-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 114. | Thio CL, Gao X, Goedert JJ, Vlahov D, Nelson KE, Hilgartner MW, O'Brien SJ, Karacki P, Astemborski J, Carrington M. HLA-Cw*04 and hepatitis C virus persistence. J Virol. 2002;76:4792-4797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 137] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 115. | Chuang WC, Sarkodie F, Brown CJ, Owusu-Ofori S, Brown J, Li C, Navarrete C, Klenerman P, Allain JP. Protective effect of HLA-B57 on HCV genotype 2 infection in a West African population. J Med Virol. 2007;79:724-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 116. | Stephens HA. HIV-1 diversity versus HLA class I polymorphism. Trends Immunol. 2005;26:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 117. | Neumann-Haefelin C, Blum HE, Chisari FV, Thimme R. T cell response in hepatitis C virus infection. J Clin Virol. 2005;32:75-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
S- Editor Ma N L- Editor Rippe RA E- Editor Ma WH