Published online Jan 21, 2007. doi: 10.3748/wjg.v13.i3.470
Revised: December 1, 2006
Accepted: December 12, 2006
Published online: January 21, 2007
A 34-year-old female complaining of abdominal fullness was diagnosed as scirrhous gastric cancer (type 4’) with peritonitis carcinomatosa in July 2002. A combined chemotherapy regimen was selected to control massive ascites; TS-1® 80 mg/m2 was given orally on d 1-14, 22-35, and paclitaxel 50 mg/m2 was administered intravenously on d 1, 8, 22 and 29. After 2 courses of this regimen, the primary tumor was markedly reduced, and ascites completely vanished. Alopecia (grade 1, since d 30), leukocytopenia (grade 2, on d 34) and anemia (grade 2, on d 34) were the only adverse events throughout the following courses. The chemotherapy was effective for 28 mo, and then it was discontinued upon the patient’s own request, and she survived for 36 mo after diagnosis.
- Citation: Koizumi Y, Obata H, Hara A, Nishimura T, Sakamoto K, Fujiyama Y. A case of scirrhous gastric cancer with peritonitis carcinomatosa controlled by TS-1® + paclitaxel for 36 mo after diagnosis. World J Gastroenterol 2007; 13(3): 470-473
- URL: https://www.wjgnet.com/1007-9327/full/v13/i3/470.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i3.470
Scirrhous gastric cancer has long been, and still is a devastating disease, especially when it is complicated with peritonitis carcinomatosa, which has no effective treatment at all up to date. On the other hand, the introduction of novel antineoplastic agents and protocols has led to certain improvement in the treatment of gastric cancer, including distant metastasis and/or malignant ascites. Here we report a case of scirrhous gastric cancer with peritonitis carcinomatosa, treated with repetitive courses of TS-1® (Tegafur-Gimeracil-Oteracil potassium) plus paclitaxel, which resulted in partial remission with disappearance of malignant ascites for over 28 mo, and survival of the patient for 36 mo after diagnosis.
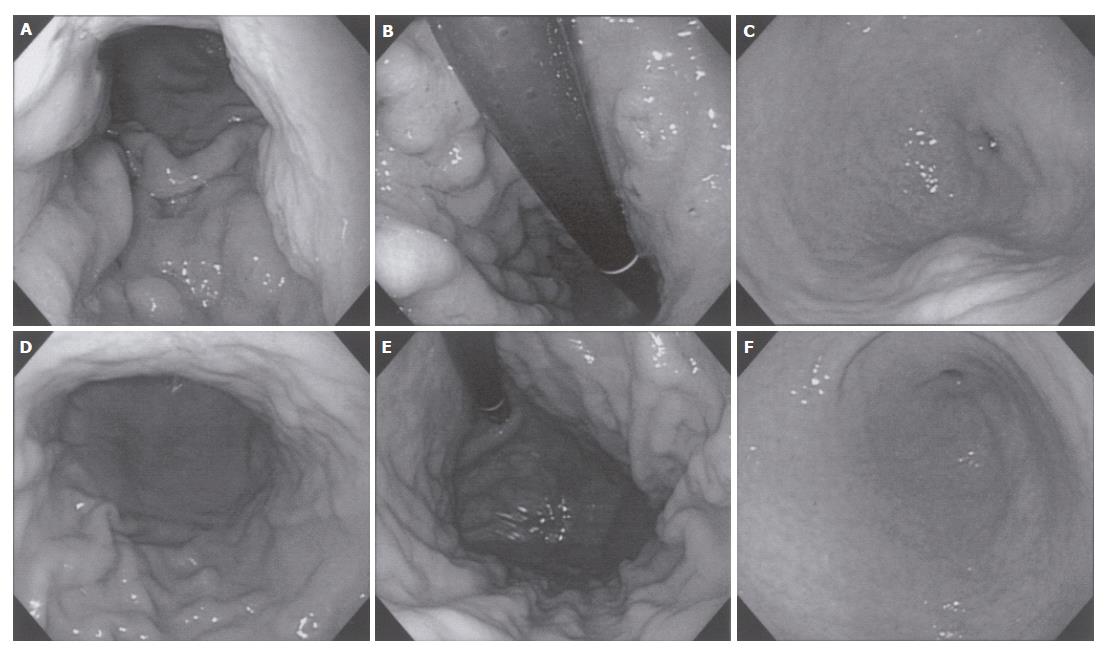
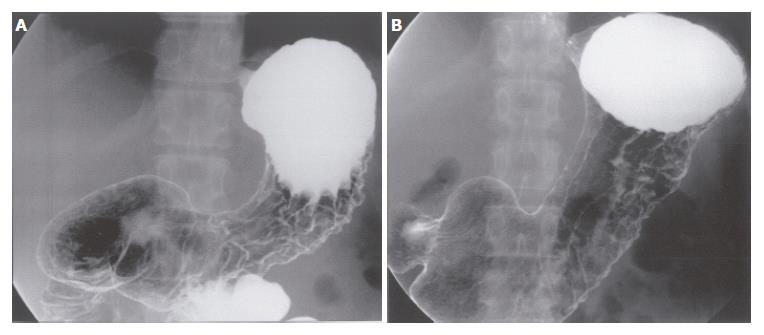
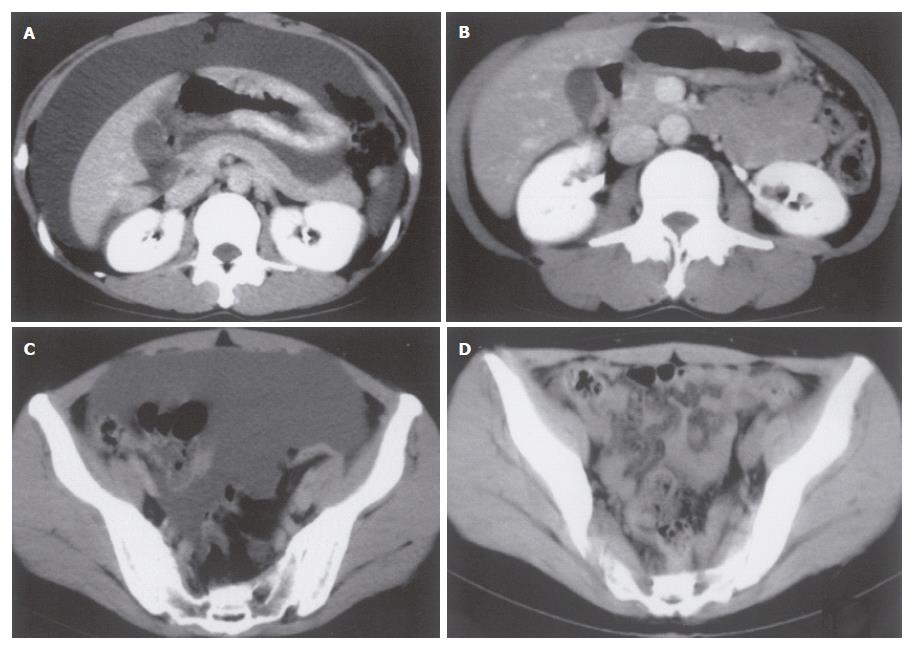
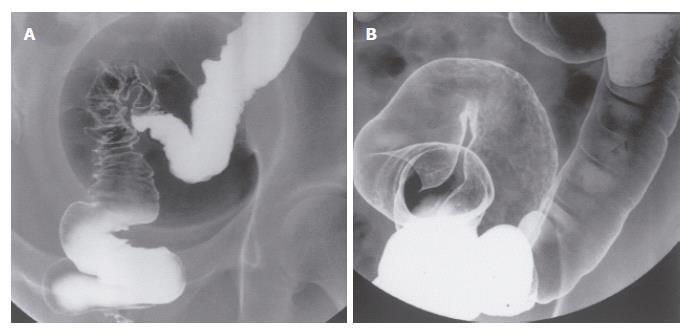
A 34-year-old female, complaining of abdominal fullness, visited our hospital on 15th July, 2002. She has been disturbed by epigastric discomfort, increasing abdominal fullness and severe constipation for about a month. And she gained weight by 5-6 kg within 2 mo. Physical examinations showed obvious abdominal distension with an abdominal circumference of 80.0 cm, and body weight of 60 kg for a height of 167.5 cm. A low grade fever (37.2°C) was the only abnormal finding in vital signs. Laboratory data revealed no major abnormalities other than hypercholesterolemia. CEA and CA19-9 were within normal range, while CA125 was slightly elevated (Table 1). A paracentesis was performed to examine the ascites, and the findings were as follows: turbid and yellow colored, specific gradient = 1.034, with 1070 cells/mm3 including many clusters of adenocarcinoma cells. A gastroscopy revealed conspicuous swelling of gastric fold with multiple erosions, and poor wall expansion exclusively at the body. The antral mucosa seemed almost intact except one submucosal tumor (SMT) like lesion at the greater curvature. Expandability at the antrum was fair (Figure 1A-C). A biopsy examination confirmed poorly differentiated adenocarcinoma and signet ring cell carcinoma. In upper GI series, the wall expansion was poor at the body (Figure 2A). Local and distant lymph node metastases were unlikely on CT scan images; yet massive ascites were detected, extending from the liver surface to the pelvic cavity (Figure 3A and B). A barium enema revealed severe stenosis of the rectosigmoid colon, probably caused by the extrinsic compression due to peritonitis carcinomatosa (Figure 4A). We therefore diagnosed this case as “scirrhous gastric cancer (type 4’) with peritonitis carcinomatosa”.
| WBC | 5.3 × 109/L | ALP | 142 IU/L |
| Neutrophil | 75.4% | Total bilirubin | 5 mg/L |
| Eosinophil | 1.9% | Glucose | 830 mg/L |
| Basophil | 0.8% | Total cholesterol | 3210 mg/L |
| Monocyte | 3.9% | Triglyceride | 770 mg/L |
| Lymphocyte | 18% | Na | 143 mEq |
| RBC | 4.12 × 1012/L | Cl | 107 mEq |
| Hematocrit | 38.1% | K | 3.7 mEq |
| Hemoglobin | 126 g/L | BUN | 153 mg/L |
| MCV | 92.5 fL | Creatinine | 8 mg/L |
| MCH | 30.6 pg | CRP | 1 mg/L |
| MCHC | 331 g/L | CEA | < 0.2 μg/L |
| Platelet | 2.6 × 1011/L | CA19-9 | 9.7 kU/L |
| Total protein | 67 g/L | CA125 | 44 kU/L |
| Albumin | 37 g/L | PT | 10.6 s (99%) |
| GOT | 23 IU/L | APTT | 29 s |
| GPT | 21 IU/L | Fbg | 2600 mg/L |
| LDH | 275 IU/L |
We selected TS-1® and paclitaxel as a combined chemotherapy regimen. TS-1® 80 mg/m2 was given orally on d 1-14 and 22-35, and paclitaxel 50 mg/m2 was administered intravenously on d 1, 8, 22 and 29. One course consisted of 35 d, and the following 2 wk were spent as a drug-free period. After 2 courses, the primary tumor was markedly reduced, and the gastric wall expandability improved (Figure 1D-F) (Figure 2C). Ascites have completely vanished (Figure 3C and D), and wall expandability of the sigmoid colon was also improved (Figure 4B).
Adverse effects were carefully monitored and evaluated according to the NCI-CTC criteria. Transient leukocytopenia (grade 2, WBC 2700/μL) and anemia (grade 2, Hb 93 g/L) were observed on d 34, however, both improved during the late courses. Alopecia (grade 1, since d 30) was the only adverse event throughout the following courses.
Since the 1st of August 2002, this regimen was performed predominantly at home, except during the days of paclitaxel infusion (2 days’ admission for each), and 27 courses have been completed with the patient in partial remission state, with neither ascites progression nor impairment of activity of daily living. In November 2004, the patient requested to stop further treatment, and the chemotherapy was disrupted in the 28th month since the initial diagnosis. After a 2 mo interval, the patient was, again, hospitalized for the recurrence of massive ascites in January 2005. TS-1® + paclitaxel regimen was resumed immediately; yet the control of ascites was difficult. Despite following several months of treatment, the patient died of peritonitis carcinomatosa in July 2005, 36 mo after the diagnosis. The primary tumor in the stomach has been reduced in size, and no metastases other than in peritoneum were detected.
The prognosis of scirrhous gastric cancer (type 4’) is extremely poor, and the 5-year survival rate is reported to be 8.7%-11.3%[1,2]. According to Green et al[3], the median survival time (MST) is estimated to be 5.4 mo with chemotherapy, and 1.1 mo with best supportive care. Peritoneal invasion is found in many advanced cases, and even after treatment most of them recur with peritonitis carcinomatosa. Many attempts have been made to treat these cases, for example, systemic/intraperitoneal administration of antineoplastic agents, immunomodulative therapy or hyperthermia, yet none of them have proved prominent efficacy.
On the other hand, TS-1®, both in monotherapy and combined chemotherapy, has shown superior results in the treatment of gastric cancer. In a phase II trial in Japan, single administration of TS-1® yielded a higher efficacy (46.5%, 60/129 cases), longer MST (8.1 mo), with a lower incidence of adverse events than historical controls (5-FU, DXR, MTX + LV, CDDP). It proved to be one of the most promising antineoplastic agents[4]. However, against poorly differentiated gastric cancer, the agents above are effective in only a certain proportion of patients. Peritonitis carcinomatosa is especially difficult to treat and is critical to the prognosis. There are several case reports demonstrating the effect of TS-1®, as a monotherapy, against malignant ascites, but evidences from clinical trials are yet to come. To achieve a higher response rate in treating peritonitis carcinomatosa, local concentrations of the agents in ascites are an important factor, and the agents should have different pharmacological effects from TS-1®. Taking these into consideration, we selected paclitaxel as the counterpart of TS-1®, based on the evidence that paclitaxel is highly infiltrative into ascites[5].
Paclitaxel is a novel antimitotic agent, which promotes tubulin polymerization and stabilizes microtubules, resulting in inhibition of mitosis. Besides, it is said to induce tumor apoptosis and inhibit tumor angiogenesis[6]. This agent has been selected as one of the second-line drugs against gastric cancer, especially for cases refractory to first-line drugs (i.e., CDDP, 5-FU) or recurrent cases after surgery. As a monotherapy, the response rate was 26% in previously treated gastric cancer and 21% in those with prior chemotherapy. And it is remarkable that paclitaxel is the only drug, used in uncombined regimens, that increased over 300 d of MST against advanced and recurrent gastric cancer, regardless of prior treatment history[7]. The administration of paclitaxel against previously untreated gastric cancer has seldom been reported, and the evaluation of combined regimens with TS-1® is still ongoing in phase I/II studies. At present, the safety of the regimen has been reported in phase I study[8], but the efficacy is yet to be proved.
In this aspect, our report suggested important findings; the combined regimen of paclitaxel + TS-1® was safely administered for previously untreated gastric cancer patient, and it was remarkably effective against massive invasion of the cancer, peritonitis carcinomatosa. With the development of many other combined regimens, the treatment against gastric cancer would have various choices. At least, TS-1® + paclitaxel could be one of the first-line regimens against far advanced gastric cancer, especially poorly differentiated adenocarcinoma complicated with peritonitis carcinomatosa.
| 1. | Kim DY, Kim HR, Kim YJ, Kim S. Clinicopathological features of patients with Borrmann type IV gastric carcinoma. ANZ J Surg. 2002;72:739-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Chen CY, Wu CW, Lo SS, Hsieh MC, Lui WY, Shen KH. Peritoneal carcinomatosis and lymph node metastasis are prognostic indicators in patients with Borrmann type IV gastric carcinoma. Hepatogastroenterology. 2002;49:874-877. [PubMed] |
| 3. | Green D, Ponce de Leon S, Leon-Rodriguez E, Sosa-Sanchez R. Adenocarcinoma of the stomach: univariate and multivariate analysis of factors associated with survival. Am J Clin Oncol. 2002;25:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Sakata Y, Ohtsu A, Horikoshi N, Sugimachi K, Mitachi Y, Taguchi T. Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer. 1998;34:1715-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 381] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 5. | Huizing MT, Rosing H, Huinink WWB. Increased Paclitaxel (P) levels in patients (PTS) with ovary cancer and ascitic (AS) fluid. Proc ASCO. 1996;15:180. |
| 7. | Yamada Y, Shirao K, Ohtsu A, Boku N, Hyodo I, Saitoh H, Miyata Y, Taguchi T. Phase II trial of paclitaxel by three-hour infusion for advanced gastric cancer with short premedication for prophylaxis against paclitaxel-associated hypersensitivity reactions. Ann Oncol. 2001;12:1133-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Ueda Y, Yamagishi H, Ichikawa D, Morii J, Koizumi K, Kakihara N, Shimotsuma M, Takenaka A, Yamashita T, Kurioka H. Phase I study of a combination of s-1 and weekly paclitaxel in patients with advanced or recurrent gastric cancer. Oncology. 2005;69:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
S- Editor Wang GP L- Editor Zhu LH E- Editor Lu W