Published online Sep 14, 2007. doi: 10.3748/wjg.v13.i34.4579
Revised: May 10, 2007
Accepted: May 12, 2007
Published online: September 14, 2007
AIM: To evaluate the prognostic value of percentage of 13C-phenylalanine oxidation (13C-PheOx) obtained by 13C-phenylalanine breath test (13C-PheBT) on the survival of patients with chronic liver failure.
METHODS: The hepatic function was determined by standard liver blood tests and the percentage of 13C-PheOx in 118 chronic liver failure patients. The follow-up period was of 64 mo. Survival analysis was performed by the Kaplan-Meier method and variables that were significant (P < 0.10) in univariate analysis and subsequently introduced in a multivariate analysis according to the hazard model proposed by Cox.
RESULTS: Forty-one patients died due to progressive liver failure during the follow-up period. The probability of survival at 12, 24, 36, 48 and 64 mo was 0.88, 0.78, 0.66, 0.57 and 0.19, respectively. Multivariate analysis demonstrated that Child-Pugh classes, age, creatinine and the percentage of 13C-PheOx (HR 0.338, 95% CI: 0.150-0.762, P = 0.009) were independent predictors of survival. When Child-Pugh classes were replaced by all the parameters of the score, only albumin, bilirubin, creatinine, age and the percentage of 13C-PheOx (HR 0.449, 95% CI: 0.206-0.979, P = 0.034) were found to be independent predictors of survival.
CONCLUSION: Percentage of 13C-PheOx obtained by 13C-PheBT is a strong predictor of survival in patients with chronic liver disease.
- Citation: Gallardo-Wong I, Morán S, Rodríguez-Leal G, Castañeda-Romero B, Mera R, Poo J, Uribe M, Dehesa M. Prognostic value of 13C-phenylalanine breath test on predicting survival in patients with chronic liver failure. World J Gastroenterol 2007; 13(34): 4579-4585
- URL: https://www.wjgnet.com/1007-9327/full/v13/i34/4579.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i34.4579
The identification of patients with poor prognosis is of crucial importance, especially since liver transplantation has emerged as an important therapy for patients with advanced cirrhosis[1]. The exact prediction of survival for an individual patient with cirrhosis is not easy. This may be one of the reasons to explain why so many studies have investigated factors which predict survival of these patients[2-4].
In recent years, growing interest has been devoted to the quantitative liver function tests, as they are expected to increase the accuracy of estimating the severity of liver disease; however, several studies on their prognostic value have shown contradictory results[5-8].
C-phenylalanine oxidation (13C-PheOx) is a valuable indicator of liver function. It represents the cytosolic enzyme activity, is a non-invasive test, easy to perform and distinguishes patients with various degrees of liver disease from otherwise healthy persons. Some studies have shown that the severity of liver cirrhosis correlates with the suppression of 13CO2 recovery after a dose of phenylalanine; other studies have reported that as liver function worsens, as defined by the Child-Pugh (CP) score, so does the phenylalanine metabolism[9-14]. Nowadays, the CP score is still the most widely used tool to estimate the severity of liver disease in patients with cirrhosis and to predict survival.
The prognostic value of 13C-phenylalnine breath test (13C-PheBT) for survival in patients with chronic liver disease is yet to be established. Therefore, the aim of this study was to evaluate the prognostic value of percentage of 13C-PheOx obtained by 13C-PheBT for the survival of patients with chronic liver failure.
Consecutive patients with chronic liver failure were studied at the Laboratory of Gastro-Hepatology of Centro Médico Nacional Siglo XXI. The study was approved by the Ethical Committee of the hospital. Patients were included according to the following criteria: age above 18 years, both genders; diagnosis of chronic liver failure based on history, clinical and biochemical findings combined with ultrasonographic results, plus liver biopsy when possible; and written informed consent of patients when entering the study. Exclusion criteria were pulmonary alterations, neurologic diseases different to encephalopathy; participation in other studies during the preceding thirty days of this study; and presence of other diseases conditioning a short prognosis by themselves (e.g. carcinoma). According to these criteria, 121 patients were selected from a group of 136 patients with chronic liver disease after having excluded 9 patients with hepatocellular carcinoma, 5 patients because of their unwillingness to participate and one patient with a percentage of 13C-PheOx > 17.
The etiology was defined on the basis of the history obtained from patients and their relatives and serological tests for viruses. The cause of liver disease was chronic alcohol consumption (≥ 30 g/d for 2 years or more) in 23 (19.5%) patients, chronic type-C hepatitis/HVC in 56 (47.5%), mixed (viral + alcohol) in 8 (6.8%) and other causes (cryptogenic, autoimmune, Budd Chiari syndrome, primary biliary cirrhosis, chronic type-B hepatitis/HBV-related cirrhosis and idiopathic) in 31 (26.3%). No patients had hemochromatosis or Wilson’s disease.
All patients were studied following the same protocol with data collected by the laboratory staff and were followed up either as outpatients or inpatients when necessary, according to general medical practice. Survival time was accounted until March 2005. The hepatic function was evaluated by 13C-PheBT and standard liver blood tests. All patients were classified by the CP score as class A (5-6 points), class B (7-9 points) or class C (10-15 points)[15]. This classification comprises albumin, total bilirubin (TB), prothrombin time (PT), ascites and encephalopathy. Ascites was classified as “absent” or “present” according to clinical examination and ultrasonographic findings. The clinical diagnosis and degree of encephalopathy were determined according to the West-Haven criteria[16]. Other tests were alanin transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (AP), creatinine and glucose. Biochemical data were evaluated by standard clinical chemical methods (Dimension, ARXL-Max DADE®, Boehringer, Germany).
13C-PheBT was measured following an overnight fast without control of prior dietary intake. A 100-mg oral dose of L-[1-13C] phenylalanine-isotopic purity 99% 13C (Isotec® Inc, Ohio, USA)-dissolved in 50 mL water was administered. Alveolar breath samples were collected while in resting position and following a normal exhalation. At each sample time, patients were asked to blow directly into a 10-mL exetainer tube (Labco Limited®, Buckinghamshire, U.K.) through a straw. Duplicate breath samples were taken before administration of the 13C-phenylalanine dose (basal), and every 10 min thereafter until completion of 1 h. Enrichment of 13CO2 was determined by isotope ratio mass spectrometry (BreathMat-plus® Finnigan Bremen, Germany). The rate of hepatic 13C-phenylalanine oxidation at each time point was calculated from the appearance of 13CO2 on exhaled air, assuming a CO2 production rate of 300 mmol/m-2 body surface area per hour, as described by Shneider et al[17]. The analytical data were expressed as percentages of the 13C-phenylalanine dose metabolized per hour (percentage of 13C-phenylalanine oxidation/13C-PheOx)[10-12]. The day when patients first presented for the registration of clinical and laboratory data and the measurement of 13C-PheBT was considered as “zero time” for the follow-up period of observation.
Results were expressed as percentages and mean ± SD. Receiving operating characteristic (ROC) analysis was used to define the optimal percentage of 13C-phenylalanine oxidation cut-off point with the highest sensitivity and specificity among thresholds. The analysis was carried out in two steps. To identify independent prognostic variables, a univariate analysis was performed with the Kaplan-Meier statistics. The curves were compared using the log-rank test. During the first stage, covariates analyzed for inclusion in the model were age, sex, etiology, pharmacologic treatment, previous hemorrhage, creatinine, glucose, 13C-phenylalanine oxidation, CP score and complications. Variables that were significant (P < 0.10) in the univariate analysis were subsequently introduced in a multivariate analysis. Then each chosen covariate was reconsidered and eliminated if P > 0.05. The procedure was performed stepwise until no further covariates could be added or removed according to the afore-mentioned criteria.
In a second step, the same Cox model analysis was performed substituting the CP score with the five variables (albumin, bilirubin, prothrombin time, ascites and encephalopathy) that define the score.
To check the proportionality of the hazard in time for the different functional classes, log of the cumulative hazard was plotted against time, demonstrating a parallel behavior in patients with low and high values for the selected predicting covariates when inspected. The goodness of fit of the model was investigated by a partial likelihood function and the Akaike’s information criterion (AIC). The decision to include or to exclude the respective regressor variables was based on a χ2 test[18-20]. Statistical analysis was carried out by using the STATA V 8.0 statistical package (StataCorp LP, College Station, Tex).
One hundred and twenty-one consecutive patients with chronic liver failure were included. Cirrhosis was confirmed by liver biopsy in 35 (28.9%) cases, and the constellation of typical physical signs, such as ascites, oesophageal varices, upper gastrointestinal bleeding, and typical laboratory findings, was accepted as evidence of chronic liver failure in the other 86 (71.0%) patients. During the follow-up period, 44 (20 males, 24 females) patients died. Causes of death were liver failure in 9 (7.6%) patients, upper gastrointestinal bleeding in 10 (8.5%), ascites in 6 (5.1%) and encephalopathy in 8 (6.8%) associated to liver failure, hepatorenal syndrome in 4 (3.4%) and hepatocellullar carcinoma in 4 (3.4%), whereas 3 patients died of other causes (accident, transplant surgery and gastric cancer). These three patients were excluded from the further analysis.
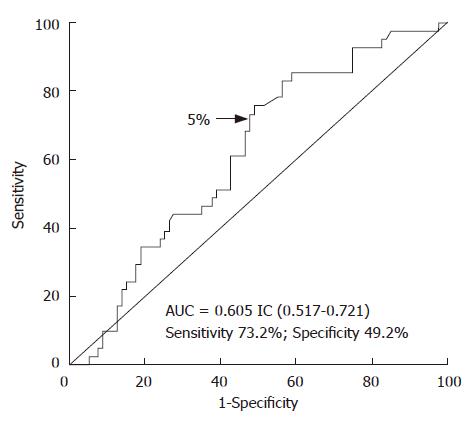
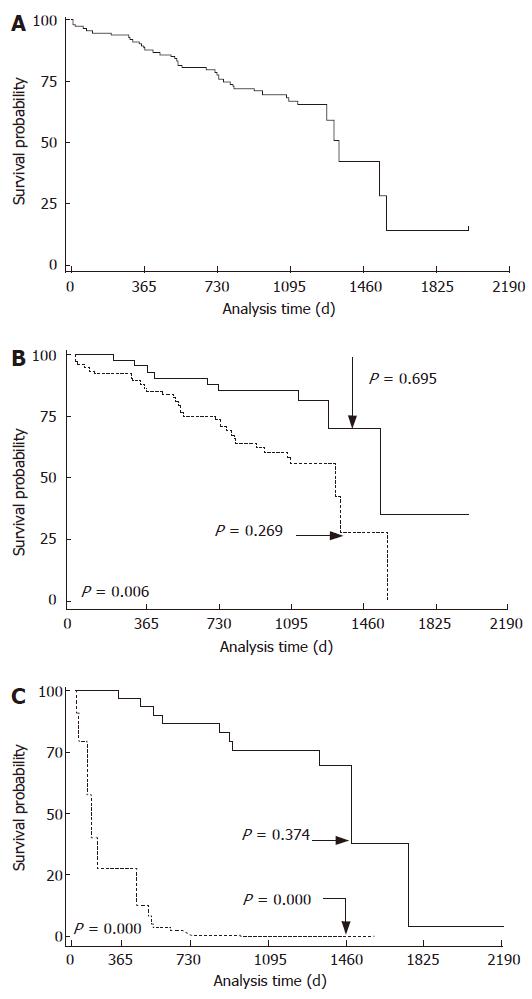
Table 1 depicts the demographic and clinical data, biochemical features and liver function tests of patients. The ROC curves showed that the optimal percentage of 13C-PheOx cut-off value for predicting survival in liver disease patients was 5.0% (sensitivity 73.2%; specificity 49.3%) (Figure 1). Serum albumin, total bilirubin and PT were clustered on the basis of the CP score. Ascites and encephalopathy were clustered as “absent” or “present”. The median probability of survival after entering the study in all the included patients was 1316 d (Figure 2A). The correlation between the percentage of C-PheOx (< 5.0% and ≥ 5.0%) and survival was highly significant (P < 0.006; Figure 2B). Likewise, the results of this study confirmed that percentage of 13C-PheOx was correlated with the CP score (r = -0.255, P = 0.005).
| Variables | Surviving | Non-surviving | P |
| n = 77 | n = 41 | value | |
| Age (yr) | 51.8 ± 10.6 | 55.8 ± 9.6 | 0.043 |
| Sex (F/M) | 49/28 | 22/19 | 0.292 |
| Etiology | |||
| Alcoholic/others | 15/62 | 8/33 | 0.997 |
| Alcohol consumption | 38 | 18 | 0.573 |
| Pharmacological treatment | 71 | 41 | 0.067 |
| History of upper GI hemorrhage | 53/24 | 28/13 | 0.952 |
| Ascites | 26 | 24 | 0.010 |
| Encephalopathy | 7 | 6 | 0.360 |
| Albumin (g/L) | 34 ± 6.0 | 29 ± 46 | 0.000 |
| Total bilirubin (mmol/L) | 27.4 ± 17.1 | 58.1 ± 99.2 | 0.059 |
| Prothrombin time (%) | 76.2 ± 18.9 | 67.6 ± 25.1 | 0.058 |
| ALT (nkat/L) | 1.2 ± 1.14 | 0.99 ± 0.81 | 0.247 |
| AST (nkat/L) | 1.3 ± 0.99 | 1.63 ± 0.81 | 0.370 |
| AP (nkat/L) | 4.18 ± 3.38 | 4.50 ± 3.41 | 0.623 |
| Creatinine (mmol/L) | 13.7 ± 3.4 | 22.2 ± 30.8 | 0.100 |
| Glucose (mmol/L) | 6.3 ± 2.4 | 5.8 ± 1.2 | 0.216 |
| Child Pugh class | |||
| Class A | 45 | 6 | |
| Class B | 23 | 26 | 0.000 |
| Class C | 9 | 9 | |
| Child-Pugh score (points) | 6.7 ± 1.9 | 8.3 ± 2.1 | 0.000 |
| 13C-phenylalanine oxidation (%) | 4.9 ± 2.9 | 3.8 ± 2.2 | 0.027 |
Eleven of the twenty investigated variables showed an independent association to poor prognosis in the univariate analysis. The variables: albumin (P < 0.000), total bilirubin (P < 0.000), prothrombin time (%) (PT) (P < 0.000), creatinine (P < 0.000), ascites at follow-up (P < 0.000), complications at follow-up (P < 0.000), encephalopathy at follow-up (P = 0.012), upper gastrointestinal bleeding at follow-up (P = 0.015), age (P = 0.034) AST (P = 0.081) and ascites when entering the study (P = 0.042). The variables with no independent association to prognosis: ALT (P = 0.135), patients’ entry to the hospital at follow-up (P = 0.146), AP (P = 0.198), treatment of portal hypertension (P = 0.213), history of encephalopathy (P = 0.424), gender (P = 0.437), history of upper gastrointestinal bleeding (P = 0.464), etiology (P = 0.478) and alcohol consumption (P = 0.804).
The multivariate analyses demonstrated that the Child-Pugh classes, age (< 60 years and ≥ 60 years), creatinine and the percentage of 13C-PheOx were independent predictors of survival. When the Child-Pugh classes were replaced by all the parameters of the score, only albumin, bilirubin, creatinine, age < 60 years and ≥ 60 years, and the percentage of 13C-PheOx were found to be independent predictors of survival. Etiology of liver disease, gender, history of gastrointestinal bleeding, ascites, encephalopathy and prothrombin time were not significant predictors of survival after adjusting for the other explanatory variables on the model. Statistical parameters for the variables included in the final Cox model are depicted in Table 2. The first Cox’s model included significant variables contained in Child-Pugh score and the second model included Child-Pugh classes.
| Variables | Coefficient | Hazard ratio1 | SE | 95% CI | Pvalue |
| 1 | |||||
| Albumin (g/L) | -1.254 | 0.285 | 0.085 | 0.158-0.512 | 0.000 |
| Total bilirubin (mmol/L) | 0.149 | 1.161 | 0.051 | 1.065-1.265 | 0.001 |
| Creatinine (mmol/L) | 0.510 | 1.666 | 0.263 | 1.222-2.270 | 0.001 |
| Age | |||||
| ≤ 60 yr | 1.0002 | ||||
| > 60 yr | 0.765 | 2.150 | 0.779 | 1.056-4.377 | 0.035 |
| 13C cumulative dose | |||||
| < 5.0% | 1.0002 | ||||
| ≥ 5.0% | -0.798 | 0.449 | 0.178 | 0.206-0.979 | 0.044 |
| 2 | |||||
| Child Pugh class | |||||
| A | 1.0002 | ||||
| B | 1.629 | 5.099 | 2.382 | 2.040-12.743 | 0.000 |
| C | 2.078 | 7.993 | 4.627 | 2.570-24.859 | 0.000 |
| Creatinine (mmol/L) | 0.417 | 1.518 | 0.200 | 1.173-1.966 | 0.002 |
| Age, | |||||
| ≤ 60 yr | 1.0002 | ||||
| > 60 yr | 1.029 | 2.799 | 1.044 | 1.347-5.816 | 0.006 |
| 13C cumulative dose | |||||
| < 5.0% | 1.0002 | ||||
| ≥ 5.0% | -1.081 | 0.338 | 0.140 | 0.150-0.762 | 0.009 |
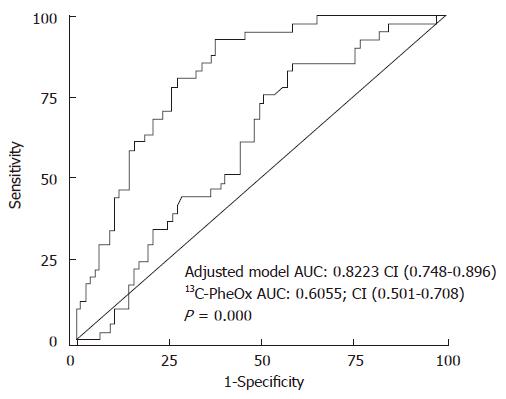
A prognostic index (PI) predicting death was derived from the best model as: PI = exp (-0.798 ×13C-PheOx) + (0.765 × age) + (-1.25 × albumin) + (0.149 × bilirubin) + (0.510 × creatinine). We attributed a value of 0 for 13C-PheOx < 5% and 1 for 13C-PheOx ≥ 5%, 0 for male sex and 1 for female sex, 0 for age < 60 years and 1 for age ≥ 60 years. The relationship between PI due to the best adjusted model and risk of death were compared with a PI for percentage of 13C-PheOx (Figure 2C). The ROC curve for PI of adjusted model for predicting death from liver failure always depicted a better performance than that obtained from percentage of 13C-PheOx alone (Figure 3). Areas under the curve were also significantly larger (AUC = 0.822, 95% CI: 0.748-0.896 vs AUC = 0.605; 95% CI: 0.501- 0.708, P = 0.000).
This study shows that the percentage of 13C-PheOx is an important prognostic factor of long-term survival in patients with chronic liver failure. In fact, values lower than 5.0% for the percentage of 13C-PheOx were associated with an elevated probability of dying and values over 5.0% were associated with a better outcome (probability 0.26 vs 0.69 at 48 mo and 0.00 vs 0.37 at 64 mo). In addition, predictive value was conserved even after adjusting for covariates (Figure 2B-C). Furthermore, percentage of 13C-PheOx added new prognostic information to that obtained by CP classification or the common clinical and biochemical data included in the CP score. The results of this study additionally confirm that percentage of 13C-PheOx correlates inversely with the CP score (r = -0.2550, P = 0.0053). These data are consistent with other studies showing that 13C-PheBT correlates with parameters reflecting the severity of hepatic diseases, including albumin, total bilirubin, PT and CP score[12,21-24].
Additionally, some studies have documented that percentage of 13C-PheOx values are significantly lower in patients with liver cirrhosis than in healthy adults[12,14,23]. It has also been suggested that the decreased ability of decompensated livers to oxidize phenylalanine may be the result of progressive liver damage, individual cellular function; low activities of phenylalanine hydroxylase (PAH) and p-hydroxyphenylpiruvate hydroxylase; the severity or the course of liver disease that produced a decrease in the total number of cells and the functioning liver cell mass with a consequent reduction in phenylalanine metabolism[10,12,14,23]. Likewise, the percentage of 13C-PheOx in patients with cirrhosis was estimated to be 20% of the normal value, suggesting that reduced enzyme levels account for a decreased metabolism in phenylalanine[13]. Hehir et al[9] described that the fractional clearance rates of aromatic amino acids in plasma of patients with acute fulminant liver disease are 2 to 10 times lower than those in normal subjects.
Other authors have demonstrated that 13C-PheBT is used to monitor the clinical course of patients with chronic liver failure[13,25], as a clinical predictor to assess postoperative early complications in patients undergoing hepatectomy[26] or to evaluate the restoration of the plasmatic phenylalanine clearance to normal during the postoperative period that is attributed to the ability of the new liver to catabolize this amino acid[9]. In an experimental model, 13C-PheBT was used to monitor the hepatic dysfunction associated with obstructive jaundice[27]. However, there is scarce information on the prognostic value of 13C-PheBT with regard to the survival of patients with cirrhosis. Our study suggests that the percentage of 13C-PheOx adds new prognostic information to that obtained by the CP score or the common clinical and biochemical data included in the CP score. Nevertheless, discrepant results were recently obtained by Koeda et al[24] who could not demonstrate a correlation of 13C-PheBT with mortality. They examined 23 patients with liver cirrhosis, 6 of them died of hepatic dysfunction in a follow-up period of 816 d. The lack of significance of 13C-PheBT in their study may have probably related, at least in part, to the fact that less end-points were analyzed. It has been clearly established that the number of end-points heavily influences the ability to detect significant effects[24,2].
In contrast to previous reports, 13C-PheOx constituted an important prognostic index in our series. In fact, mortality due to liver-related causes was best predicted by a prognostic index containing albumin, bilirubin, creatinine, age, and 13C-PheBT than by a prognostic index containing the CP score, creatinine, age and 13C-PheBT, as assessed by a partial likelihood function and the Akaike’s information criterion (AIC). However, there are no more data available on the prognostic value of 13C-PheOx.
A limitation in our study could be that it was not possible to calculate a model for end-stage liver disease (MELD)[28] because we did not perform the international normalized ratio of prothrombin time (INR) in all patients. MELD score is superior to CP score as a predictor of intermediate mortality is unclear[29].
Even when the use of 13C-breath tests with various substrates is not a novelty in hepatology, the clinical usefulness of these tests that explore the hepatocellular subfunctions (microsomal, cytosolic or mithocondrial) as well as the superiority of some quantitative prognostic liver function tests compared with the CP score are still unclear[30,31].
Amynopyrine breath test (ABT) has been used to predict short-term prognosis and mortality in patients with alcoholic hepatitis. There are contradictory results of ABT for predicting survival in patients with cirrhosis. Some studies showed that ABT is better than the Child-Turcotte score, whilst others demonstrate that ABT is not better. However, some other studies seem to provide additional prognostic information to the CP score[31-34].
Galactose elimination capacity (GEC), a valuable indicator of liver function, is dependent on hepatic blood supply and inhibited by alcohol consumption. It has been demonstrated that GEC adds some more prognostic information when the CP score is not included; whereas, other studies reported that, GEC is unable to improve the prognostic ability of GEC[8,35-37].
The caffeine breath test (CBT) represents a valid indicator of plasma caffeine clearance (CC) and correlates with the varying degrees of liver dysfunction. However, both tests do not appear to add prognostic information beyond that provided by the CP classification. CBT and CC may decrease with increasing age, cigarette smoking and disease state[38-40].
Indocyanine green elimination (IGC) provides some prognostic information related to survival of patients with cirrhosis, but does not improve the predictive ability of clinical information considered for the CP score. It is also sensible to hepatic blood flow, and adversely affected by cardiovascular drugs (calcium channel blockers) and is significantly reduced after portosystemic shunting[6,8,41,42].
On the other hand, the interest in search for accurate prognostic tests for patients with chronic liver disease is still of utmost importance since the emergence of liver transplantation is an important therapy for patients with advanced cirrhosis[1].
In conclusion, 13C-PheBT is a strong predictor of survival in patients with chronic liver failure that adds information which may not be available from the common clinical and biochemical data included in the CP score for the assessment of the risk of death due to liver disease. Should these data be confirmed in other studies and different settings, the 13C-PheBT could prove to be a useful clinical tool to routinely evaluate the prognosis of patients with chronic liver disease in addition to the common clinical and biochemical data, because of its non-invasiveness and safety.
We thank professor Salvador Zamora Muñoz for his helpful advice regarding this manuscript.
In recent years, there has been a growing interest in quantitative liver function tests, including breath tests, since they are expected to increase the accuracy of estimating the severity of liver disease. L-[1-13C] phenylalanine breath test 13C-PheBT distinguishes patients with various degrees of liver disease from healthy persons. However, the prognostic value of 13C-PheBT for survival of patients with chronic liver disease is yet to be established.
Even when the use of 13C-breath tests with various substrates is not a novelty in hepatology, the clinical usefulness of these tests that explore the hepatocellular subfunctions (microsomal, cytosolic or mithocondrial) and measure of hepatocyte functional capacity in liver diseases as well as the prognostic value of 13C-breath tests compared with the Child-Pugh (CP) or model for end-stage liver disease (MELD) are still unclear.
The current study provides evidence that 13C-PheBT is a strong predictor of survival in patients with chronic liver failure and adds information which may not be available from the common clinical and biochemical data included in the CP score for the assessment of the risk of death due to liver disease.
13C-PheBT could prove to be a useful clinical tool to routinely evaluate the prognosis of patients with chronic liver disease in addition to the common clinical and biochemical data, because of its non-invasiveness and safety.
L-[1-13C] phenylalanine breath test measures hepatocyte functional capacity by estimating the oxidation of L-[1-13C] phenylalanine and represents the hepatic cytosolic enzyme activity.
This manuscript describes the prospective evaluation of 13C-phenylalanine breath test in the evaluation of prognosis in cirrhotic patients.
| 1. | National Institutes of Health Consensus Development Conference Statement: liver transplantation--June 20-23, 1983. Hepatology. 1984;4:107S-110S. [PubMed] |
| 2. | Infante-Rivard C, Esnaola S, Villeneuve JP. Clinical and statistical validity of conventional prognostic factors in predicting short-term survival among cirrhotics. Hepatology. 1987;7:660-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 221] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Orrego H, Israel Y, Blake JE, Medline A. Assessment of prognostic factors in alcoholic liver disease: toward a global quantitative expression of severity. Hepatology. 1983;3:896-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 129] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Christensen E. Prognostic models including the Child-Pugh, MELD and Mayo risk scores--where are we and where should we go? J Hepatol. 2004;41:344-350. [PubMed] |
| 5. | Becker M. 13C breath test for measurement of liver function. Gut. 1998;43 Suppl 3:S25-S27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Merkel C, Bolognesi M, Finucci GF, Angeli P, Caregaro L, Rondana M, Gatta A. Indocyanine green intrinsic hepatic clearance as a prognostic index of survival in patients with cirrhosis. J Hepatol. 1989;9:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Salerno F, Borroni G, Moser P, Sangiovanni A, Almasio P, Budillon G, Capuano G, Muraca M, Marchesini G, Bernardi M. Prognostic value of the galactose test in predicting survival of patients with cirrhosis evaluated for liver transplantation. A prospective multicenter Italian study. AISF Group for the Study of Liver Transplantation. Associazione Italiana per lo Studio del Fegato. J Hepatol. 1996;25:474-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Albers I, Hartmann H, Bircher J, Creutzfeldt W. Superiority of the Child-Pugh classification to quantitative liver function tests for assessing prognosis of liver cirrhosis. Scand J Gastroenterol. 1989;24:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 184] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Hehir DJ, Jenkins RL, Bistrian BR, Wagner D, Moldawer LL, Young VR, Blackburn GL. Abnormal phenylalanine hydroxylation and tyrosine oxidation in a patient with acute fulminant liver disease with correction by liver transplantation. Gastroenterology. 1985;89:659-663. [PubMed] |
| 10. | Ishii Y, Asai S, Kohno T, Suzuki S, Ishii M, Hosoi I, Fujii M, Iwai S, Ishikawa K. 13CO(2) peak value of L-[1-(13)C]phenylalanine breath test reflects hepatopathy. J Surg Res. 1999;86:130-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Ishii Y, Suzuki S, Kohno T, Aoki M, Kohno T, Ito A, Takayama T, Asai S. L-[1-13C] phenylalanine breath test reflects histological changes in the liver. J Surg Res. 2003;114:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Burke PA, Stack JA, Wagner D, Lewis DW, Jenkins RL, Forse RA. L-[1-(13)C] Phenylalanine oxidation as a measure of hepatocyte functional capacity in end-stage liver disease. Am J Surg. 1997;173:270-273; discussion 270-273;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Heberer M, Talke H, Maier KP, Gerok W. Metabolism of phenylalanine in liver diseases. Klin Wochenschr. 1980;58:1189-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Lara Baruque S, Razquin M, Jimenez I, Vazquez A, Gisbert JP, Pajares JM. 13C-phenylalanine and 13C-methacetin breath test to evaluate functional capacity of hepatocyte in chronic liver disease. Dig Liver Dis. 2000;32:226-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5827] [Article Influence: 109.9] [Reference Citation Analysis (2)] |
| 16. | Conn HO, Leevy CM, Vlahcevic ZR, Rodgers JB, Maddrey WC, Seeff L, Levy LL. Comparison of lactulose and neomycin in the treatment of chronic portal-systemic encephalopathy. A double blind controlled trial. Gastroenterology. 1977;72:573-583. [PubMed] |
| 17. | Schneider JF, Schoeller DA, Nemchausky B, Boyer JL, Klein P. Validation of 13CO2 breath analysis as a measurement of demethylation of stable isotope labeled aminopyrine in man. Clin Chim Acta. 1978;84:153-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Kleinbaum GD, Survival Analysis. A Self-Learning Text. New York: Springer 2000; 1-170. |
| 19. | Collet D. Modelling Survival Data in Medical Research. 2nd ed. Washington DC: Chapman & Hall/CRC 2003; 391. |
| 20. | Seppo Pynn�nen, Department of mathematics and Statistics, University of Vaasa. Detection of Outliers in Regression Analysis by Information Criteria 1992, cited 2004-08-11. Available from: www.uwasa.fi/sjp/. |
| 21. | Festi D, Capodicasa S, Sandri L, Colaiocco-Ferrante L, Staniscia T, Vitacolonna E, Vestito A, Simoni P, Mazzella G, Portincasa P. Measurement of hepatic functional mass by means of 13C-methacetin and 13C-phenylalanine breath tests in chronic liver disease: comparison with Child-Pugh score and serum bile acid levels. World J Gastroenterol. 2005;11:142-148. [PubMed] |
| 22. | Ishii T, Furube M, Hirano S, Takatori K, Iida K, Kajiwara M. Evaluation of 13C-phenylalanine and 13C-tyrosine breath tests for the measurement of hepatocyte functional capacity in patients with liver cirrhosis. Chem Pharm Bull (Tokyo). 2001;49:1507-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Kobayashi T, Imamura H, Takayama T, Makuuchi M. The role of preoperative phenylalanine breath test in hepatectomy. Hepatogastroenterology. 2003;50:1124-1127. [PubMed] |
| 24. | Koeda N, Iwai M, Kato A, Suzuki K. Validity of 13C-phenylalanine breath test to evaluate functional capacity of hepatocyte in patients with liver cirrhosis and acute hepatitis. Aliment Pharmacol Ther. 2005;21:851-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Ishii Y, Suzuki S, Kohno T, Aoki M, Goto I, Kohno T, Ito A, Asai S. Patients with severe liver cirrhosis followed up by L-[1-(13)C] phenylalanine breath test. J Gastroenterol. 2003;38:1086-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Kobayashi T, Kubota K, Imamura H, Hasegawa K, Inoue Y, Takayama T, Makuuchi M. Hepatic phenylalanine metabolism measured by the [13C]phenylalanine breath test. Eur J Clin Invest. 2001;31:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Aoki M, Ishii Y, Ito A, Khono T, Takayama T. Phenylalanine breath test as a method to evaluate hepatic dysfunction in obstructive jaundice. J Surg Res. 2006;130:119-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3779] [Article Influence: 151.2] [Reference Citation Analysis (2)] |
| 29. | Said A, Williams J, Holden J, Remington P, Gangnon R, Musat A, Lucey MR. Model for end stage liver disease score predicts mortality across a broad spectrum of liver disease. J Hepatol. 2004;40:897-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 283] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 30. | Saunders JB, Wright N, Lewis KO. Predicting outcome of paracetamol poisoning by use 14C-aminopyrine breath test. Br Med J. 1980;280:279-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Schneider JF, Baker AL, Haines NW, Hatfield G, Boyer JL. Aminopyrine N-demethylation: a prognostic test of liver function in patients with alcoholic liver disease. Gastroenterology. 1980;79:1145-1150. [PubMed] |
| 32. | Merkel C, Bolognesi M, Bellon S, Bianco S, Honisch B, Lampe H, Angeli P, Gatta A. Aminopyrine breath test in the prognostic evaluation of patients with cirrhosis. Gut. 1992;33:836-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 65] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Villeneuve JP, Infante-Rivard C, Ampelas M, Pomier-Layrargues G, Huet PM, Marleau D. Prognostic value of the aminopyrine breath test in cirrhotic patients. Hepatology. 1986;6:928-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Urbain D, Muls V, Thys O, Ham HR. Aminopyrine breath test improves long-term prognostic evaluation in patients with alcoholic cirrhosis in Child classes A and B. J Hepatol. 1995;22:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Tygstrup N. The galactose elimination capacity in relation to clinical and laboratory findings in patients with cirrhosis. Acta Med Scand. 1964;175:291-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Henderson JM, Kutner MH, Bain RP. First-order clearance of plasma galactose: the effect of liver disease. Gastroenterology. 1982;83:1090-1096. [PubMed] |
| 37. | Merkel C, Gatta A, Zoli M, Bolognesi M, Angeli P, Iervese T, Marchesini G, Ruol A. Prognostic value of galactose elimination capacity, aminopyrine breath test, and ICG clearance in patients with cirrhosis. Comparison with the Pugh score. Dig Dis Sci. 1991;36:1197-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Lewis FW, Rector WG. Caffeine clearance in cirrhosis. The value of simplified determinations of liver metabolic capacity. J Hepatol. 1992;14:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Joeres R, Klinker H, Heusler H, Epping J, Zilly W, Richter E. Influence of smoking on caffeine elimination in healthy volunteers and in patients with alcoholic liver cirrhosis. Hepatology. 1988;8:575-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Park GJ, Katelaris PH, Jones DB, Seow F, Le Couteur DG, Ngu MC. Validity of the 13C-caffeine breath test as a noninvasive, quantitative test of liver function. Hepatology. 2003;38:1227-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Pomier-Layrargues G, Huet PM, Infante-Rivard C, Villeneuve JP, Marleau D, Duguay L, Tanguay S, Lavoie P. Prognostic value of indocyanine green and lidocaine kinetics for survival and chronic hepatic encephalopathy in cirrhotic patients following elective end-to-side portacaval shunt. Hepatology. 1988;8:1506-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 42. | Navasa M, García-Pagán JC, Bosch J, Rodés J. Prognostic value of hepatic clearance of indocyanine green in patients with liver cirrhosis and hemorrhage of esophageal varices. Med Clin (Barc). 1992;98:290-294. [PubMed] |
S- Editor Ma N L- Editor Wang XL E- Editor Liu Y