Published online Jan 21, 2007. doi: 10.3748/wjg.v13.i3.403
Revised: November 5, 2006
Accepted: December 11, 2006
Published online: January 21, 2007
AIM: To evaluate the effect of age and acoustic stress on gastric myoelectrical activity (GMA) and autonomic nervous system function.
METHODS: Twenty-one male subjects (age range 22-71 years, mean 44 years) were recruited and exposed, in random order, to three auditory stimuli (Hospital noise, conversation babble and traffic noise) after a 20-min baseline. All periods lasted 20 min and were interspersed with a 10 min of recovery. GMA was obtained using a Synectics Microdigitrapper. Autonomic nerve function was assessed by monitoring blood pressure and heart rate using an automatic recording device.
RESULTS: Dominant power tended to decrease with increase of age (P < 0.05). The overall percentage of three cycle per minute (CPM) activity decreased during exposure to hospital noise (12.0%, P < 0.05), traffic noise (13.9%, P < 0.05), and conversation babble (7.1%). The subjects in the younger group (< 50 years) showed a consistent reduction in the percentage of 3 CPM activity during hospital noise (22.9%, P < 0.05), traffic noise (19.0%, P < 0.05), and conversation babble (15.5%). These observations were accompanied by a significant increase in bradygastria: hospital noise (P < 0.05) and traffic noise (P < 0.05). In contrast, the subjects over 50 years of age did not exhibit a significant decrease in 3 CPM activity. Regardless of age, noise did not alter blood pressure or heart rate.
CONCLUSION: GMA changes with age. Loud noise can alter GMA, especially in younger individuals. Our data indicate that even short-term exposure to noise may alter the contractility of the stomach.
- Citation: Castle JS, Xing JH, Warner MR, Korsten MA. Environmental noise alters gastric myoelectrical activity: Effect of age. World J Gastroenterol 2007; 13(3): 403-407
- URL: https://www.wjgnet.com/1007-9327/full/v13/i3/403.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i3.403
Excessive noise has been linked to a variety of health issues, including hearing loss[1] mental illness[2,3], sleep disturbance[4], hypertension[5,6] and cardiovascular diseases[7-9]. In terms of gastrointestinal tract, non-specific abdominal complaints[10,11], gastritis and peptic ulcers[12,13] have been noted in populations, including workers, that are exposed to high levels of environmental noise. Despite this relationship with gastric disorders, little is known regarding the effects of loud sounds on gastrointestinal function. On theoretical grounds, however, an interaction between noise and gastrointestinal function would seem likely given the abundant neural connections between the human auditory system, the autonomic nervous system and the gastrointestinal tract[1]. To the extent that noise has an influence on the autonomic nervous system, an effect on many digestive functions, including peristalsis and acid secretion, would be expected. In this respect, Tomei et al[13] observed that noise increased gastric acid production in humans with normal baseline levels of gastric acid, and Gue et al[14] have shown that noises slows the emptying of food from canine stomach.
The motility of the stomach is controlled by gastric myoelectrical activity (GMA) that results in contractions of its muscular component. GMA is measured and recorded using surface electrodes. The information so obtained is termed an electrogastrogram (EGG). The EGG is obtained using standard EKG electrodes placed on the skin of the upper abdominal wall. These signals are amplified and filtered prior to computer analysis. The validity of such cutaneous measurements has been validated by simultaneously obtaining direct measurements form the wall of the stomach[15,16]. In humans, the normal GMA is an approximately 3 cycles per minute (CPM) sinusoidal wave, and these waves may result in gastric contractions at a similar frequency. Additionally, under normal conditions, most of the voltage (termed “power”) measured during EGG is concentrated around the 3 CPM activity.
From a clinical standpoint, EGG recordings have been employed to investigate patients with dyspepsia whose complaints cannot be defined by routine tests, such as barium X-rays and endoscopy. In this respect, a dominant frequency of GMA greater or less than 3 CPM or a shift in the relative distribution of power away from 3 CPM activity are associated with abnormalities of gastric motor activity[17]. These alterations are thought to be the basis of many stomach complaints collectively referred to as “indigestion” or “dyspepsia”.
In light of above observations, the hypothesis evolved that loud noise might alter the EGG, perhaps by a mechanism involving the autonomic nervous system. To test this possibility, we exposed healthy volunteers to a variety of noises while recording the myoelectrical activity of their stomachs and monitoring their blood pressure and heart rate. A secondary hypothesis of this study was that advancing age would attenuate the effects of noise inasmuch as the reactivity of the autonomic nervous system declines with age.
Under the conditions employed in our study, we found that noise was capable of decreasing the percentage of normal 3 CPM activity and these changes were less pronounced in older individuals.
This investigation was approved by the Human Studies Subcommittee (IRB) of the James J. Peters VA Medical Center and informed consent was obtained prior to initiating the studies herein described. A screening hearing test was performed several days prior to the study which assessed the ability to identify sounds at a volume equal or less that 25 dB hearing level at 500, 1000, and 2000 Hz in the both ears and a sound at a volume equal to or less than 45 dB at 4000 Hz in the both ears. If the subjects could not correctly identify each sound, they were excluded from the study and were referred for further workup to the audiology service. All subjects were considered healthy and had no underlying medical problems. Volunteers were excluded from the study if detailed questioning revealed a previous history of chronic gastrointestinal discomforts including heartburn, abdominal pain, constipation or diarrhea. In addition, entry into the study required a normal baseline EGG with a dominant frequency between 2.4-3.7 CPM. Of the 26 screened candidates, 21 male subjects (age range 22-71 years, mean 44 years) met the criteria for inclusion into the study.
All subjects were requested not to eat or drink within 4 h prior to the start of the study. They were seated in a special sound room in a reclining chair and instructed not to move or talk for the remainder of the study. The sound room was double walled and approximately thirty-five square feet in area. The ambient noise levels were well within the standards of the American National Standards Institute. The subjects were observed through a one-way viewing window so as to discourage any movement during the study. The subjects were fitted with both an automatic blood pressure recording device and three electrodes that measured GMA.
The duration of the study was 110 min. During this period, the subjects wore stereophonic headphones continuously and were exposed in a random pattern to three periods of different types of auditory stimuli. A twenty-minute baseline period of silence preceded the auditory stimuli. Each stimulus lasted 20 min and was interspersed with a 10 min of silence as a recovery period to minimize potential carry-over effect from previous period. The 10-min recovery time was assumed to be adequate as our preliminary study had shown the effect of noise on GMA did not go beyond this period of time. The three auditory stimuli were as follows: (1) unedited hospital noise recorded during the afternoon of a weekday in the Intensive Care Unit of the James J. Peters Veterans Affairs Medical Center and played back at a level of 87.4 dBA (SPL), a level comparable to that of actual ambient level meter; (2) conversation babble (Auditec, St. Louis, MO) played back at a sound level of 91.3 dBA (SPL); and (3) traffic noise in New York City played back at a sound level of 85.6 dBA. All sound pressure levels were measured with a Lason-Davis 800 B precision sound level meter.
Since GMA may change with age, and many responses to auditory stimuli are believed to decline with age, we further divided subjects into two groups, those under 50 years of age (age range 22-47 years, mean 34 years, n = 12), and those over 50 years of age (age range 51-71 years, mean 56 years, n = 9). The baseline characteristics of GMA of these two age groups were compared and the effect of noise on these two particular age groups was analyzed.
Gastric myoelectrical activity was recorded using a Microdigitrapper (Medtronic-Synectics, Shoreview, Minnesota). Three surface electrodes were placed on the anterior wall of the abdomen. The first electrode was placed midway between the xiphoid and the umbilicus. The second electrode was placed 5 cm to the right of the first electrode. The third electrode was placed five centimeters to the left and thirty degrees above the first electrode. Excess hair was shaved from the immediate area, the skin was gently abraded with an alcohol swab, and conducting gel was applied. All recordings were made at a sampling frequency of 4 Hz, with a cutoff frequency of 15 Hz. The recorded data were uploaded into a computer, transformed (fast-Fourier) and analyzed using a dedicated software (Multigram Version 6.31, Medtronic’s-Synectics, Shoreview, Minnesota). Visual inspection of the raw data was performed to detect motion artifacts prior to analysis. Several quantitative parameters were derived including the percentage of 3 CPM activity (defined as normal myoelectrical activity in the range of 2.4-3.7 CPM), bradygastria (< 2.4 CPM) and tachygastria (> 3.7 CPM), dominant frequency (DF) and dominant power (DP). DF refers to the frequency at which the EGG power spectrum has a peak power in the range of 0.5-9.0 CPM, which equals to the frequency of the gastric slow wave measured from the implanted serosal electrodes[15,16]. The power at the DF in the power spectrum of the EGG was defined as the DP. The relative change of the EGG dominant power may reflect gastric contractility[16,18].
An automatic blood pressure recording device was applied via an arm cuff placed around the upper arm of each participant. Recordings were made automatically every 5 min during the whole course of study. Systolic pressure, diastolic pressure and heart rate were analyzed to see whether noise affected autonomic nerve activity.
Data were presented either as percent change relative to baseline (percentage of 3 CPM activity, bradygastria and tachygastria) or means ± SE (DF and DP). Student’s t test or one way RM ANOVA was used for comparison wherever appropriate. A P value less than 0.05 was considered statistically significant.
We subjectively divided participants into two age groups, using age 50 as a cutoff line. Percentage of 3 CPM activity, DF and DP were derived from the twenty-minute baseline period and comparisons were made between these two age groups. Detailed data are presented in Table 1. In brief, though no significant difference was observed in DF and percentage of 3 CPM activity during the baseline, younger people tended to have obviously higher dominant power (401.4 ± 03.8) than those above age 50 (115.1 ± 45.7, P < 0.05).
| Dominantfrequency | Dominantpower | 3 CPMactivity (%) | |
| Age < 50 yr (n = 12) | 3.0 ± 0.1 | 401.4 ± 103.8 | 87.9 ± 5.1 |
| Age > 50 yr (n = 9) | 3.1 ± 0.3 | 115.1 ± 45.7 | 77.7 ± 7.6 |
| P value | P = 0.724 | P < 0.05 | P = 0.259 |
During exposure to hospital noise and traffic noise, the percentage of 3 CPM activity (range 2.4-3.7 CPM) was decreased by 12.0% (P < 0.05) and 13.9% (P < 0.05), respectively, in our overall population (n = 21). Reduction in percentage of 3 CPM activity was also observed during exposure to conversation babble (7.1%), but was not statistically significant.
In the younger group (n = 12; age range 22-47 years, mean 34 years), the percentage of 3 CPM activity decreased to a greater degree during exposure to hospital noise (22.9%, P < 0.05) and traffic noise (19.0%, P < 0.05). A non-significant decrease was also seen during conversation noise (15.5%). In the older group (n = 9; age range 51-71 years, mean 56 years) showed no such a trend. In fact, during exposure to hospital noise and conversation babble, their percent activity of 3 CPM actually increased by 2.5% and 4%, respectively. During exposure to traffic noise, the percent activity of 3 CPM fell in this group by 7.1%.
Decreases in 3 CPM activity were accompanied mainly by rises in bradygastria activity. In the younger group, bradygastria activity rose during hospital and traffic noise by 16.3% (P < 0.05) and 13.4% (P < 0.05), respectively.
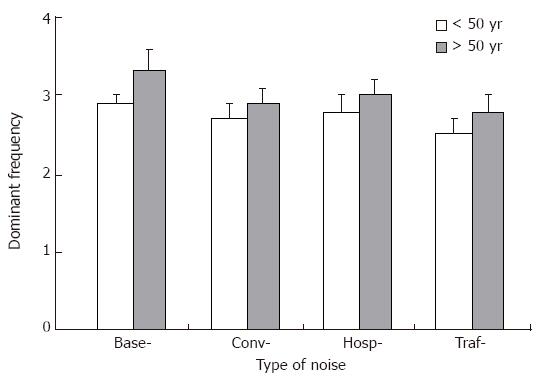
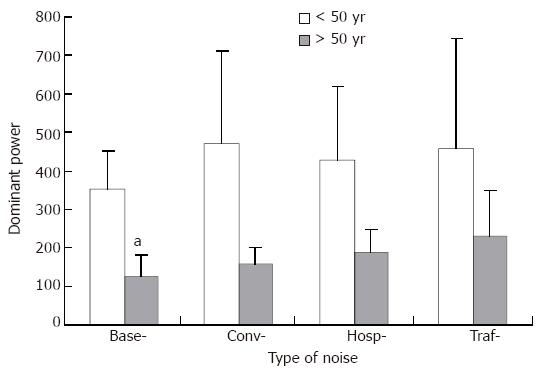
Trends toward a decrease in DF (Figure 1) and an elevation in DP (Figure 2) were consistently observed in the both age groups (> 50 years and < 50 years), but none of them reached statistical significance.
No effect of noise was seen either on diastolic, systolic pressure or on heart rate (Table 2). For the total population in the study, systolic pressure changed by 1.6 mmHg, -0.5 mmHg, and 1.4 mmHg for hospital, conversation, and traffic noise, respectively. Diastolic pressure changed by -0.9 mmHg, -1.6 mmHg, and -0.2 mmHg, respectively (Table 1). Finally, heart rate changed by 3.2 beats per minute (bpm), 2.5 bpm, and 1.5 bpm, respectively.
| Baseline | ICU | Babble | Traffic | ||
| Systolic | 122.2 ± 11.3 | 122.1 ± 19.3 | 119.3 ± 13.2 | 122.2 ± 12.6 | |
| Age < 50 yr | Diastolic | 78.1 ± 11.5 | 76.1 ± 14.8 | 74.3 ± 12.9 | 79.2 ± 12.6 |
| (n = 12) | Pulse | 73.6 ± 9.5 | 75.6 ± 10.2 | 76.4 ± 14.8 | 75.5 ± 9.8 |
| Systolic | 132.9 ± 18.8 | 136.5 ± 15.6 | 135.1 ± 19.3 | 136.0 ± 14.8 | |
| Age > 50 yr | Diastolic | 85.4 ± 11.4 | 85.7 ± 11.3 | 86.3 ± 13.1 | 83.6 ± 12.3 |
| (n = 9) | Pulse | 70.7 ± 16.5 | 75.4 ± 14.8 | 72.9 ± 15.1 | 71.7 ± 15.0 |
Our results indicate that GMA may change with advancing age, manifested mainly by a decline in dominant power. In addition, the average percentage of 3 CPM activity declined during exposure to noise in the younger subjects. Together with these decreases in 3 CPM activity, bradygastria became more prominent. A trend toward a decrease in DF and an elevation in DP was consistently observed during exposure to noises in both age groups. None of the noises employed in this study had a significant effect on autonomic nerve function reflected by blood pressure and heart rate.
Many non-auditory effects of noise have been described and have been reviewed in detail elsewhere[1]. In general terms, these non-auditory responses can be considered reflexive in nature and tend to increase the level of alertness and arousal. These reflexes included a focused posture directed to the source of noise, a shunting blood away from vegetative regions like the stomach, a rise in adrenalin, noradrenalin, and 17-hydroxycorticosteroids, a release of glucose into the blood stream, and an increase in blood pressure[19,20].
Many non-auditory responses to noise have been linked to activation of the autonomic nervous system. However, in the present study, noise did not alter blood pressure or heart rate. These are parameters that one would have expected to reflect enhancement of the autonomic nervous system and which have previously been shown to be affected by loud noise[5,6,11]. Our negative results in this regard suggest that, under the present conditions, alterations in GMA may occur at levels of autonomic stimulation less intense than that required to alter the circulatory system.
Our findings that exposure to noise decreases the percentage of normal 3 CPM activity is consistent with a recent report[21] in which the authors found that loud music and household noise all tended to alter the rhythmicity of gastric slow waves by an increase in bradygastria and a concurrent reduction in the normal 2-4 CPM activity. As in the present study, there were no signs of autonomic activation.
Workers in noisy jobs commonly report nausea and are known to have an increased incidence of digestive disorders including gastritis and ulcers[10,13]. As noted before, acoustic stress has been linked to increased gastric acid secretion[13]. An additional mechanism is suggested by the present study. Our finding that the percentage of normal 3 CPM activity decreased in younger subjects after exposure to noises raised the possibility that digestive problems, at least in part, might reflect alterations in contractile activity of the stomach. In this respect, it has been demonstrated that reduction in the normal GMA, the presence of bradygastria or arrhythmias is associated with reduced or absent gastric contractions[22]. Decreased movement of gastrointestinal tract has long been considered a sine qua non of the “fight or flight” response and, as noted above, probably arises from autonomic (specifically, sympathetic) stimulation. Our findings are consistent with studies by Gue et al[14], showing that the noise slowed the emptying of food by the canine stomach.
The relationship between age and characteristics of GMA has been investigated. It is clear that gastric slow waves are absent at birth and go through periods of maturation after birth[23]. Whether GMA changes with the advancement of age at adulthood is still inconclusive but studies have reported that aging increases the instability of electrical frequency[24], or the frequencies of the rhythm of surface EGG[25]. In our study, it is interesting to note that the dominant power was significantly lower in the older subjects than those of younger group. Although the significance of this phenomenon is unclear, it is possible that the aging stomach might be less active in motility and strength.
Advancing age is accompanied by a deterioration of autonomic nervous function[26]. This may, in part, explain why GMA of older individuals is diminished during exposure to stimuli such as meal[27], or head out of water immersion[28]. Finally, in an animal study by Pharm and Willott[29], it was determined that the “threshold” auditory stimulus required to initiate an acoustic startle response grew with age. These studies may clarify why older subjects in our study showed a diminished gastric electrical response to noise that did the younger subjects.
In previous studies, noise has been observed to both increase[5,19,30] and have no effect[31,32] on blood pressure and heart rate. Our own negative results in this respect are consistent with the latter findings. However, the duration, intensity and quality of the noise undoubtedly determine whether and to what extent an effect on blood pressure and heart rate will be observed.
To understand the influence of environmental noises on GI physiological functions.
Environmental noises have been linked to cardiovascular and neurological disorders. However, the effect of noise on the GI system has been minimally explored.
This is the first study that to show that noise can affect physiological gastric electrical activity in healthy subjects and that the degree of this effect seems to be age-related.
Prevention of over-exposure to environmental noises may prevent some negative health issues.
Electrogastrogram: Gastric myoelectrical activity is recorded using a portable device via surface electrodes placed on the anterior wall of the abdomen.
It is a well-written study. Electrogastrography is an evolving field, and worth of further investigation.
| 1. | Kryter K. The handbook of hearing and the effects of noise. New York: Academic Press 1994; . |
| 2. | Abey-Wickrama I, A'Brook MF, Gattoni FE, Herridge CF. Mental-hospital admissions and aircraft noise. Lancet. 1969;2:1275-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 36] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Meecham WC, Smith HG. Effects of jet aircraft noise on mental hospital admissions. Br J Audiol. 1977;11:81-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Ohrström E. Sleep disturbances caused by road traffic noise - Studies in laboratory and field. Noise Health. 2000;2:71-78. [PubMed] |
| 5. | Andrén L, Hansson L, Björkman M, Jonsson A. Noise as a contributory factor in the development of elevated arterial pressure. A study of the mechanisms by which noise may raise blood pressure in man. Acta Med Scand. 1980;207:493-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Belli S, Sani L, Scarficcia G, Sorrentino R. Arterial hypertension and noise: a cross-sectional study. Am J Ind Med. 1984;6:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Cohen S, Krantz DS, Evans GW, Stokols D. Cardiovascular and behavioral effects of community noise. Am Sci. 1981;69:528-535. [PubMed] |
| 8. | Knipschild P. VIII. Medical effects of aircraft noise: review and literature. Int Arch Occup Environ Health. 1977;40:201-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Tomei F, Tomao E, Papaleo B, Baccolo TP, Alfì P. Study of some cardiovascular parameters after chronic exposure to noise. Int J Cardiol. 1991;33:393-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Berland T. The fight for quiet. Englewood Cliffs, New Jersey: Prentice-Hall 1970; . |
| 11. | Falk SA, Woods NF. Hospital noise--levels and potential health hazards. N Engl J Med. 1973;289:774-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 104] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Karagodina L. Effect of aircraft noise on the population near airports. Hyg Sanit (USSR). 1969;34:182-187. |
| 13. | Tomei F, Papaleo B, Baccolo TP, Persechino B, Spanò G, Rosati MV. Noise and gastric secretion. Am J Ind Med. 1994;26:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Gué M, Peeters T, Depoortere I, Vantrappen G, Buéno L. Stress-induced changes in gastric emptying, postprandial motility, and plasma gut hormone levels in dogs. Gastroenterology. 1989;97:1101-1107. [PubMed] |
| 15. | Hamilton JW, Bellahsene BE, Reichelderfer M, Webster JG, Bass P. Human electrogastrograms. Comparison of surface and mucosal recordings. Dig Dis Sci. 1986;31:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 141] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Chen JD, Schirmer BD, McCallum RW. Serosal and cutaneous recordings of gastric myoelectrical activity in patients with gastroparesis. Am J Physiol. 1994;266:G90-G98. [PubMed] |
| 17. | Telander RL, Morgan KG, Kreulen DL, Schmalz PF, Kelly KA, Szurszewski JH. Human gastric atony with tachygastria and gastric retention. Gastroenterology. 1978;75:497-501. [PubMed] |
| 18. | Smout AJ, van der Schee EJ, Grashuis JL. What is measured in electrogastrography? Dig Dis Sci. 1980;25:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 248] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 19. | Andrén L, Hansson L, Eggertsen R, Hedner T, Karlberg BE. Circulatory effects of noise. Acta Med Scand. 1983;213:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Arguelles AE, Ibeas D, Ottone JP, Chekherdemian M. Pituitary-adrenal stimulation by sound of different frequencies. J Clin Endocrinol Metab. 1962;22:846-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Chen DD, Xu X, Wang Z, Chen JD. Alteration of gastric myoelectrical and autonomic activities with audio stimulation in healthy humans. Scand J Gastroenterol. 2005;40:814-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Xing J, Qian L, Chen J. Experimental gastric dysrhythmias and its correlation with in vivo gastric muscle contractions. World J Gastroenterol. 2006;12:3994-3998. [PubMed] |
| 23. | Chen JD, Co E, Liang J, Pan J, Sutphen J, Torres-Pinedo RB, Orr WC. Patterns of gastric myoelectrical activity in human subjects of different ages. Am J Physiol. 1997;272:G1022-G1027. [PubMed] |
| 24. | Pfaffenbach B, Adamek RJ, Kuhn K, Wegener M. Electrogastrography in healthy subjects. Evaluation of normal values, influence of age and gender. Dig Dis Sci. 1995;40:1445-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Nishimura N, Hongo M, Yamada M, Kawakami H, Toyota T. Gastric myoelectrical activities in elderly human subjects--surface electrogastrographic observations. J Smooth Muscle Res. 1995;31:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Stansberry KB, Hill MA, Shapiro SA, McNitt PM, Bhatt BA, Vinik AI. Impairment of peripheral blood flow responses in diabetes resembles an enhanced aging effect. Diabetes Care. 1997;20:1711-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Parkman HP, Harris AD, Miller MA, Fisher RS. Influence of age, gender, and menstrual cycle on the normal electrogastrogram. Am J Gastroenterol. 1996;91:127-133. [PubMed] |
| 28. | Miwa C, Mano T, Saito M, Iwase S, Matsukawa T, Sugiyama Y, Koga K. Ageing reduces sympatho-suppressive response to head-out water immersion in humans. Acta Physiol Scand. 1996;158:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Parham K, Willott JF. Acoustic startle response in young and aging C57BL/6J and CBA/J mice. Behav Neurosci. 1988;102:881-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Eggertsen R, Svensson A, Magnusson M, Andrén L. Hemodynamic effects of loud noise before and after central sympathetic nervous stimulation. Acta Med Scand. 1987;221:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Malchaire JB, Mullier M. Occupational exposure to noise and hypertension: a retrospective study. Ann Occup Hyg. 1979;22:63-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Santana VS, Barberino JL. Occupational noise exposure and hypertension. Rev Saude Publica. 1995;29:478-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
S- Editor Liu Y L- Editor Kumar M E- Editor Bi L