Published online Sep 7, 2007. doi: 10.3748/wjg.v13.i33.4504
Revised: June 13, 2007
Accepted: June 18, 2007
Published online: September 7, 2007
AIM: To investigate whether changes in the frequency of peripheral natural killer T (NKT) cells were correlated with liver disease in patients who had metabolic predispositions to nonalcoholic fatty liver disease (NAFLD).
METHODS: Peripheral blood samples were obtained from 60 Chinese NAFLD patients and 60 age and gender matched healthy controls. The frequency of peripheral NKT cells was detected by flow cytometry. Clinical and laboratory data were collected for further analysis.
RESULTS: NAFLD patients had a lower frequency of peripheral NKT cells than healthy controls (1.21% ± 0.06% vs 1.62% ± 0.07%, P < 0.001). Further analysis revealed that the frequency of peripheral NKT cells was negatively correlated with body mass index, waist circumference and serum levels of alanine aminotransferase. Logistic regression analysis revealed that elevated body mass index [hazard ratio (HR): 2.991], aspartate aminotransferase levels (HR: 1.148) and fasting blood sugar (HR: 3.133) increased the risk of NAFLD, whereas an elevated frequency of peripheral NKT cells (HR: 0.107) decreased the risk.
CONCLUSION: Changes in the frequency of peripheral NKT cells were correlated with NAFLD and a decreased frequency of peripheral NKT cells was a risk factor for NAFLD.
- Citation: Xu CF, Yu CH, Li YM, Xu L, Du J, Shen Z. Association of the frequency of peripheral natural killer T cells with nonalcoholic fatty liver disease. World J Gastroenterol 2007; 13(33): 4504-4508
- URL: https://www.wjgnet.com/1007-9327/full/v13/i33/4504.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i33.4504
Nonalcoholic fatty liver disease (NAFLD), ranging from nonalcoholic steatosis to nonalcoholic steatohepatitis (NASH) and cirrhosis, may be the most common liver disease in western countries, with a prevalence of 20%-30% in the general population[1,2]. Nonalcoholic steatosis is considered to be a benign condition, but NASH can progress to cirrhosis and liver failure, and the 5-year survival rate for an individual diagnosed with NASH is estimated to be 67%[3]. Although NAFLD has been extensively studied in recent years, the exact pathogenesis of this disease remains unknown.
Natural killer T (NKT) cells were originally defined in mice in the last decade as a lymphocyte subtype that co-expresses natural killer receptors together with T cell receptors[4]. A striking characteristic of these T cells is the recognition of lipid antigens presented by the restrictive non-classical, non-polymorphic MHC class I-like CD1d molecule[5]. The capacity for rapid secretion of cytokines, such as interleukin-4, interferon-γ, interleukin-10 and interleukin-13, assures these cells an immuno-modulatory role in autoimmune, allergic, antimicrobial and anti-tumor immune responses[6].
Recently, several lines of evidence from animal experiments have suggested a link between NKT cell deficiency and NAFLD[7-10]. Hepatic NKT cells were reduced in leptin-deficient ob/ob mice[7] and in mice fed with a high fat diet[8]. Adoptive transfer of NKT cells[9] or oral administration of liver-extracted proteins[10] ameliorated steatosis and glucose intolerance in leptin-deficient ob/ob mice. These metabolic improvements were partly associated with an increase in hepatic NKT cell numbers[9,10].
These experimental results support a regulatory role for NKT cells; however, their role in the clinical setting of NAFLD remains unclear. This observation prompted this investigation of the possible role of NKT cells in NAFLD patients. The present study questions whether changes in the frequency of peripheral NKT cells are correlated with liver disease in patients with a metabolic predisposition to NAFLD.
This study was carried out at the First Affiliated Hospital of Zhejiang University School of Medicine. All subjects were volunteers attending their annual examination at our hospital from Sep 5 to Oct28, 2005. Informed consent was obtained from all subjects and the study protocol was approved by the hospital Ethics Committee.
The diagnosis of NAFLD was based on the criteria established by the Fatty Liver and Alcoholic Liver Disease Study Group of the Chinese Liver Disease Association[11]. The exclusion criteria specific to this study included persons with a self-reported history of acute infection or tissue injury in the previous 3 mo, patients with a history of a malignant tumor or autoimmune disease, and patients above 65 years old or below 20 years old. A total of 60 eligible NAFLD patients were enrolled (50 males and 10 females, median age 40.0 years, range from 24 years to 65 years).
For each NAFLD patient, one control was enrolled with matching gender and age (within 3 years). A total of 60 healthy controls were enrolled (50 males and 10 females, median age 42.0 years, range from 25 years to 65 years). All controls were free of viral hepatitis and autoimmune disease and had alcohol consumption within “sensible” limits (less than 30 g/d for men and less than 20 g/d for women). Exclusion criteria were the same as for the patient group.
The clinical examinations were administered in the mornings after an overnight fast, and the subjects were also instructed to refrain from exercise during the day before their examination. The examination consisted of a physical examination by a physician, blood draw, blood pressure measurement, anthropometry and a health habit inventory. Body mass index (BMI, kg/m2), used as an index of body fat, was calculated as weight in kilograms divided by height in meters squared. The waist to hip ratio was calculated as waist circumference divided by hip circumference.
Blood samples were obtained from an antecubital vein and the samples were used for the analysis of biochemical values and NKT cells frequency. Biochemical values were measured by the Hitachi autoanalyzer model 7600 (Hitachi Corp, Japan). The biochemical values included alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride, total and high-density lipoprotein cholesterol and fasting blood sugar (FBS).
The frequency of peripheral NKT cells was measured by flow cytometry as previously described[12,13]. In brief, two-color flow cytometric analysis was performed with an EPICS-XL flow cytometer (Beckman-Coulter Corp, USA) using System II software. FITC-conjugated anti-Vα24 monoclonal antibody (Immunotech, France) was used to label NKT cells, and isotype matched controls were also used in all experiments. Vα24+ T cells were considered to be NKT cells in the present study.
Dates are presented as mean ± SE. The data were analyzed by SPSS11.5 statistical software. The Mann-Whitney U test or Student’s t-test were used for comparisons of the data. Spearman correlation analysis was used to estimate the relationship between the frequency of peripheral NKT cells and other variables. Logistic regression analysis (Backward: Wald; Entry: 0.05, Removal: 0.10) was used to evaluate the risk factors for NAFLD. P < 0.05 (2-tailed test) was considered statistically significant.
NAFLD patients and healthy controls were different in terms of weight, BMI, waist circumference, hip circumference, waist to hip ratio, systolic blood pressure, diastolic blood pressure, ALT, AST, triglyceride, total-lipoprotein cholesterol and FBS, while there was no difference in terms of age, gender, height, or high-density lipoprotein cholesterol between the two groups (Table 1).
| Variable | Healthy controls | NAFLD patients |
| Age (yr) | 43.0 ± 1.3 | 43.0 ± 1.3 |
| Height (cm) | 166.7 ± 6.2 | 167.1 ± 6.9 |
| Weight (kg) | 62.6 ± 7.6 | 75.3 ± 10.4 b |
| Body mass index (kg/m2) | 22.50 ± 0.29 | 26.91 ± 0.37 b |
| Waist circumference (cm) | 80.2 ± 0.8 | 92.0 ± 1.1b |
| Hip circumference (cm) | 93.7 ± 0.5 | 100.3 ± 0.7 b |
| Waist-hip ratio | 0.856 ± 0.006 | 0.916 ± 0.006 b |
| Systolic blood pressure (mmHg) | 119.5 ± 2.2 | 130.3 ± 2.0 b |
| Diastolic blood pressure (mmHg) | 73.3 ± 1.4 | 81.0 ± 1.2 b |
| Alanine aminotransferase (U/L) | 21.5 ± 1.8 | 46.9 ± 3.6 b |
| Aspartate aminotransferase (U/L) | 24.4 ± 1.0 | 36.0 ± 2.8 b |
| Triglyceride (mmol/L) | 1.712 ± 0.142 | 2.632 ± 0.144 b |
| Total cholesterol (mmol/L) | 4.583 ± 0.111 | 4.973 ± 0.117 d |
| High density lipoprotein cholesterol (mmol/L) | 1.264 ± 0.277 | 1.277 ± 0.033 |
| Fasting blood sugar (mmol/L) | 4.356 ± 0.068 | 5.567 ± 0.311b |
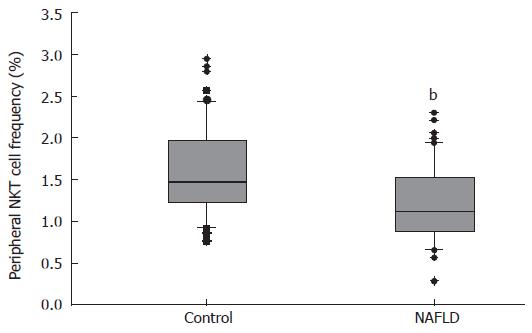
NAFLD patients had a lower frequency of peripheral NKT cells, 1.21% ± 0.06% in NAFLD patients versus 1.62% ± 0.07% in healthy controls (P < 0.001) (Figure 1).
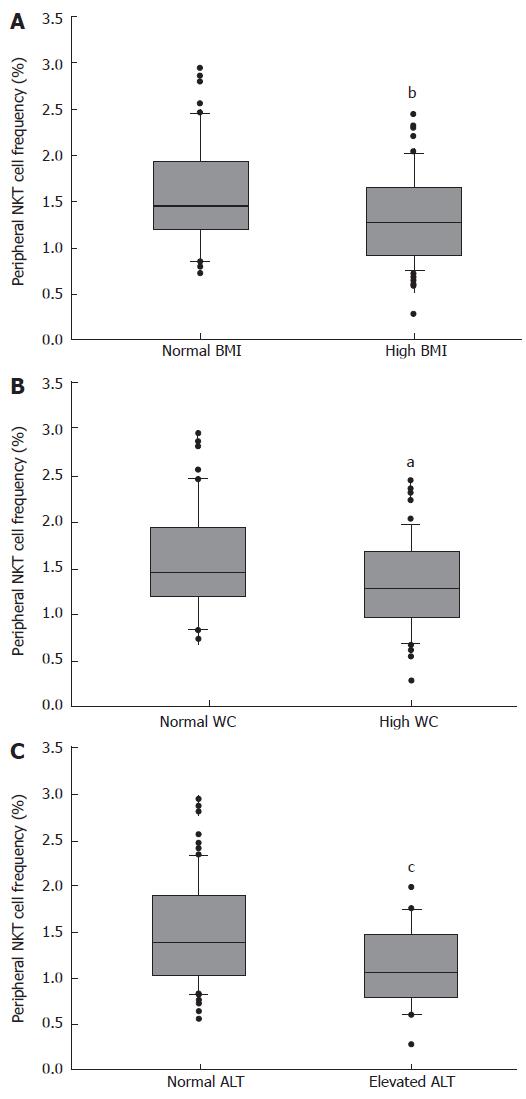
Spearman correlation analysis revealed that there was a poor correlation between the frequency of peripheral NKT cells and BMI (r = -0.322, P = 0.001), waist circumference (r = -0.237, P = 0.021) and ALT levels (r = -0.217, P = 0.035).
We further analyzed the relationship between the frequency of peripheral NKT cells and the three variables. As shown in Figure 2, the frequency of peripheral NKT cells was decreased in the subjects with high BMI (BMI ≥ 24 kg/m2), or in the subjects with high waist circumference (waist circumference ≥ 90 cm for male and ≥ 85 cm for female), or in the subjects with elevated ALT (ALT ≥ 50 U/L). This result partially indicated that the frequency of peripheral NKT cells was negatively correlated with BMI, waist circumference and ALT.
Stepwise regression analysis was performed on the 11 variables that were different between the two groups (weight and hip circumference were excluded) using the dichotomous variable logistic regression model. The results showed that four features were closely associated with the risk for NAFLD, including the frequency of peripheral NKT cells, BMI, AST and FBS (Table 2).
| Variable | β | SE | Wald value | P-value | OR | 95% CI of OR |
| NKT | -2.239 | 0.984 | 5.181 | 0.023 | 0.107 | 0.016-0.733 |
| BMI | 1.096 | 0.370 | 8.755 | 0.003 | 2.991 | 1.448-6.181 |
| AST | 0.138 | 0.062 | 4.960 | 0.026 | 1.148 | 1.017-1.296 |
| FBS | 1.142 | 0.459 | 6.186 | 0.013 | 3.133 | 1.274-7.707 |
| Constant | -53.119 | 14.751 | 12.967 | < 0.001 | < 0.001 | - |
These results indicate that changes in the frequency of peripheral NKT cells are correlated with liver disease in patients with a metabolic predisposition to NAFLD. This finding was supported by three main results. First, NAFLD patients had a lower frequency of peripheral NKT cells, a unique T lymphocyte subtype that shares some characteristics with natural killer cells[14]. NKT cells have been shown to play important regulatory roles in various liver diseases such as viral hepatitis[15], autoimmune liver disease[16], metabolic liver disease[17] and hepatic malignant tumor[18]. The frequency of peripheral NKT cells was decreased in patients with hepatitis C[15] and autoimmune hepatitis[16]. The decreased frequency of peripheral NKT cells may be caused by down-regulation of T cell receptors, apoptosis of the cells and/or compartmentalization into peripheral organs[15]. The decreased frequency of peripheral NKT cells in NAFLD patients in the present study indicates that NAFLD may represent another liver disease related to a decreased frequency of peripheral NKT cells.
The second result was that the frequency of peripheral NKT cells was negatively correlated with BMI, waist circumference and ALT. NAFLD has been shown to be strongly associated with excess body weight and central adiposity in particular[19-21]. Most NAFLD patients are overweight, and the prevalence of NAFLD is much more common in obese than in non-obese individuals (76% vs 16%)[19]. BMI and waist circumference, used as indicators for obesity and central obesity, are closely related with NAFLD[19-22]. ALT is also closely related with NAFLD, and excluding causes such as chronic hepatitis and alcohol-induced liver disease, NAFLD explains 80% to 90% of the remaining cases of elevated ALT[23]. Therefore, elevated ALT has been used as a noninvasive surrogate marker for NAFLD[21,24]. In the present study, the frequency of peripheral NKT cells was found to be significantly correlated with BMI, waist circumference and ALT, but the r values were very low, indicating that the correlation between these terms was very weak. However, as BMI, waist circumference and ALT were all strongly associated with NAFLD, the present findings might suggest that peripheral NKT cells are correlated with NAFLD.
The third result supporting the correlation of NKT cells with NAFLD was that the decreased frequency of peripheral NKT cells was a risk factor for NAFLD. Previous studies revealed that overweight, diabetes mellitus and elevated liver enzymes were risk factors for NAFLD in agreement with our results[1,19,25,26]. In addition, this study showed that the decreased frequency of peripheral NKT cells was another risk factor for NAFLD. This association may due, at least in part, to a diminished protective effect of the lower number of NKT cells against vulnerable factors such as lipopolysaccharide (LPS)[8]. This finding also suggests that NKT cells are associated with NAFLD, and could lead to immune manipulations of NKT cells as a therapeutic tool in NAFLD in the future. Interestingly, data from animal experiments confirmed the therapeutic role of NKT cells in NAFLD[9,10].
This study had some limitations, however. The first limitation was regarding the methodology used to evaluate peripheral NKT cells. Until recently, NKT cells could not be unambiguously identified. αGalCer/CDld tetramers and Vα24/Vβ11 double-staining are considered to be two standard methods for NKT cell identification[27]. Vα24 staining alone as used in the present study may overestimate the numbers of Vα24 and Vβ11 double-positive NKT cells. However, because NKT cells represent a subtype of T lymphocytes, staining NKT cells with Vα24 and CD3 reduces the possibility of overestimation, and the same method has also been used by previous studies[12,13]. The second limitation was that hepatic NKT cells were not studied in NAFLD patients or in healthy controls. NKT cells arise mainly in the thymus and migrate to peripheral tissues, such as the liver and pancreas, where they accumulate in large numbers[28]. It would be much more meaningful to study hepatic NKT cells in NAFLD patients, but it was not convenient to get enough liver tissue samples from NAFLD subjects, in part due to ethical reasons. The third limitation is that the involvement of peripheral NKT cells in NAFLD was not studied. NKT cells have several subtypes such as CD4+CD8-, CD4-CD8- (DN) and CD4-CD8+, and different subtypes have different functions[6]. Further studies are needed to understand the relationship between the subtypes of NKT cells and NAFLD.
In conclusion, these results suggest that changes in the frequency of peripheral NKT cells were correlated with liver disease in patients who had a metabolic predisposition to NAFLD, and a decreased frequency of these cells was a risk factor for NAFLD. These findings suggest a potential role for peripheral NKT cells in the pathogenesis of NAFLD.
The authors thank all subjects for their valuable contributions to the present work. We are also grateful to Dr. Wei Wu for his technical assistance in flow cytometry and Professor Yi Shen for his excellent assistance in statistical analysis.
Nonalcoholic fatty liver disease (NAFLD) has been extensive studied in recent years, but the mechanism of this disease remains unclear. The most popular two-hit hypothesis keeps that hepatic fat accumulation is the first hit and concomitant hepatic inflammation is the second hit. Several potential pathogenic factors including insulin resistance, mitochondrial dysfunction, lipid peroxidation and immunity abnormality have been intensively investigated.
To explore the role of NKT cells in the clinical setting of NAFLD.
Recent evidences from animal experiments indicated an association between NKT cells and NAFLD. It was observed that hepatic NKT cells were reduced in leptin-deficient ob/ob mice and in mice fed with a high fat diet. Adoptive transfer of NKT cells or oral administration of liver-extracted proteins ameliorated steatosis and glucose intolerance in leptin-deficient ob/ob mice.
Previous evidences supporting the association between NKT cells and NAFLD were all from animal experiments. Whether NKT cells also play important regulatory role in the clinical setting of NAFLD remains unclear. In this study, the authors found that changes in the frequency of peripheral NKT cells were correlated with NAFLD and a decreased frequency of peripheral NKT cells was a risk factor for NAFLD.
The results may provide new theoretic and experimental evidences for the study of the pathogenesis of NAFLD, and new therapeutic approaches for NAFLD by regulating the balance of NKT cells in the body.
NKT cells were originally defined in mice in the last decade as a lymphocyte subtype that co-expresses natural killer receptors together with T cell receptors. A striking characteristic of these T cells is the recognition of lipid antigens presented by the restrictive non-classical, non-polymorphic MHC class I-like CD1d molecule. NKT cells play an important immuno-modulatory role in autoimmune, allergic, antimicrobial and anti-tumor immune responses.
The paper by Xu et al describes that a reduced number of peripheral NKT cells is associated with NAFLD. The topic of the paper is highly interesting. The patients have to be much better characterized.
S- Editor Liu Y L- Editor Rippe RA E- Editor Wang HF
| 1. | Jimba S, Nakagami T, Takahashi M, Wakamatsu T, Hirota Y, Iwamoto Y, Wasada T. Prevalence of non-alcoholic fatty liver disease and its association with impaired glucose metabolism in Japanese adults. Diabet Med. 2005;22:1141-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 312] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 2. | Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1488] [Cited by in RCA: 1484] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 3. | Reid AE. Nonalcoholic steatohepatitis. Gastroenterology. 2001;121:710-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 354] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 4. | Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1064] [Cited by in RCA: 1032] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 5. | Vincent MS, Gumperz JE, Brenner MB. Understanding the function of CD1-restricted T cells. Nat Immunol. 2003;4:517-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Berzins SP, Smyth MJ, Godfrey DI. Working with NKT cells--pitfalls and practicalities. Curr Opin Immunol. 2005;17:448-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Guebre-Xabier M, Yang S, Lin HZ, Schwenk R, Krzych U, Diehl AM. Altered hepatic lymphocyte subpopulations in obesity-related murine fatty livers: potential mechanism for sensitization to liver damage. Hepatology. 2000;31:633-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 149] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Li Z, Soloski MJ, Diehl AM. Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology. 2005;42:880-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 241] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 9. | Elinav E, Pappo O, Sklair-Levy M, Margalit M, Shibolet O, Gomori M, Alper R, Thalenfeld B, Engelhardt D, Rabbani E. Adoptive transfer of regulatory NKT lymphocytes ameliorates non-alcoholic steatohepatitis and glucose intolerance in ob/ob mice and is associated with intrahepatic CD8 trapping. J Pathol. 2006;209:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Elinav E, Pappo O, Sklair-Levy M, Margalit M, Shibolet O, Gomori M, Alper R, Thalenfeld B, Engelhardt D, Rabbani E. Amelioration of non-alcoholic steatohepatitis and glucose intolerance in ob/ob mice by oral immune regulation towards liver-extracted proteins is associated with elevated intrahepatic NKT lymphocytes and serum IL-10 levels. J Pathol. 2006;208:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Diagnostic criteria of nonalcoholic fatty liver disease. Zhonghua GanZangBing ZaZhi. 2003;11:71. [PubMed] |
| 12. | Wan JJ, Zeng YY, He XH, Xu LH, Cai XC. The characteristics of TCRVa24 NKT cells in response to in vitro stimulation. Zhongguo Bingli Shengli Zazhi. 2002;18:774-777. |
| 13. | DelaRosa O, Tarazona R, Casado JG, Alonso C, Ostos B, Peña J, Solana R. Valpha24+ NKT cells are decreased in elderly humans. Exp Gerontol. 2002;37:213-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what's in a name? Nat Rev Immunol. 2004;4:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 882] [Cited by in RCA: 963] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 15. | Lucas M, Gadola S, Meier U, Young NT, Harcourt G, Karadimitris A, Coumi N, Brown D, Dusheiko G, Cerundolo V. Frequency and phenotype of circulating Valpha24/Vbeta11 double-positive natural killer T cells during hepatitis C virus infection. J Virol. 2003;77:2251-2257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Cherñavsky AC, Paladino N, Rubio AE, De Biasio MB, Periolo N, Cuarterolo M, Goñi J, Galoppo C, Cañero-Velasco MC, Muñoz AE. Simultaneous expression of Th1 cytokines and IL-4 confers severe characteristics to type I autoimmune hepatitis in children. Hum Immunol. 2004;65:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Kinebuchi M, Matsuura A, Ohya K, Abo W, Kitazawa J. Contribution of Va24Vb11 natural killer T cells in Wilsonian hepatitis. Clin Exp Immunol. 2005;139:144-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Shibolet O, Alper R, Zlotogarov L, Thalenfeld B, Engelhardt D, Rabbani E, Ilan Y. Suppression of hepatocellular carcinoma growth via oral immune regulation towards tumor-associated antigens is associated with increased NKT and CD8+ lymphocytes. Oncology. 2004;66:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Bellentani S, Saccoccio G, Masutti F, Crocè LS, Brandi G, Sasso F, Cristanini G, Tiribelli C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 842] [Cited by in RCA: 867] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 20. | Church TS, Kuk JL, Ross R, Priest EL, Biltoft E, Blair SN. Association of cardiorespiratory fitness, body mass index, and waist circumference to nonalcoholic fatty liver disease. Gastroenterology. 2006;130:2023-2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 195] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 21. | Ruhl CE, Everhart JE. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2003;124:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 408] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 22. | Eguchi Y, Eguchi T, Mizuta T, Ide Y, Yasutake T, Iwakiri R, Hisatomi A, Ozaki I, Yamamoto K, Kitajima Y. Visceral fat accumulation and insulin resistance are important factors in nonalcoholic fatty liver disease. J Gastroenterol. 2006;41:462-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 225] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 23. | Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 655] [Article Influence: 27.3] [Reference Citation Analysis (2)] |
| 24. | Suzuki A, Lindor K, St Saver J, Lymp J, Mendes F, Muto A, Okada T, Angulo P. Effect of changes on body weight and lifestyle in nonalcoholic fatty liver disease. J Hepatol. 2005;43:1060-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 215] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 25. | Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999-2002. Am J Gastroenterol. 2006;101:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 233] [Article Influence: 11.7] [Reference Citation Analysis (1)] |
| 26. | Chen QK, Chen HY, Huang KH, Zhong YQ, Han JA, Zhu ZH, Zhou XD. Clinical features and risk factors of patients with fatty liver in Guangzhou area. World J Gastroenterol. 2004;10:899-902. [PubMed] |
| 27. | Metelitsa LS. Flow cytometry for natural killer T cells: multi-parameter methods for multifunctional cells. Clin Immunol. 2004;110:267-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. A thymic precursor to the NK T cell lineage. Science. 2002;296:553-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 415] [Article Influence: 17.3] [Reference Citation Analysis (0)] |