Published online Jan 21, 2007. doi: 10.3748/wjg.v13.i3.480
Revised: October 28, 2006
Accepted: December 2, 2006
Published online: January 21, 2007
A 54-year old man with a family history of hyperlipidemia was admitted with a 12 h history of severe generalized abdominal pain associated with nausea, vomiting and abdominal distension. Examination of the abdomen revealed tenderness in the periumblical area with shifting dullness. Serum pancreatic amylase was 29 IU/L and lipase 44 IU/L, triglyceride 36.28 mmol/L. Ultrasound showed ascites. CT of the abdomen with contrast showed inflammatory changes surrounding the pancreas consistent with acute pancreatitis. Ultrasound (US) guided abdomen paracentesis yielded a milky fluid with high triglyceride content consistent with chylous ascites. The patient was kept fasting and intravenous fluid hydration was provided. Meperidine was administered for pain relief. On the following days the patient’s condition improved and he was gradually restarted on a low-fat diet, and fat lowering agent (gemfibrozil) was begun, 600 mg twice a day. On d 14, abdomen US was repeated and showed fluid free peritoneal cavity. The patient was discharged after 18 d of hospitalization with 600 mg gemfibrozil twice a day. At the time of discharge, the fasting triglyceride was 4.2 mmol/L. After four weeks the patient was seen in the clinic, he was well.
- Citation: Khan FY, Matar I. Chylous ascites secondary to hyperlipidemic pancreatitis with normal serum amylase and lipase. World J Gastroenterol 2007; 13(3): 480-482
- URL: https://www.wjgnet.com/1007-9327/full/v13/i3/480.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i3.480
There are many etiologic factors leading to pancreatitis. Hypertriglyceridemia (HTG) is seldom diagnosed as one of these factors. Pancreatitis secondary to HTG, presents typically as an episode of acute pancreatitis (AP) or recurrent AP, rarely as chronic pancreatitis. Although AP often requires clinicians to rely on laboratory tests, such as serum amylase and lipase, as diagnostic aids, patients with acute pancreatitis secondary to HTG can have normal amylase[1] and lipase levels. Furthermore, acute pancreatitis is a recognized cause of chylous ascites[2]. Association of normal amylase and lipase acute pancreatitis secondary to HTG with chylous ascites is extremely rare. A PubMed search of the literature revealed no cases with such association. We report here an exceptionally rare case of a 54-year old man with chylous ascites secondary to hyperlipidemic pancreatitis with normal serum amylase and lipase.
A 54-year old man was admitted with a 12 h history of severe generalized abdominal pain associated with nausea, vomiting and abdominal distension. His history was negative for weight loss, malignancy, recent abdominal surgery, travel abroad, abdominal trauma, and underlying liver or kidney diseases. He had a positive family history of hyperlipidemia. Initial examination was notable for a temperature of 37°C, pulse 113 beats/min, respiratory rate 16/min, and blood pressure 130/85 mmHg. The patient appeared ill and examination of the abdomen revealed tenderness in the periumblical area with shifting dullness but without organomegaly. Rectal examination was normal. The remainder of the examination was unremarkable.
Initial investigations showed hemoglobin level of 150 g/L, total leucocyte count 12 × 109/L (60% neutrophils, 31% lymphocytes) and adequate number of platelets. Blood chemistry, liver function test and coagulation profile were normal. Serum pancreatic amylase was 29 IU/L (normal, 15-55 IU/L) and lipase 44 IU/L (normal, 13- 60 IU/L), triglyceride 36.28 mmol/L, total cholesterol 5.2 mmol/L. On the next day serum pancreatic amylase was 45 IU/L, lipase 50.2 IU/L, and fasting triglyceride 25 mmol/L. An antibody to human immunodeficiency virus was negative.
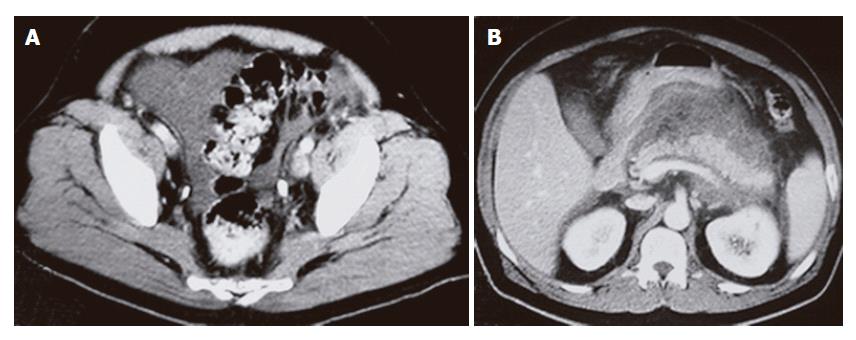
Ultrasound showed fluid collection (ascites) but could not visualize the pancrease. CT of the abdomen and pelvis with contrast showed pelvic ascites (Figure 1A) and inflammatory changes surrounding the pancreas consistent with acute pancreatitis (Figure 1B). The pancreas was well demarcated and homogenous with no focal lesions. No abnormal masses or enlarged lymph nodes were found.
Acute pancreatitis secondary to hypertriglyceridaemia was diagnosed despite normal plasma amylase and lipase concentrations. Ultrasound-guided abdomen paracentesis yielded a milky fluid with the following biochemical composition: 3.2 g/L triglycerides, 0.85 g/L cholesterol, 49 g/L total protein, 30 g/L albumin, 0.72 g/L glucose and 121 U/L LDH. Cell count of the fluid was 230 × 106/L and no acid-fast bacilli (AFB) were present in smear. This high triglyceride ascites was consistent with chylous ascites.
The patient was kept fasting from the first hospital day, and intravenous fluid hydration was provided. Meperidine was administered for pain relief. On the 5th hospital day the pain reduced and the serum pancreatic amylase was 48 IU/L, lipase 55 IU/L, and triglyceride 16.2 mmol/L. On the 6th d a low fat diet was resumed and fat lowering agent (gemfibrozil) was initiated, 600 mg, twice a day. On 14th d abdomen ultrasound (US) was repeated and showed fluid free peritoneal cavity. Workup for lymphoma and malignancy including chest CT, colonoscopy and upper endoscopy were negative. A culture of ascitic fluid for TB infection was negative. The patient was discharged after 18 d of hospitalization with gemfibrozil 600 mg twice a day. At the time of discharge, the serum pancreatic amylase was 15 IU/L, lipase 33 IU/L, and triglyceride 4.2 mmol/L.
On follow-up evaluation approximately 4 wk after the patient was discharged from the hospital, the patient was symptom free. Repeat physical examination was unremarkable. Laboratory evaluation yielded serum pancreatic amylase of 8 IU/L and lipase of 20 IU/L, triglyceride of 3.2 mmol/L. Abdomen ultrasound and CT showed fluid free peritoneal cavity with normal pancreas.
Acute pancreatitis is an important cause of acute upper abdominal pain. Because its clinical features are similar to a number of other acute illnesses, it is difficult to make a diagnosis only on the basis of symptoms and signs. Acute pancreatitis can be suspected clinically, but requires biochemical and radiologic and sometimes histologic evidence to confirm the diagnosis.
The diagnosis is most often confirmed by evaluation of serum amylase and lipase levels. The diagnosis of acute pancreatitis is made by a serum amylase activity four times above normal (or by a lipase activity greater than twice the upper limit of normal)[3].
The sensitivity and specificity of amylase and lipase are reported to be considerably dependent on the detection method used, ranging from 70% to 100% and 33% to 89% for serum amylase, and from 74% to 100% and 34% to 100% for serum lipase, respectively[4]. Recognizing factors that reduce the sensitivity and specificity of serum amylase and lipase can help prevent misdiagnosis and allow for appropriate treatment. Factors that can lead to normal amylase and lipase values are hypertriglyceridemia (as in this patient) and extensive pancreatic necrosis (acute fulminant or acute chronic pancreatitis)[5].
In such patients (as in this patient) CT scanning provides an accurate confirmation of clinical and laboratory findings and offers excellent anatomic and morphologic representation of the pancreas and peripancreatic tissue.
It was observed that plasma triglyceride levels higher than 500 mg/dL interfere with in vitro determination of the actual amylase level by preventing the calorimetric reading of the assay end point[4]. However, the reasons for normal serum amylase and lipase level in patients with hypertriglyceridemia-associated pancreatitis are still a dilemma. We reviewed the files of the patients admitted to our hospital with acute pancreatitis, from January 2004 to December 2005. The number of patients labeled as hypertriglyceridemia-associated pancreatitis was 18, of which only one patient (5.5%) had normal serum amylase and lipase level. Serial dilutions of the patient’s sample with the assay buffer to reduce interference of light transmission by hyperlipidemic serum can reveal an abnormal amylase value that was previously masked by the lactescent plasma[1].
Overall, although pancreatitis caused by hypertriglyceridemia has the same prognosis as other causes of the acute episode[6], early recognition and treatment have been shown to hasten clinical recovery[7,8].
True chylous ascites is defined as the presence of ascitic fluid with high fat (triglyceride) content, usually higher than 2 g/L, although some authors use a cutoff value of 1.1 g/L[9-12]. True chylous ascites must be distinguished from chyliform and pseudochylous effusions, in which the turbid, milky appearance is due to cellular degeneration caused by bacterial peritonitis or malignancy. A low triglyceride level is characteristic of these effusions.
There are multiple causes of chylous ascites. The most common causes in Western countries are abdominal malignancy and cirrhosis, which account for over two-thirds of all cases. In contrast, infectious etiologies (i.e., tuberculosis and filariasis) are responsible for the majority of cases in developing countries. Other causes include congenital, inflammatory (e.g., acute and chronic pancreatitis), post-operative, traumatic, and miscellaneous disorders.
Kelley and Butt of the Mayo Clinic[13] reported that 62 (87%) of 71 cases of chylous ascites, were secondary to malignancy.
Chylous ascites might occur due to different mechanisms[14]: (1) obstruction of the lymph flow caused by external pressure (mass) causing leakage from dilated subserosal lymphatics into the peritoneal cavity; (2) exudation of lymph through the walls of dilated retroperitoneal vessels lacking valves, which leak fluid through a fistula into the peritoneal cavity as in congenital lymphangiectasia; and (3) traumatic thoracic duct obstruction causing direct leakage of chyle through a lymphoperitoneal fistula.
Basically, acute or chronic pancreatitis, can cause compression of adjacent lymphatic channels resulting in chylous ascites[15].
Conservative medical treatment should be the first step in managing all patients with chylous ascites. The treatment should be directed at the underlying disorder. In this patient, cure of acute pancreatitis and reduction in triglyceride level led to resolution of chylous ascites. General measures include withholding oral feedings and starting total parenteral nutrition to minimize pancreatic exocrine secretion[16]. One-third of patients can improve on conservative management and do not require any further intervention[17]. Treatment with somatostatin or octreotide together with diuretics and repeated paracentesis may be beneficial for some patients[18,19]. Some patients fail medical therapy, ultimately requiring surgery[20].
In conclusion, relying solely on high serum amylase and/or lipase level to establish the diagnosis of acute pancreatitis is unjustified and should be abandoned, because hypertriglyceridemia can cause spuriously normal amylase and lipase levels. Consequently, abdominal computer tomography scan might be useful in establishing the diagnosis of acute pancreatitis when hypertriglyceridemia interferes with the evaluation of pancreatic enzyme activities and ultrasound examination provides poor pancreatic visualization.
| 1. | Fallat RW, Vester JW, Glueck CJ. Suppression of amylase activity by hypertriglyceridemia. JAMA. 1973;225:1331-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 51] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Ben-Ami H, Nagachandran P, Assalia A, Edoute Y. Acute transient chylous ascites associated with acute biliary pancreatitis. Am J Med Sci. 1999;318:122-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Steinberg W, Goldstein S, Davis N. Diagnostic assays in acute pancreatitis. Ann Intern Med. 1985;103:475-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (1)] |
| 4. | Wong EC, Butch AW, Rosenblum JL. The clinical chemistry laboratory and acute pancreatitis. Clin Chem. 1993;39:234-243. [PubMed] |
| 5. | Greenberger NJ, Toskes PP. Approach to the patient with pancreatic disease. Harrison's principles of internal medicine. New York: McGraw Hill 1987; 1368-1372. |
| 6. | Fortson MR, Freedman SN, Webster PD. Clinical assessment of hyperlipidemic pancreatitis. Am J Gastroenterol. 1995;90:2134-2139. [PubMed] |
| 7. | Piolot A, Nadler F, Cavallero E, Coquard JL, Jacotot B. Prevention of recurrent acute pancreatitis in patients with severe hypertriglyceridemia: value of regular plasmapheresis. Pancreas. 1996;13:96-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Tenner S, Banks PA. Acute pancreatitis: nonsurgical management. World J Surg. 1997;21:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Cárdenas A, Chopra S. Chylous ascites. Am J Gastroenterol. 2002;97:1896-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 198] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Press OW, Press NO, Kaufman SD. Evaluation and management of chylous ascites. Ann Intern Med. 1982;96:358-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 221] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Runyon BA, Akriviadis EA, Keyser AJ. The opacity of portal hypertension-related ascites correlates with the fluid's triglyceride concentration. Am J Clin Pathol. 1991;96:142-143. [PubMed] |
| 12. | Jüngst D, Gerbes AL, Martin R, Paumgartner G. Value of ascitic lipids in the differentiation between cirrhotic and malignant ascites. Hepatology. 1986;6:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 39] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Kelley ML, Butt HR. Chylous ascites: an analysis of its etiology. Gastroenterology. 1960;39:161-170. [PubMed] |
| 14. | Browse NL, Wilson NM, Russo F, al-Hassan H, Allen DR. Aetiology and treatment of chylous ascites. Br J Surg. 1992;79:1145-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 138] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Goldfarb JP. Chylous effusions secondary to pancreatitis: case report and review of the literature. Am J Gastroenterol. 1984;79:133-135. [PubMed] |
| 16. | Variyam EP. Central vein hyperalimentation in pancreatic ascites. Am J Gastroenterol. 1983;78:178-181. [PubMed] |
| 17. | Stone LD. Pancreatic ascites. Br J Hosp Med. 1986;35:252-253. [PubMed] |
| 18. | Oktedalen O, Nygaard K, Osnes M. Somatostatin in the treatment of pancreatic ascites. Gastroenterology. 1990;99:1520-1521. [PubMed] |
| 19. | Uhl W, Anghelacopoulos SE, Friess H, Büchler MW. The role of octreotide and somatostatin in acute and chronic pancreatitis. Digestion. 1999;60 Suppl 2:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 20. | Gómez-Cerezo J, Barbado Cano A, Suárez I, Soto A, Ríos JJ, Vázquez JJ. Pancreatic ascites: study of therapeutic options by analysis of case reports and case series between the years 1975 and 2000. Am J Gastroenterol. 2003;98:568-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
S- Editor Pan BR L- Editor Wang XL E- Editor Bi L