INTRODUCTION
Hepatitis C virus (HCV) infection has become a major problem of public health with more than 100 million infected individuals worldwide, including over 30 million in China. Unfortunately, up to 60%-80% of HCV infected adults will develop chronic liver disease, although the clinical phenomena may vary greatly from being asymptomatic to liver cirrhosis or heptocellular carcinoma (HCC)[1]. Interferon is still the major drug against chronic hepatic HCV infection, but sustained virologic response (SVR) to interferon alone remains low, especially in HCV 1b infected patients. Although a combination of peglated IFN and ribovirin may greatly improve the SVR and now become the standard therapy, some patients still remain unresponsive. HCV proteins which may be related to interferon resistance include core protein C[2], envelope protein E[3], nonstructural protein NS3/4A[4] and NS5A[5]. As one of the HCV nonstructural proteins, NS5A plays an important role in the interferon response, viral replication, and hepatic carcinogenesis[6]. Janus kinase-signal transducer and activator of the transcription (JAK-STAT) signaling pathway is a major interferon-induced signal pathway which executes the anti-virus function. STAT1 is a member of the STAT family, which exists in the cytoplasm as an inactivated monomer. Induced by interferon, STAT1 is Tyr701 phosphorylated and dimerized, then translocated into the nucleus to stimulate ISGs (interferon stimulated genes) transactivation and anti-viral protein expression. The anti-viral effectiveness of interferon may be inhibited when this STAT pathway is damaged. HCV NS5A protein is supposed to play an important role in HCV resistance to IFN, and one of the mechanisms is to interact with PKR[5,7,8], but it is still uncertain whether HCVNS5A has any influence on the STAT signal. In this experiment we found that HCV NS5A may inhibit STAT1 Tyr701 phosphorylation and nuclear translocation, which may be a new way of HCV NS5A to interfere with IFN function.
MATERIALS AND METHODS
Cell lines and plasmids transfection
Plasmid PRC/CMV is a gift from Professor Siddiqui (University of Colorado, USA) Plasmids pCNS5A is an eukaryotic expression vector made in Dr. Siddiqui’s laboratory, which was constructed by cloning HCV type 1b NS5A cDNA into PRC/CMV plasmid[9]. The liver carcinoma cell lines Huh7 (preserved at the Institute of Hepatology, The Second Xiangya Hospital, Central South University) were grown in Dulbecco’s modified eagle medium (Life Technologies, USA) with 10% fetal bovine serum at 37°C and 5% CO2. The cells were transfected by the individual plasmids with Lipofectin reagent (Life Technologies, USA) when they became 60%-70% confluent. Forty-eight h after transfection, the cells were harvested for the detection of HCV NS5A protein expression by immunocytochemistry, and the analysis of STAT1 Tyr701 phosphorylation and nuclear translocation by immunocytofluoresence and Western blot.
Western blot experiment
Total cellular proteins of Huh7 cells induced by IFNα-2b (Harbin Pharmaceutical Company, China) at different time points (0.25, 0.5, 1, 2, 4 and 8 h) were extracted as follows: Huh7 cells were collected and 100 μL lysate solution [150 mmol/L NaCl, 1 mmol/L EGTA, 1 mmol/L NaF, 50 mmol/L Tris-HCl (pH7.4), 1% NP-40, 0.1 mmol/L NaN3, 50 μg/mL PMSF] was added. After 30 min incubation on ice and centrifugation at 4°C, 12 000 ×g for 10 min, the supernatant was saved as total cellular protein for further studies. The same amount of total protein was used to load onto 10% SDS-PAGE, and then transferred onto a NC membrane (Schleicher & Schuell Company). After blocking in 5% fat-free milk for 1 h, the membrane was probed by anti-phosphorylated STAT1 (Tyr701) (Cell Signaling Technology), washed and incubated with the second HRP-labeled antibody (Boster, China), and then observed by DAB staining (KPL, USA), and anti-actin (Santa Cruz) served as the control.
Immunocytochemistry and immunofluorescence assay
For immunocytochemistry, PCNS5A and PRC/CMV-transfected and non- transfected Huh7, cells were washed three times and fixed with 4% paraformaldehyde, then blocked with 5% normal goat serum. Serum from HCV infected patients as primary antibody was added to bind HCV NS5A protein, followed by HRP-labeled antibody incubation and DAB staining. For immunofluorecent assay, PCNS5A- and PRC/CMV-transfected and non-transfected Huh7 cells induced by IFNα-2b for 30 min were washed and fixed as described in immunocytochemistry. Anti-STAT1 (Boster, China), anti-phosphorylated STAT1 (Tyr701, Cell Signaling Technology, USA) and FITC-labeled antibody (KPL, USA) were employed, and the staining was observed under fluorescent microscope.
RESULTS
HCV NS5A expression was detected in PCNS5A-transfected cells
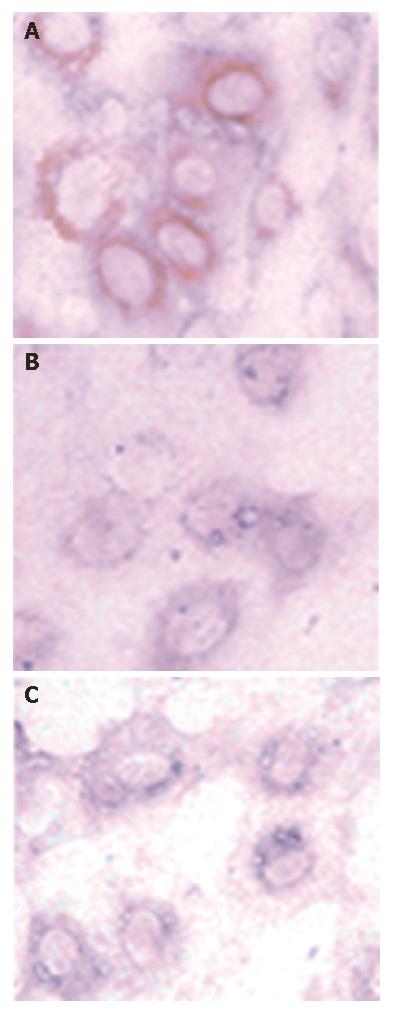
HCV NS5A protein was found in brown staining, and distributed in the cytoplasm around the nucleus in some PCNS5A-transfected huh7 cells, but no staining was observed in PRC/CMV transfected or non-transfected cells (Figure 1). This result indicates that NS5A plasmid was successfully transfected into Huh 7 cells and NS5A protein expressed.
Figure 1 HCV NS5A protein staining of Huh7 cells after 48 h transfection (DAB, × 400).
A: PCNS5A-transfected cells; B: PRC/CMV-transfected cells; C: Non-transfected cells.
STAT1 Tyr701 phosphorylation after induction of IFNα-2b
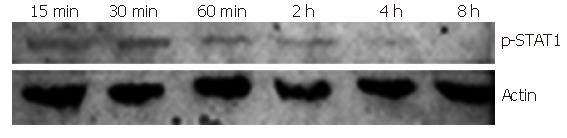
STAT1 Tyr701 was mostly phosphorylated after 30-min of induction of IFNα-2b. Western blot showed that the time point of 30 min after IFNα-2b induction was the best for STAT 1Tyr701 phosphorylation, though early to 15 min and later till 4 h after IFN stimulation, the STAT1 phosphorylation was still detected (Figure 2). We therefore chose the 30 min of IFN induction as the time point for further experiments.
Figure 2 Tyr701 phosphorylated STAT1 in Huh7 cells induced by IFNα-2b (10 000 U/mL) (Western blot).
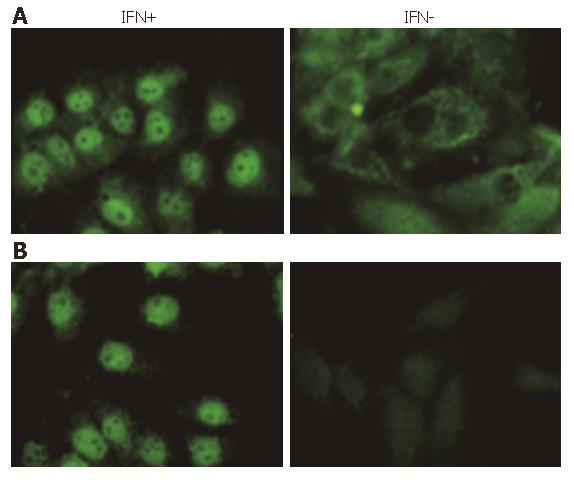
Immunoflurorescence assay was used to identify the STAT1 phosphorylation and nuclear translocation more clearly. Under fluorescent microscopy, whole STAT1 was found in the plasm of Huh7 cells, but not in nucleus without IFN stimulation. After 30-min induction of IFNα-2b, almost all of the STAT1 translocated into the nuclei (Figure 3A). When anti-phosphorylated STAT1 (Tyr701) was employed as the first antibody, as we anticipated, there was no staining of STAT1 in the cytoplasm of the cells without IFN stimulation, indicating that STAT1 found in the plasma was un-phosphorylated. It was also demonstrated in this experiment that the STAT1 in nucleus was totally stained after 30 min of IFN induction, indicating the STAT1 in the nucleus was phosphorylated (Figure 3B). These results showed that IFNα-2b was able to induce STAT1 phosphorylation and sequential nuclear import.
Figure 3 STAT1 or Phosphorylated STAT1 (Tyr701) in Huh7 cells stimulated by IFNα-2b (10 000 U/mL) for 30 min (FITC, × 400).
A: STAT1; B: Tyr701.
IFNα-2b-induced STAT1 phosphorylation and nuclear translocation inhibited by HCV NS5A protein
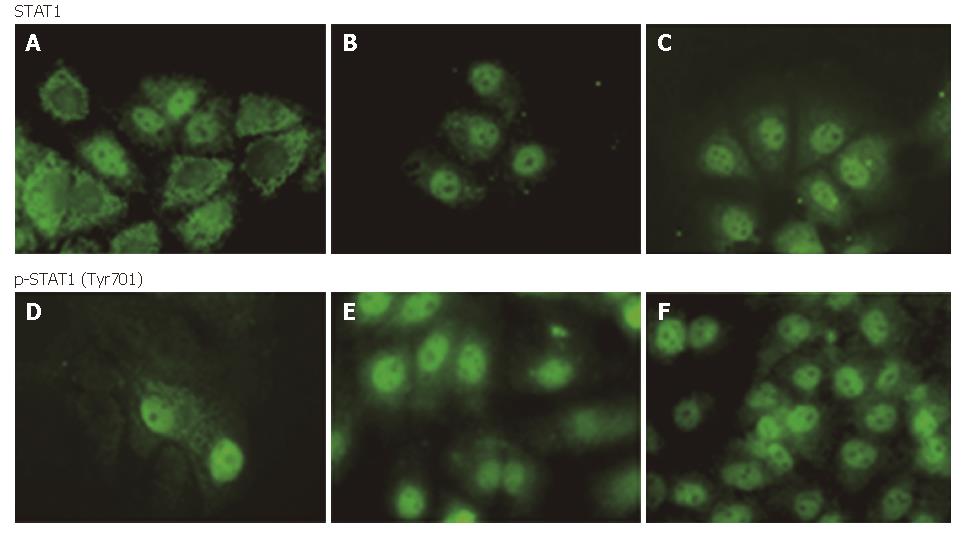
When the JAK-STAT signal pathway was activated by IFN, STAT1 molecules originally distributed in the plasma were phosphorylated at Tyr701 and translocated into the nucleus in a short time. In our experiment, Huh7 cells were induced by IFNα-2b (10 000 U/mL) for 30 min. We found that PRC/CMV-transfected and non-transfected cell nuclei were stained for STAT1, and no difference was observed between the two-groups of cells. On the contrary, most PCNS5A -transfected cells were nuclei negative while plasma positive for STAT1, indicating the inhibition of HCV NS5A on IFN-induced STAT1 nuclear import. With regard to the test using anti-phosphorylated STAT1 as the first antibody, most of the NS5A-expressed cell nuclei were not stained for STAT1, suggesting that HCV NS5A inhibited STAT1 phosporylation. Some cell’s nuclei were stained for STAT1, possibly because these cells were not successfully transfected by the NS5A expression plasmid. In the other two groups, all the cell nuclei were positive and plasma was negative for p-STAT1 (Figure 4).
Figure 4 STAT1 or phosphorylated STAT1 in cytoplasm and nuclei of Huh7 cells after 30 min IFN induced (FITC, × 400): A and D: PCNS5A-transfected; B and E: PRC/CMV-transfected; C and F: non-transfected.
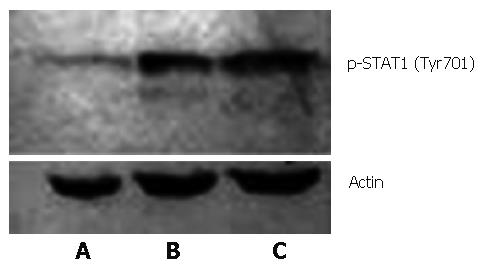
Western blot was performed to further confirm NS5A's inhibition of STAT1 phosphorylation induced by IFN. It turned out that phosphorylated STAT1 (Tyr701) expression was remarkably lower in the presence of HCV NS5A compared with the other two groups with no NS5A protein expression (Figure 5), indicating that HCV NS5A had an inhibitive effect on STAT1 Tyr701 phosphorylation.
Figure 5 Phosphorylated STAT1 from IFN induced Huh7 cells (FITC, × 400): A: PCNS5A-transfected; B: PRC/CMV-transfected; C: Non-transfected.
DISCUSSION
HCV is a positive single-stranded RNA virus belonging to the Flaviridae family, and its genome only contains a single long open reading frame encoding a large poly- protein precursor which is thereafter processed by a combination of cellular and viral proteases into several mature proteins, including three or four structural proteins (core, E1, E2/P7) and at least 6 nonstructural proteins (NS): NS2, NS3, NS4A, NS4B, NS5A, NA5B[10]. HCV NS5A is a phosphoprotein that exists mostly in the cytoplasm in two different forms of Mr 56 000 and 58 000 with modifications of serine residues. Mr 58 000 form is produced by additional phosphorylation of the Mr 56 000 form[11]. In 1995, Enomoto et al[12] first discovered the association between HCV NS5A and sensitivity to interferon therapy. Since then, a lot of work has been done and showed that HCV NS5A might affect an interferon therapeutic effect through several ways, including variation of amino acid in the HCV NS5A ISDR (interferon sensitivity determining region)[12] or variable region V3[13,14], interaction between antiviral protein PKR(double-stranded RNA-dependent protein kinase)[5,7,8] or 2’, 5’-OAS (2’, 5’-oligoadenylate synthetase)[15] and others[16,17]. But it is still uncertain whether HCV NS5A has any effect on the IFN-induced STAT signal pathyway. Recently, NS5 protein from other viruses such as Langat virus (LGTV) and Japanese encephalitis virus have been reported to inhibit IFN-induced STAT signaling[23,24]. This aroused our interest in knowing if HCV NS5A has similar function.
It is well known that the interferon mediated antiviral effect is mainly through a JAK-STAT pathway, in which STAT1 plays an important role as molecular messenger. STAT1 exists in two isomerides, STAT1α and splice variant STAT1β/STAT2. STAT1α gene knockout rats shows no response to interferon α or γ[18], indicating the criticality of STAT1 in interferon antiviral activity. STAT1 molecules originally distribute in cytoplasm. Induced by interferon, the 701 amino acid residue in the C terminal of STAT1 is phosphorylated and interferon stimulatory gene factors are formed, followed by nuclear import with importin α 5[19], binding to ISRE (interferon-stimulated response element), and regulation of transactivation and expression of antiviral proteins. So in our experiment, we mainly focused on the possible effect of HCV NS5A on STAT1 phosphrylation and nuclear translocation. The results showed that STAT1 phosphorylation became strong gradually when induced by interferon from 15 to 30 min and decreased quickly in a few hours, which is in accordance with other reports[19]. When HCV NS5A protein was introduced by transient transfection, IFN-induced STAT1 nuclear translocation was greatly decreased. Since STAT1 Tyr701 phosphorylation is the prerequisite of IFN-induced STAT1 nuclear import, we further proved that STAT1 Tyr701 phophorylation and sequential nuclear translocation were both reduced in the presence of HCV NS5A, indicating that HCV NS5A interferes with the interferon signaling pathway. It might be one of the HCV NS5A related molecular mechanism in interferon resistance. Interestingly, almost in the same time, Lan also reported the similar result that HCV NS5A protein inhibited IFN-induced STAT1 phosphorylation and nuclear translocation in three heptocyte-derived cell lines, and found HCV NS5A protein could interact with the N-terminal of STAT1, which would be the molecular mechanism by which HCV NS5A inhibits STAT1 phosphorylation[25]. They used full-length HCV and HCV subgenomic constructs to transiently transfect Huh-T7 cells. HCV NS5A protein may be affected by other HCV proteins such as NS5B[26]. Ours and other’s results suggested that HCV NS5A protein may inhibit IFN-induced STAT1 phosphorylation and the subsequent nuclear import. One of the mechanisms might be the binding of HCV NS5A and STAT1.
JAK-STAT and MAPK pathway “cross-talk” with each other. Serine phosphorylation can modulate tyrosine phosphorylation, activation and DNA-binding activity of STATs while in the MAPK pathway, activated ERK phosphorylates the conserved Ser727 on STAT3 and the serine phosphorylation enhances tyrosine phosphorylation on Tyr701[20-22]. Can HCV NS5A interact with the MAPK pathway and indirectly depress STAT1 phosphorylation in the JAK-STAT pathway? Can HCV NS5A disturb the function of importin α 5? These questions need to be addressed in future studies.