Published online Sep 14, 2007. doi: 10.3748/wjg.v13.i34.4560
Revised: June 13, 2007
Accepted: June 18, 2007
Published online: September 14, 2007
AIM: To investigate the different impact of genotypes B and C on the development of liver cirrhosis (LC) among different age groups of patients with chronic hepatitis B (CH-B).
METHODS: We examined the outcome of 121 patients with CH-B, divided by age and genotype. Univariate analyses were used to compare different groups. The Cox proportional hazard model was employed to evaluate factors affecting the development of LC.
RESULTS: In patients < 30 years old, there were no significant predictors for development of LC. However, in patients ≥ 30 years old, genotype C was the only significant predictor. In the genotype C group, 8 of 12 patients who progressed to LC were 30-49 years old at initial diagnosis of chronic hepatitis (7 patients were positive for HBeAg). In the genotype B group, 4 of 8 patients who developed LC were ≥ 50 years old at initial diagnosis and were HBeAg-negative.
CONCLUSION: The rate of development of LC was comparable in patients infected with genotypes B and C when CH-B occurred at < 30 years old. However, CH-B patients infected with genotype C showed poor prognosis if they were 30-49 years old and were positive for HBeAg. Age-specific natural course of CH-B should be considered when patients with CH-B are treated with antiviral drugs.
- Citation: Maeshiro T, Arakaki S, Watanabe T, Aoyama H, Shiroma J, Yamashiro T, Hirata T, Hokama A, Kinjo F, Nakayoshi T, Nakayoshi T, Mizokami M, Fujita J, Sakugawa H. Different natural courses of chronic hepatitis B with genotypes B and C after the fourth decade of life. World J Gastroenterol 2007; 13(34): 4560-4565
- URL: https://www.wjgnet.com/1007-9327/full/v13/i34/4560.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i34.4560
Hepatitis B virus (HBV) is a double-stranded DNA virus. Chronic HBV infection is associated with different forms of liver diseases, encompassing inactive carrier, chronic hepatitis (CH), liver cirrhosis (LC) and hepatocellular carcinoma (HCC)[1].
It has been recently reported that HBV genotypes influence disease severity and clinical outcome of HBV infection[2-6]. HBV genotypes are classified into 8 groups, from A to H, based on a sequence difference greater than 8%, and are variously distributed in the world[2,5,7-14]. In Japan, genotypes B and C are predominant, as in other Asian countries, but > 90% of chronic carriers have genotype C[3,5,8,15].
We previously reported that HBeAg seroconversion rate in genotype B was significantly higher compared with genotype C[6]. Moreover, it has been reported that progression to LC is more frequently seen in genotype C patients than in genotype B patients[2-6]. Therefore, it seems that infection with genotype C is associated with a poor prognosis.
However, our previous study[16] and another study[17] showed that association of genotype C with advanced liver disease was not seen in patients over 50 years old. Therefore, the influence of HBV genotype on the prognosis of CH may vary depending on the age groups.
The aim of the present study was to investigate the different impact of HBV genotypes B and C on the development of LC among patients with chronic hepatitis B (CH-B) belonging to different age groups.
From January 1977 to January 2005, we enrolled 145 consecutive patients with CH-B who were admitted to our institute and related facilities followed for more than 6 mo, i.e. for 7 to 207 mo (mean ± SD: 79.8 ± 48.4). In 124 patients, diagnosis of CH was made by liver biopsy after written informed consent, and histological classification was carried out according to standard criteria[18]. The degree of hepatic inflammation and the stage of fibrosis were scored by a modified Knodell histological index[19]. The inflammation score (grading) was obtained by combining the scores for the first three components of the index: portal, periportal, and lobular inflammation. The score could range from 0 to 18. The degree of inflammation was graded as 4 to 8, mild; 9 to 18, moderate to severe. The fibrosis score (staging) was graded as 0 to 1, no-mild; 2 to 3, moderate-severe. In the remaining 21 patients, diagnosis of CH was made clinically and was characterized by persistent alanine aminotransferase (ALT) elevation over for at least 6 mo and by ultrasound findings using a cirrhosis score (< 8 points)[20]. Diagnosis of LC was made mainly by clinical assessment including serial determination of serum ALT levels, platelet count, ultrasound examination (using the above mentioned cirrhosis score, ≥ 8 points)[20] and computed tomography. For the diagnosis of HCC, we added the measurement of serum alpha-fetoprotein to the above diagnostic criteria for LC. Patients positive for antibodies to hepatitis C virus (HCV), antibodies to hepatitis D virus (HDV) and with a history of alcohol or drug abuse were excluded from the study. Patients treated with antiviral drugs (lamivudine or interferons) were also excluded from this study. Patients were observed every 1 to 3 mo or more frequently if required. Routine follow-up studies included clinical evaluation, conventional liver function tests for ALT, aspartate aminotransferase (AST), and γ-glutamyl transpeptidase (γGTP) and serological markers of HBV infection including hepatitis B e antigen (HBeAg) and antibodies to HBeAg (anti-HBe). Ultrasound examination and computed tomography were done every 6-12 mo or more often if necessary. Hepatitis B surface antigen (HBsAg), HBeAg, and anti-HBe antibody were tested using commercially available enzyme immunoassays (EIA) (Enzygnost, Behring, Germany). Anti-HCV was assayed by a second-generation EIA (Ortho Diagnostics, Raritan, USA). Anti-HDV was examined by a commercially available EIA (Dinabot, Tokyo, Japan). HBV genotype was determined by the restriction fragment length polymorphism (RFLP) method on an S-gene sequence amplified by polymerase chain reaction (PCR) with nested primers, as previously described by Mizokami et al[21].
The chi-square test, Fisher's exact test, and Student's t-test were used to compare differences between groups, when appropriate. Cumulative developing rates of LC were calculated by the Kaplan-Meier method and differences were determined by the log rank test. In a multivariate analysis, the Cox proportional hazard model was employed to evaluate factors affecting development of LC. Statistical comparisons were done using SPSS for Windows version 11 package (SPSS Inc., Japan). All analyses were two sided, and P-values less than 0.05 were considered statistically significant.
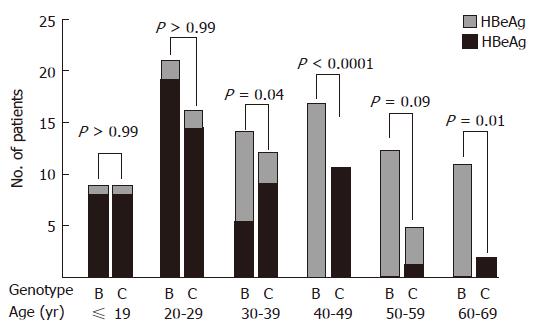
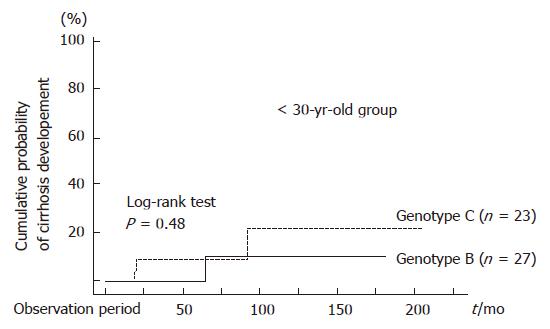
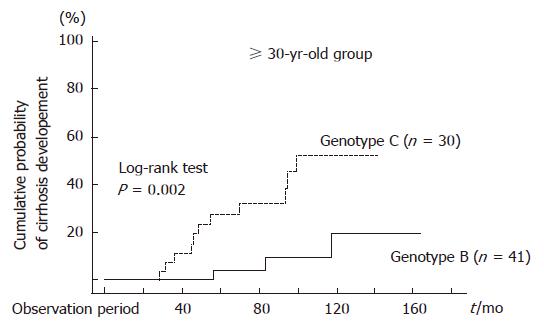
Figure 1 shows age-specific HBeAg status at entry in the study for patients with CH-B infected with genotype B or C. For patients < 30 years old, most individuals were HBeAg-positive, and no difference was seen in HBeAg-positive rate between patients infected with genotype B and those infected with genotype C. However, for patients ≥ 30 years old, the majority of genotype C patients were positive for HBeAg, whereas most patients infected with genotype B were HBeAg-negative: no patient infected with genotype B showed a positive reaction for HBeAg in the age group greater than 40 years old. In addition, for patients > 50 years old, the percentage of patients infected with genotype B was increased. Since the proportion of patients positive for HBeAg was different between the group < 30 years old and that ≥ 30 years old, we divided the subjects into 2 groups (< 30 years old and ≥ 30 years old) to compare the natural course of CH-B in patients infected with genotypes B vs C (Table 1). There were no differences in LC development rates between genotypes B and C among the group < 30 years old using the Kaplan-Meier method and the log rank test (P = 0.48, Figure 2). In addition, when using the Cox proportional hazard model, which included HBeAg, gender, degree of fibrosis and genotype as associated variables, there was no significant predictor for development of LC (Table 2). On the other hand, in the group ≥ 30 years old, only genotype was found to contribute to LC development by the Kaplan-Meier method: in fact, the rate was higher in patients infected with genotype C than in those infected with genotype B (P = 0.002, Figure 3). The Cox proportional hazard model also indicated that HBV genotype C was the only significant predictor of the development of LC (Relative risk 5.75; 95% CI: 0.033-0.916, P = 0.039, Table 2).
| < 30-yr-old group | ≥ 30-yr-old group | |||
| Genotype B | Genotype C | Genotype B | Genotype C | |
| Number of patients | 27 | 23 | 41 | 30 |
| Age (yr, mean ± SD) | 21.6 ± 5.3 | 22.2 ± 4.4 | 45.5 ± 10.8 | 42.7 ± 8.7 |
| Gender (Male/Female) | 19/8 | 16/7 | 37/4 | 22/8 |
| HBeAg (+/-) | 24/3 | 21/2 | 4/37 | 19/11b |
| Observation period | 69.0 ± 53.4 | 62.8 ± 45.9 | 77.9 ± 50.1 | 87.0 ± 50.6 |
| (mo, mean ± SD) | ||||
| ALT (IU/L, mean ± SD) | 266.3 ± 262.1 | 245.8 ± 292.4 | 223.1 ± 251.7 | 265.8 ± 359.8 |
| Degree of fibrosis (0/1/2/3) | 4/11/5/1 | 2/13/2/2 | 6/13/10/4 | 2/13/6/7 |
| < 30-yr-old group | ≥ 30-yr-old group | |||||
| Variables | Relativerisk | 95% CI | P | Relativerisk | 95% CI | P |
| Gender (Male/Female) | 0 | - | 0.972 | 0 | 0.000-3.993 | 0.969 |
| HBeAg | ||||||
| Positive | 1 | 0.028-6.469 | 0.538 | 1 | 0.185-3.250 | 0.727 |
| Negative | 2.36 | 1.29 | ||||
| Degree of fibrosis | ||||||
| Mild-moderate | 1 | 0.080-1.030 | 0.053 | 1 | 0.296-3.238 | 0.972 |
| Severe | 10.87 | 1.02 | ||||
| Genotypes | ||||||
| B | 1 | 0.019-2.833 | 0.258 | 1 | 0.033-0.916 | 0.039 |
| C | 4.24 | 5.75 | ||||
Patients who progressed to LC during the observation period are shown in Table 3. Eight patients progressed to LC in patients infected with genotype B. Five of these 8 patients were ≥ 40 years old at initial diagnosis, and all these 5 patients were HBe Ag-negative. Three patients < 30 years old who progressed to LC showed a positive reaction for HBeAg at enrollment. All 3 patients experienced a severe hepatic flare during the observation period and thereafter progressed to LC. However, following development of LC, they followed a benign clinical course of burn-out hepatitis: disappearance of HBeAg and persistently normal or nearly normal ALT levels. On the other hand, in the genotype C group, 8 of 12 patients who progressed to LC were 30-49 years old at initial diagnosis of CH, and 7 of the 8 patients were HBeAg-positive. These 8 patients rapidly progressed to LC within 2 to 8 years, and all of them were less than 50 years old at the development of LC. Three of 8 patients died from HCC or liver failure shortly after development of LC, with periods ranging from 1 to 7 years.
| Genotype B Age (yr) | Genotype C Age (yr) | ||||
| At entry | Developmentof LC | Outcome | At entry | Developmentof LC | Outcome |
| 17 M e+ | 18 e+ | 24 e- | 18 M e+ | 24 e+ | 31 e+ dead (HCC) |
| 23 M e+ | 27 e- | 36 e- | |||
| 28 M e+ | 30 e+ | 38 e- | |||
| 31 M e+ | 33 e+ | 40 e+ dead (hepatic failure) | |||
| 31 M e+ | 39 e+ | 44 e- | |||
| 33 M e+ | 40 e+ | 48 e+ | |||
| 36 M e+ | 40 e- | 46 e- | |||
| 38 M e+ | 45 e- | 48 e- dead (HCC) | |||
| 41 M e- | 48 e- | 49 e- | 41 M e+ | 43 e+ | 51 e- |
| 42 M e+ | 46 e- | 48 e- | |||
| 45 M e- | 48 e- | 49 e- dead (HCC) | |||
| 58 M e- | 64 e- | 70 e- | 50 M e- | 54 e- | 57 e- |
| 58 M e- | 72 e- | 73 e- | 50 M e- | 52 e- | 53 e- dead (hepatic failure) |
| 60 M e- | 61 e- | 66 e- | |||
| 66 M e- | 70 e- | 71 e- | 73 F e+ | 79 e+ | 90 e+ |
In East Asia, most of HBV carriers are infected with genotype B or C[2,5,22]. HBV genotype C is the predominant genotype in Japan, accounting for approximately 70% of HBV carriers living in Japan[5].
The island Okinawa prefecture is located south-west of the main Japanese islands. Although people living in the Okinawa prefecture are Japanese, two-thirds of HBV carriers are infected with genotype B. In Okinawa prefecture, prevalence of HBsAg among blood donors is 3.5%, which is twice the average for all areas of Japan (1.5%), and is the highest in Japan (from the annual report of the Japanese Red Cross Center). In contrast, mortality rates due to cirrhosis and HCC associated with HBV infection are the lowest in Japan[23,24]. This paradoxical phenomenon may be due to the fact that the majority of HBV carriers in Okinawa are infected with genotype B, and carriers of genotype B have a more benign clinical phenotype than those with genotype C (the predominant genotype in Japan).
In the present study, in the group < 30 years old, the majority of patients with CH-B were positive for HBeAg and the rate of positivity for HBeAg was not significantly different between patients infected with genotypes B and C. However, HBeAg was not detected in the majority of patients infected with genotype B in the group ≥ 30 years old. In contrast, most patients infected with genotype C were positive for HBeAg in the group ≥ 30 years old.
It has been reported that the seroconversion rate of HBeAg was higher in CH patients infected with genotype B than those infected with genotype C[6,25,26]. However, in this study, LC development rate were not significantly different between patients with genotypes B and C in the group < 30 years old. Only one CH patient infected with genotype C progressed to LC during the observation period, whereas LC developed in 3 patients infected with genotype B. Therefore, prognoses of CH patients infected with genotypes B and C were comparable in the group < 30 years old. In contrast, LC development rate among CH patients infected with genotype C was significantly higher than that of genotype B in the group ≥ 30 years old. These patients progressed to LC within a short period of time after diagnosis of CH. In the group ≥ 30 years old, there was a significant difference in prevalence rates of HBeAg between patients infected with genotypes B and C. Therefore, it was presumed that difference in LC developing rate between genotypes B and C was responsible for differences in HBeAg positive rates. However, only genotype C was a significant predictor for development of LC using the Cox proportional hazard model, while HBeAg was not a significant predictor.
Among the 55 patients infected with genotype B in the group ≥ 30 years old, 5 were HBeAg positive at initial diagnosis of CH. These 5 patients didn’t progress to LC during the observation period (data not shown). Further stratification of the patients according to HBeAg status showed that development of LC was more frequently seen in patients infected with genotype C than in those with genotype B in the group ≥ 30 years old. As mentioned above, difference in prognosis between genotypes B and C was significantly different. In particular, prognosis was very poor if patients were infected with genotype C, were positive for HBeAg, and were ≥ 30 years old. Therefore, we believe that antiviral therapy (lamivudine, adefovir dipivoxil or entecavir) is beneficical if patients are ≥ 30 years old, positive for HBeAg and infected with genotype C.
Kao et al[26] reported that among 270 chronic HBV carriers in Taiwan, 53% belonged to genotype B and 32% to genotype C. Genotype C was significantly more common in patients with LC and/or HCC who were older than 50 years, when compared to age-matched inactive carriers, suggesting that genotype C was associated with more severe liver diseases. However, genotype B was more frequently seen in younger patients (< 35 years old) with HCC, most of whom did not have LC. While Kao et al showed that genotype C was associated with poor prognosis in older age groups, as confirmed in our study, the predominance among younger patients with HCC in genotype B was quite different. These discrepancies may be responsible for differences in subgenotypes among patients infected with genotype B between Taiwan and Okinawa. Sugauchi et al determined subgenotypes among 274 carriers of HBV infected with genotype B and reported that all 177 genotype B isolates from the carriers living in Asian countries other than Japan belong to the genotype Ba[27,28]. On the contrary, they also reported that of the 97 carriers infected with genotype B living in Japan, only 7 (7%) possessed genotype Ba, and that the remaining majority was of genotype Bj without recombinations. In addition, it has been reported that loss of HBeAg occurrs earlier and is more frequent in carriers of genotype Bj than genotype Ba or genotype C, particularly after they have reached 30 years or older[27,28]. In this study, not all patients with genotype B were subgenotyped, but approximately 80% of them were infected with genotype Bj. Therefore, it is possible to speculate that differences in subgenotypes of HBV genotype B isolates may be responsible for differences in the natural course of patients infected with genotype B between Taiwan and Okinawa.
In this study, development of LC was commonly seen in patients with genotype B, and the majority of patients were HBeAg-negative at initial diagnosis of CH. Most of HBV carriers infected with genotype B seroconverted from HBeAg to anti-HBe under 30 years old and became HBeAg-negative inactive carriers[5,16]. Status of HBeAg and anti-HBe antibody correlated with mutations in pre-core lesions[29,30]. Most patients infected with genotype B showed the pre-core mutation before 30 years old, stopping HBeAg production, followed by reduction in HBV-DNA level[5]. However, after clearance of HBeAg, CH occurred in some patients, and occurrence was associated with increase in serum HBV-DNA level. HBeAg-negative CH was more frequently seen in patients infected with genotype B compared with genotype C. LC developed in some patients with genotype B, the majority of whom were ≥ 40 years old at diagnosis of CH. Therefore, CH-B has to be treated in consideration of the above described natural course of HBV infection. We believe that antiviral therapy (lamivudine, adefovir dipivoxil or entecavir) is highly recommended for the following patients: (1) HBeAg-positive CH infected with genotype C aged ≥ 30 years; (2) HBeAg-negative CH infected with genotype B aged ≥ 40 years, especially if showing advanced fibrosis (F2 or F3).
In conclusion, when HBeAg-positive CH-B was diagnosed in the group ≥ 30 years old, the rate of development of LC was higher in patients infected with genotype C than in those with genotype B. Therefore, we believe that these cases should be treated with antiviral drugs. In addition, development of LC is not rare in patients infected with genotype B if HBeAg-negative CH occurs at ≥ 40 years of age.
We thank Dr. Hiroki Nakasone for his helpful discussion and suggestions.
Chronic infection by hepatitis B virus (HBV) causes various liver diseases. HBV genotypes are classified into 8 groups, from A to H. Genotypes B and C are predominant in Asia and > 90% of chronic carriers show genotype C, which is associated with poor prognosis. However, prior studies showed that association of genotype C with advanced liver disease was not seen in patients over 50 years old.
HBV genotypes, distributed differently in the world, influence disease severity and clinical outcomes. Therefore, studies of genotypes have been thoroughly investigated to improve disease outcomes.
Prior studies showed that infection with genotype C was associated with poor prognosis. This study has focused on different age groups of chronic hepatitis B for different influences of genotype B and C and unrevealed the age-specific natural course of the disease.
This study has clearly shown the age-specific natural course of the chronic hepatitis B with genotype B and C in Japan. These results should be helpful for determining the indication of antiviral drugs.
HBV genotype: HBV genotypes are classified into 8 groups, from A to H, according to differences in base sequences greater than 8%, and are differently distributed in the world. In Japan, genotypes B and C are predominant as in other Asian countries, but > 90% of chronic carriers show genotype C.
This population study by Maeshiro and colleagues describes the natural history of chronic hepatitis B of genotypes B and C. A moderate number of patients were seen over a long time period of 28 years and an even lower number of 121 patients was included in the analysis. Nevertheless, the study shows strong evidence for a higher progression rate towards cirrhosis for patients infected with HBV genotype C. This phenomemon was associated with a lower HBe conversion rate in genotype C patients.
| 1. | Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1728] [Cited by in RCA: 1714] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 2. | Kao JH, Chen PJ, Lai MY, Chen DS. Hepatitis B genotypes correlate with clinical outcomes in patients with chronic hepatitis B. Gastroenterology. 2000;118:554-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 684] [Cited by in RCA: 700] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 3. | Kikuchi K, Niitsuma H, Ishii M, Cervantes JG, Hong S, Ojima T, Suzuki C, Kobayashi T, Ueno Y, Kobayashi K. Genoepidemiology and its relationship to clinical features in patients infected chronically with hepatitis B virus (HBV). Hepatol Res. 2000;17:43-55. [DOI] [Full Text] |
| 4. | Ding X, Mizokami M, Yao G, Xu B, Orito E, Ueda R, Nakanishi M. Hepatitis B virus genotype distribution among chronic hepatitis B virus carriers in Shanghai, China. Intervirology. 2001;44:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 140] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Orito E, Ichida T, Sakugawa H, Sata M, Horiike N, Hino K, Okita K, Okanoue T, Iino S, Tanaka E. Geographic distribution of hepatitis B virus (HBV) genotype in patients with chronic HBV infection in Japan. Hepatology. 2001;34:590-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 315] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 6. | Nakayoshi T, Maeshiro T, Nakayoshi T, Nakasone H, Sakugawa H, Kinjo F, Orito E, Mizokami M. Difference in prognosis between patients infected with hepatitis B virus with genotype B and those with genotype C in the Okinawa Islands: a prospective study. J Med Virol. 2003;70:350-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Okamoto H, Tsuda F, Sakugawa H, Sastrosoewignjo RI, Imai M, Miyakawa Y, Mayumi M. Typing hepatitis B virus by homology in nucleotide sequence: comparison of surface antigen subtypes. J Gen Virol. 1988;69:2575-2583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 773] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 8. | Stuyver L, De Gendt S, Van Geyt C, Zoulim F, Fried M, Schinazi RF, Rossau R. A new genotype of hepatitis B virus: complete genome and phylogenetic relatedness. J Gen Virol. 2000;81:67-74. [PubMed] |
| 9. | Lindh M, Andersson AS, Gusdal A. Genotypes, nt 1858 variants, and geographic origin of hepatitis B virus--large-scale analysis using a new genotyping method. J Infect Dis. 1997;175:1285-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 330] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 10. | Magnius LO, Norder H. Subtypes, genotypes and molecular epidemiology of the hepatitis B virus as reflected by sequence variability of the S-gene. Intervirology. 1995;38:24-34. [PubMed] |
| 11. | Norder H, Hammas B, Lee SD, Bile K, Couroucé AM, Mushahwar IK, Magnius LO. Genetic relatedness of hepatitis B viral strains of diverse geographical origin and natural variations in the primary structure of the surface antigen. J Gen Virol. 1993;74:1341-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 256] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Norder H, Couroucé AM, Magnius LO. Complete genomes, phylogenetic relatedness, and structural proteins of six strains of the hepatitis B virus, four of which represent two new genotypes. Virology. 1994;198:489-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 587] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 13. | Arauz-Ruiz P, Norder H, Robertson BH, Magnius LO. Genotype H: a new Amerindian genotype of hepatitis B virus revealed in Central America. J Gen Virol. 2002;83:2059-2073. [PubMed] |
| 14. | Alvarado-Esquivel C, Sablon E, Conde-González CJ, Juárez-Figueroa L, Ruiz-Maya L, Aguilar-Benavides S. Molecular analysis of hepatitis B virus isolates in Mexico: predominant circulation of hepatitis B virus genotype H. World J Gastroenterol. 2006;12:6540-6545. [PubMed] |
| 15. | Usuda S, Okamoto H, Iwanari H, Baba K, Tsuda F, Miyakawa Y, Mayumi M. Serological detection of hepatitis B virus genotypes by ELISA with monoclonal antibodies to type-specific epitopes in the preS2-region product. J Virol Methods. 1999;80:97-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 226] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 16. | Sakugawa H, Nakasone H, Nakayoshi T, Orito E, Mizokami M, Yamashiro T, Maeshiro T, Kinjo F, Saito A, Miyagi Y. Preponderance of hepatitis B virus genotype B contributes to a better prognosis of chronic HBV infection in Okinawa, Japan. J Med Virol. 2002;67:484-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Sumi H, Yokosuka O, Seki N, Arai M, Imazeki F, Kurihara T, Kanda T, Fukai K, Kato M, Saisho H. Influence of hepatitis B virus genotypes on the progression of chronic type B liver disease. Hepatology. 2003;37:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 315] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 18. | Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1582] [Cited by in RCA: 1518] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 19. | Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2558] [Cited by in RCA: 2519] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 20. | Lin DY, Sheen IS, Chiu CT, Lin SM, Kuo YC, Liaw YF. Ultrasonographic changes of early liver cirrhosis in chronic hepatitis B: a longitudinal study. J Clin Ultrasound. 1993;21:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 151] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Mizokami M, Nakano T, Orito E, Tanaka Y, Sakugawa H, Mukaide M, Robertson BH. Hepatitis B virus genotype assignment using restriction fragment length polymorphism patterns. FEBS Lett. 1999;450:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 184] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Kao JH, Chen PJ, Lai MY, Chen DS. Clinical and virological aspects of blood donors infected with hepatitis B virus genotypes B and C. J Clin Microbiol. 2002;40:22-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Sakugawa H. Correlation between hepatitis B virus infection and chronic liver disease in Okinawa. Kansenshogaku Zasshi. 1992;66:14-21. [PubMed] |
| 24. | Japanese Ministry of Health Welfare. Age-adjusted death rates by prefecture. A Special Report of Vital Statistics. Tokyo, Japan: Statistics and information department, Japanese Ministry of Health and Welfare 2000; 278-279. |
| 25. | Chu CJ, Hussain M, Lok AS. Hepatitis B virus genotype B is associated with earlier HBeAg seroconversion compared with hepatitis B virus genotype C. Gastroenterology. 2002;122:1756-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 360] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 26. | Kao JH, Chen PJ, Lai MY, Chen DS. Hepatitis B virus genotypes and spontaneous hepatitis B e antigen seroconversion in Taiwanese hepatitis B carriers. J Med Virol. 2004;72:363-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 140] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | Sugauchi F, Orito E, Ichida T, Kato H, Sakugawa H, Kakumu S, Ishida T, Chutaputti A, Lai CL, Ueda R. Hepatitis B virus of genotype B with or without recombination with genotype C over the precore region plus the core gene. J Virol. 2002;76:5985-5992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 233] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 28. | Sugauchi F, Orito E, Ichida T, Kato H, Sakugawa H, Kakumu S, Ishida T, Chutaputti A, Lai CL, Gish RG. Epidemiologic and virologic characteristics of hepatitis B virus genotype B having the recombination with genotype C. Gastroenterology. 2003;124:925-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 165] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 29. | Lindh M, Hannoun C, Dhillon AP, Norkrans G, Horal P. Core promoter mutations and genotypes in relation to viral replication and liver damage in East Asian hepatitis B virus carriers. J Infect Dis. 1999;179:775-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 244] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 30. | Carman WF, Jacyna MR, Hadziyannis S, Karayiannis P, McGarvey MJ, Makris A, Thomas HC. Mutation preventing formation of hepatitis B e antigen in patients with chronic hepatitis B infection. Lancet. 1989;2:588-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 866] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
S- Editor Zhu LH L- Editor Negro F E- Editor Liu Y