Published online Jan 21, 2007. doi: 10.3748/wjg.v13.i3.457
Revised: November 15, 2006
Accepted: December 4, 2006
Published online: January 21, 2007
AIM: To investigate the effect of N-desulfated heparin on tumor metastasis and angiogenesis, and expression of vascular endothelial growth factor (VEGF) of orthotopic implantation of human gastric carcinoma in male severe combined immune deficiency (SCID) mice.
METHODS: Human gastric cancer SGC-7901 cells were orthotopically implanted into the stomach of SCID mice. The mice were randomly divided into normal saline group and N-desulfated heparin group. One week after operation, the mice in N-desulfated heparin group received i.v. injections of N-desulfated heparin (Shanghai Institute of Cell Biology, Chinese Academy of Sciences, 10 mg/kg.d) twice weekly for 3 wk. The mice in normal saline group received i.v. injections of normal saline (100 μL) twice weekly for 3 wk. The mice were sacrificed six weeks after implantation. Tumor metastasis was evaluated histologically for metastasis under microscope. Intratumoral microvessel density (MVD) and VEGF expression were evaluated immuohistochemically. VEGF mRNA expression in gastric tissue of SCID mice was detected by real time PCR.
RESULTS: The tumor metastasis rate was 80% in normal saline group and 20% in N-desulfated heparin group (P < 0.05). MVD was 8.0 ± 3.1 in normal saline group and 4.3 ± 1.8 in N-desulfated heparin group (P < 0.05). VEGF positive immunostaining was found in cytoplasm of cancer cells. The rate of VEGF positive expression was higher in normal saline group than in N-desulfated heparin treated group (90% vs 20%, P < 0.05). VEGF mRNA expression was significantly inhibited by N-desulfated heparin and was higher in normal saline group than in N-desulfated heparin group (Ct value 19.51 ± 1.01 vs 22.55 ± 1.36, P < 0.05). N-desulfated heparin significantly inhibited the expression of VEGF mRNA in cancer cells. No bleeding occurred in N-desulfated heparin group.
CONCLUSION: N-desulfated heparin can inhibit metastasis of gastric cancer by suppressing tumor VEGF expression and tumor angiogenesis, but has no obvious anticoagulant activity.
- Citation: Chen JL, Hong J, Lu JL, Chen MX, Chen WX, Zhu JS, Chen NW, Chen GQ, Geng JG. Effect of non-anticoagulant N-desulfated heparin on expression of vascular endothelial growth factor, angiogenesis and metastasis of orthotopic implantation of human gastric carcinoma. World J Gastroenterol 2007; 13(3): 457-461
- URL: https://www.wjgnet.com/1007-9327/full/v13/i3/457.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i3.457
Gastric carcinoma is one of the most frequent malignancies and is the leading cause of cancer deaths in China. Up to now, there is no effective treatment for metastasis of tumor. Recent studies showed that angiogenesis plays a crucial role in tumor growth and metastasis. The regulation of angiogenesis is balanced by proangiogenic and antiangiogenic factors and is associated with prognosis of patients with tumors. Inhibition of angiogenesis can control tumor metastasis and improve the prognosis[1-4]. Vascular endothelial growth factor (VEGF) and fibroblast growth factor-2 (FGF-2) are the main factors promoting angiogenesis, and anti-VEGF therapy is effective in inhibiting angiogenesis and metastasis of tumor[5-7].
Heparin, a highly sulfated proteoglan, has been extensively used as an anticoagulant drug for a long time. Aside from its anticoagulant action, heparin binds to various growth factors, cytokines, and extracellular proteins and consequently is able to affect migration of cancer cells and angiogenesis in tumors. In vitro study[8] indicates that heparin can inhibit VEGF and FGF-2-induced proliferation of vascular endothelial cells and vascular formation, while in vivo study[9] has demonstrated that heparin has antimetastatic activity. However, clinical use of heparin in treatment of tumor is limited by its strong anticoagulant activity, which may cause severe bleeding complications. Chemically modified heparin shows a significantly reduced anticoagulant activity and enhanced ability to interact with FGF, VEGF and hepatocyte growth factor, which are known to stimulate angiogenesis[10]. In this study, we investigated the effect of N-desulfated heparin on tumor metastasis in mouse models of human gastric cancer constructed by orthotopic implantation of histologically intact tumor tissue.
Goat anti-human CD34 antibody and goat anti-human VEGF antibody were obtained from Santa Cruz Biotechnical Company. VEGF probe for real time PCR was provided by Daan Gene Company of Zhongshan University.
Male severe combined immune deficiency (SCID) mice were obtained from Shanghai Experimental Animal Center of Chinese Academy of Sciences. Animals used were 6 wk old and weighed 20-25 g. Human gastric cancer SGC-7901 (Shanghai Cancer Institute), a poorly-differentiated adenocarcinoma line, was originally derived from a primary tumor and maintained by passage in the subcutis of nude mice. Animal models were made using orthotopic implantation of histologically intact tissue of human gastric carcinoma[11]. Tumors were resected aseptically. Necrotic tissues were cut and the remaining healthy tumor tissues were scissor minced into pieces (about 5 mm × 7 mm in diameter) in Hank’s balanced salt solution. Each tumor piece was weighed and adjusted to be 150 mg. Mice were anesthetized with 4.3% trichloraldehyde hydrate. An incision was made through the left upper abdominal pararectal line. Then peritoneal cavity was carefully exposed and a part of serosal membrane in the middle of the greater curvature of stomach was mechanically injured using scissors. A tumor piece of 150 mg was fixed on each injured site of the serosal surface. The stomach was returned to the peritoneal cavity, and the abdominal wall and skin were closed.
After metastatic models were made, the mice were randomly divided into N-desulfated heparin group (n = 10) and normal saline group (n = 10). One week after operation, the mice in N-desulfated heparin group received i.v. injections of N-desulfated heparin (Shanghai Institute of Cell Biology, Chinese Academy of Sciences, 10 mg/kg.d) twice weekly for 3 wk. The mice in normal saline group received i.v. injections of normal saline (100 μL) twice weekly for 3 wk. The mice were weighed twice weekly.
All animals were sacrificed 6 wk after implantation. An incision was made through the abdominal wall, and then peritoneal cavity was carefully exposed. Tumors growing on the stomach wall were removed and fixed in 10% formalin, and processed for routine paraffin embedding. Tissues from all organs and lymph nodes were collected and fixed in 10% formalin, and processed for routine paraffin embedding after careful macroscopic examination. Four-micron-thick sections were stained with hematoxylin and eosin, and evaluated histologically for liver metastasis or lymph node metastasis or other organ metastasis under microscope.
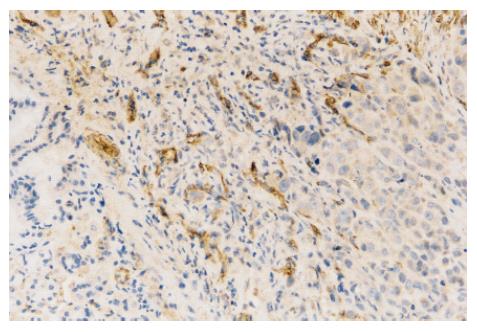
Immunostaining was performed using a labeled streptavidin biotin method. Four-micron-thick sections were deparaffined in xylene and rehydrated with graded alcohol. Immunohistochemical staining was carried out to detect CD34 expression following the manufacturer’s protocol. The concentration of anti-CD34 antibody was 1:300. MVD (CD34-positive microvessels) was calculated under 200 fold microscope. The modified Weidner’s method was used for the evaluation of MVD according to CD34 endothelial cell immunostaining. For the microvessel counting, positive stainings for MVD in 5 most highly vascularized areas in each section were counted in 200 ×fields. MVD was expressed as average of the microvessel count in the areas.
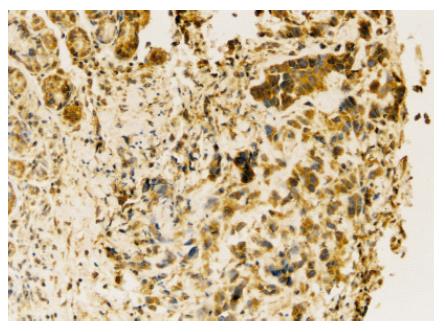
Immunostaining was performed using a labeled streptavidin biotin method. Four-micron-thick sections were deparaffined in xylene and rehydrated with graded alcohol. Immunohistochemical staining was carried out to detect VEGF expression following the manufacturer’s protocol. The concentration of anti-VEGF antibody was 1:60. Positive cells under 10% were defined as positive +, over 10% as positive ++. Positive expression was defined as positive + or positive ++. VEGF primers and probe used are VEGF f: GTTCGAGGAAAGGGAAAGGGTC, VEGF r: GCGAGTCTGTGTTTTTGCAGGA, VEGF probe: AGCGCAAGAAATCCCGGTTTAAATC.
VEGF RNA was isolated by method of Trizol. Synthesis of the first strand cDNA was performed according to the instructions delivered with reverse transcription kit, using human VEGF antisense strand primers and reverse transcriptase. After 1 h incubation at 37°C, samples were heat inactivated for 3 min at 95°C and kept at -80°C until use.
Aliquots of 5 μL of cDNA were amplified in a final volume of 50 μL using PCR buffer at the presence of 1 μL of Taq DNA polymerase and 0.5 μL of VEGF probe. Samples were amplified at 93°C for 2 min, at 93°C for 0.5 min and at 55°C for 1min followed by 40 cycles. Real time PCR was carried out in an automated real time PCR cycler (American ABI 7000).
All data were expressed as mean ± SD. Student’s t test and χ2 precise method were used to determine changes in different groups. P < 0.05 was considered statistically significant.
All mice developed localized tumors at the implanted site which were poorly-differentiated adenocarcinomas under microscope. Tumor growth did not differ significantly between the animals treated with normal saline or with N-desulfated heparin. Of the 10 animals treated with normal saline, 8 developed metastatic tumors in regional lymph nodes, 6 in liver, and 6 in other organs. However, after the mice were treated with N-desulfated heparin for 3 wk, metastasis of tumor was inhibited significantly. Of the 10 animals treated with N-desulfated heparin, 2 developed metastatic tumors in liver. The metastatic rate was higher in mice treated with normal saline than in those treated with N-desulfated heparin (80% vs 20%, P < 0.05).
N-desulfated heparin had no significant effect on body changes in SCID mice. No bleeding complications were found in N-desulfated heparin group.
In normal saline-treated mice, many CD34 positively stained vessels were diffusely located and formed tube-like structures in tumor (Figure 1). However, they were almost absent in N-desulfated heparin- treated mice. The MVD was significantly lower in N-desulfated heparin-treated mice than in normal saline-treated mice (4.3 ± 1.8 vs 8.0 ± 3.1, P < 0.05).
Under microscope, VEGF positive immunostaining was found in cytoplasm of cancer cells (Figure 2). The rate of VEGF positive expression was higher in normal saline group than in N-desulfated heparin group (P < 0.05, Table 1).
| Groups | n | - | + | ++ | Positive rate (%) |
| Normal saline group | 10 | 1 | 2 | 7 | 90 |
| N-desulfated heparin group | 10 | 8 | 1 | 1 | 20a |
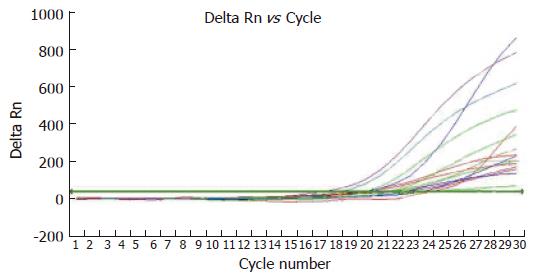
VEGF mRNA expression in gastric tissue of SCID mice detected by real time PCR was higher in normal saline group than in N-desulfated heparin group (Ct value 19.51 ± 1.01 vs 22.55 ± 1.36, P < 0.05) (Figure 3).
Recent studies showed that angiogenesis is a critical determinant of solid tumor metastasis, and antiangiogenic therapy plays an important role in improving prognosis of patients with gastric carcinoma[12-14]. It was reported that low molecular weight heparin has significantly-reduced anticoagulant activity and enhanced ability to bind to FGF-2 and VEGF, thus inhibiting angiogenesis of tumor[15].
N-desulfated heparin, a modified heparin, is known to have more significantly-reduced anticoagulant activity (1/76 of heparin) than O-desulfated heparin (5%-30% of heparin) or N-acetylated heparin (10% of heparin)[16]. There is no report so far on the effect of N-desulfated heparin on tumor metastasis. Therefore, the effect of N-desulfated heparin on tumor metastasis, angiogenesis and VEGF expression was observed in mouse model of orthotopic implantation of human gastric carcinoma tissue.
In the present study, tumor metastasis was inhibited significantly by N-desulfated heparin. Szende et al[17] found that fraxiparine has a significant effect on lung metastases, while heparin does not influence metastasis. These data suggest that low molecular heparin may be of antimetastatic activity[18]. Kragh et al[19] reported that non-anticoagulant heparin can inhibit tumor metastasis.
To evaluate the effect of N-desulfated heparin on angiogenesis, immunohistochemical staining of CD34 in tumors was carried out. The results showed that N-desulfated heparin significantly inhibited angiogenesis in these tumors. Mousa et al[20] have demonstrated anti-angiogenic activity of the low molecular weight heparin, tinzaparin. Naggi et al[10] reported that N-acetylatedand and glycol-split heparins are potential antiangiogenic and antimetastatic agents which are more effective than unmodified heparin, suggesting that N-desulfated heparin can inhibit tumor metastasis by inhibiting angiogenesis.
In the present study, the rate of VEGF positive expression was higher in normal saline group than in N-desulfated heparin group and VEGF mRNA expression was higher in normal saline group than in N-desulfated heparin group, demonstrating that N-desulfated heparin can significantly inhibit the expression of VEGF in cancer cells. Kakeji et al[21] showed that VEGF is expressed in early and advanced gastric cancer. Multivariate analysis has revealed that VEGF is an independent prognostic factor and an independent risk factor for liver metastasis. Fondevila et al[22] demonstrated that VEGF expression is an independent predictor of tumor recurrence and survival following curative resection of gastric cancer. Pisano et al[23] showed that undersulfated, low-molecular-weight glycol-split heparin may be an antiangiogenic VEGF antagonist. In the present study, hemorrhage was never observed in N-desulfated heparin treated mice, suggesting that N-desulfated heparin has no obvious anticoagulant activity.
In conclusion, VEGF produced by cancer cells is an angiogenic factor in human cancer tissue and plays an important role in tumor metastasis. N-desulfated heparin inhibits tumor metastasis by inhibiting expression of VEGF and angiogenesis and can be used in the treatment of tumor metastasis.
| 1. | Klerk CP, Smorenburg SM, Otten HM, Lensing AW, Prins MH, Piovella F, Prandoni P, Bos MM, Richel DJ, van Tienhoven G. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol. 2005;23:2130-2135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 445] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 2. | Castelli R, Porro F, Tarsia P. The heparins and cancer: review of clinical trials and biological properties. Vasc Med. 2004;9:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Shinkaruk S, Bayle M, Laïn G, Déléris G. Vascular endothelial cell growth factor (VEGF), an emerging target for cancer chemotherapy. Curr Med Chem Anticancer Agents. 2003;3:95-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 152] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Tsujitani S, Saito H, Maeta Y, Yamaguchi K, Tatebe S, Kondo A, Kaibara N. Neoangiogenesis in patients with gastric carcinoma in relation to the expression of vascular endothelial growth factor and thymidine phosphorylase. Anticancer Res. 2004;24:1853-1859. [PubMed] |
| 5. | Reinmuth N, Parikh AA, Ahmad SA, Liu W, Stoeltzing O, Fan F, Takeda A, Akagi M, Ellis LM. Biology of angiogenesis in tumors of the gastrointestinal tract. Microsc Res Tech. 2003;60:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Petralia GA, Lemoine NR, Kakkar AK. Mechanisms of disease: the impact of antithrombotic therapy in cancer patients. Nat Clin Pract Oncol. 2005;2:356-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Jung YD, Mansfield PF, Akagi M, Takeda A, Liu W, Bucana CD, Hicklin DJ, Ellis LM. Effects of combination anti-vascular endothelial growth factor receptor and anti-epidermal growth factor receptor therapies on the growth of gastric cancer in a nude mouse model. Eur J Cancer. 2002;38:1133-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 185] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 8. | Tóvári J, Bereczky B, Gilly R, Skopál J, Vágó A, Tímár J. Heparin inhibits metastatization of experimental melanoma. Magy Onkol. 2004;48:235-241. [PubMed] |
| 9. | Bobek V, Kovarík J. Antitumor and antimetastatic effect of warfarin and heparins. Biomed Pharmacother. 2004;58:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Naggi A, Casu B, Perez M, Torri G, Cassinelli G, Penco S, Pisano C, Giannini G, Ishai-Michaeli R, Vlodavsky I. Modulation of the heparanase-inhibiting activity of heparin through selective desulfation, graded N-acetylation, and glycol splitting. J Biol Chem. 2005;280:12103-12113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 179] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 11. | Chen JL, Chen WX, Zhu JS, Chen NW, Zhou T, Yao M, Zhang DQ, Wu YL. Effect of P-selectin monoclonal antibody on metastasis of gastric cancer and immune function. World J Gastroenterol. 2003;9:1607-1610. [PubMed] |
| 12. | Mousa SA. Low-molecular-weight heparin in thrombosis and cancer. Semin Thromb Hemost. 2004;30 Suppl 1:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Zacharski LR. Anticoagulants in cancer treatment: malignancy as a solid phase coagulopathy. Cancer Lett. 2002;186:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Ranieri G, Coviello M, Chiriatti A, Stea B, Montemurro S, Quaranta M, Dittadi R, Paradiso A. Vascular endothelial growth factor assessment in different blood fractions of gastrointestinal cancer patients and healthy controls. Oncol Rep. 2004;11:435-439. [PubMed] |
| 15. | Ono K, Ishihara M, Ishikawa K, Ozeki Y, Deguchi H, Sato M, Hashimoto H, Saito Y, Yura H, Kurita A. Periodate-treated, non-anticoagulant heparin-carrying polystyrene (NAC-HCPS) affects angiogenesis and inhibits subcutaneous induced tumour growth and metastasis to the lung. Br J Cancer. 2002;86:1803-1812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Zhou T, Chen JL, Song W, Wang F, Zhang MJ, Ni PH, Geng JG. Effect of N-desulfated heparin on hepatic/renal ischemia reperfusion injury in rats. World J Gastroenterol. 2002;8:897-900. [PubMed] |
| 17. | Szende B, Paku S, Rácz G, Kopper L. Effect of Fraxiparine and heparin on experimental tumor metastasis in mice. Anticancer Res. 2005;25:2869-2872. [PubMed] |
| 18. | Amirkhosravi A, Mousa SA, Amaya M, Francis JL. Antimetastatic effect of tinzaparin, a low-molecular-weight heparin. J Thromb Haemost. 2003;1:1972-1976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Kragh M, Binderup L, Vig Hjarnaa PJ, Bramm E, Johansen KB, Frimundt Petersen C. Non-anti-coagulant heparin inhibits metastasis but not primary tumor growth. Oncol Rep. 2005;14:99-104. [PubMed] |
| 20. | Mousa SA, Mohamed S. Anti-angiogenic mechanisms and efficacy of the low molecular weight heparin, tinzaparin: anti-cancer efficacy. Oncol Rep. 2004;12:683-688. [PubMed] |
| 21. | Kakeji Y, Koga T, Sumiyoshi Y, Shibahara K, Oda S, Maehara Y, Sugimachi K. Clinical significance of vascular endothelial growth factor expression in gastric cancer. J Exp Clin Cancer Res. 2002;21:125-129. [PubMed] |
| 22. | Fondevila C, Metges JP, Fuster J, Grau JJ, Palacín A, Castells A, Volant A, Pera M. p53 and VEGF expression are independent predictors of tumour recurrence and survival following curative resection of gastric cancer. Br J Cancer. 2004;90:206-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 143] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Pisano C, Aulicino C, Vesci L, Casu B, Naggi A, Torri G, Ribatti D, Belleri M, Rusnati M, Presta M. Undersulfated, low-molecular-weight glycol-split heparin as an antiangiogenic VEGF antagonist. Glycobiology. 2005;15:1C-6C. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
S- Editor Liu Y L- Editor Wang XL E- Editor Bi L