INTRODUCTION
Ulcerative colitis (UC) is a chronic inflammatory disease of colon and rectum. The underlying aetiology and exact pathogenesis remain unknown. Current hypothesis favours dysregulation of gastrointestinal immune system in a genetically predisposed individual[1]. Extra-intestinal manifestations of inflammatory bowel disease (IBD) are common and may occur in 40% of patients[2]. It mainly involves skin, eye, joint, and the biliary system. Many of them are postulated to have autoimmune mechanisms[3]. Renal manifestations such as glomerulopathy associated with IBD were rarely reported, whereas psoriasis was found to occur more frequently in patients with IBD and their relatives[4]. Minimal change disease (MCD) causes more than 90% of cases of nephrotic syndrome in children and 10%-15% cases in adults[5]. The aetiology and pathogenesis of both MCD and psoriasis are also unknown and presumed to have an immunological basis. We report a case of fulminant ulcerative colitis which developed soon after withdrawal of immunosuppressive treatment for steroid-resistant MCD in a patient with a history of psoriasis.
CASE REPORT
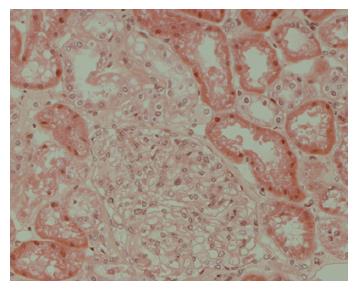
A 43-year-old Chinese patient had a 3 years history of plaque psoriasis follow up in dermatology clinic. He was treated by topical steroid and vitamin D analogue (calcipitriol) only. He presented to our hospital with a 2 wk history of generalized oedema involving his face, limbs, and scrotum in January 2002. His renal function was normal but he was found to have significant proteinuria (18 gm/day). His serum albumin level was low, 18 gm/L, and fasting blood glucose was 4.5 mmol/L. Investigations for the underlying cause of his nephrotic syndrome including antinuclear antibody, antineutrophil cytoplasmic antibody, immunoglobulin pattern, hepatitis B and C serology, anti-streptolysin O titre, and serum mercury level were all negative or within the normal range, respectively. He denied taking any other medications in particular non-steroidal anti-inflammatory drugs or herbs. Subsequent renal biopsy confirmed minimal change disease (Figure 1). He was treated with high dose prednisolone (60 mg daily) but his disease was found to be steroid resistant. A second renal biopsy was performed 4 mo later to rule out alternative diagnosis. The histological finding was still consistent with MCD. Treatment with cyclophosphamide and then azathioprine was started by the nephrologist to keep his disease in remission. Steroid and all immunosuppressants were stopped in August 2003 after his renal disease has been continued to be quiescent for 6 mo.
Figure 1 Renal biopsy showed features consistent with minimal change disease.
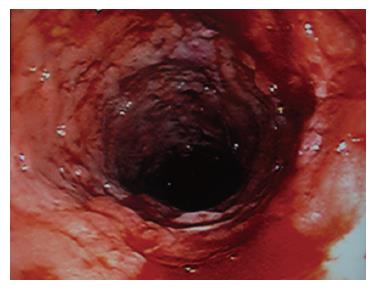
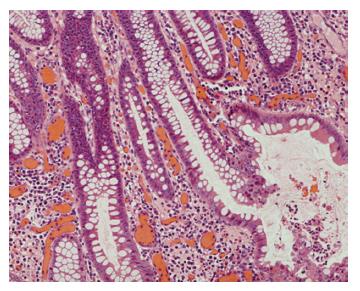
One month later, he was admitted to the gastro-enterology unit because of right lower quadrant abdominal pain and passing bloody diarrhoea 8 to 10 times per day. At that time, he was afebrile and his vital signs were stable. Blood result showed that he had significant hypochromic microcytic anaemia with haemoglobin of 8.6 gm/dL. Renal and liver function was normal. His serum albumin was 28 gm/L and his 24 h urine for protein was 0.2 gm per day only. Stool for common bacterial and parasitic pathogens and clostridial difficle toxin were negative. Blood for cytomegalovirus pp65 antigen was negative. Abdominal X-ray did not reveal features of toxic megacolon. Ileo-colonoscopy was performed and showed severe pancolitis without terminal ileum involvement (Figure 2). Histology showed active colitis with crypt distortion and crypt abscess without granuloma or pathogen seen (Figure 3). The clinical picture was compatible with fulminant ulcerative colitis. High dose intravenous hydrocortisone (100 mg thrice daily) and mesalamine were started but his condition did not improve. At that moment, he refused surgery and therefore, intravenous cyclosporine A infusion (4 mg/kg per day) was commenced. His bloody diarrhoea started to improve after 1 wk of treatment and he was discharged with close follow up in the outpatient clinic. During the subsequent 6-mo follow up, his intestinal symptoms remained poorly controlled and bloody diarrhoea persisted despite optimal immunomodulatory therapy. Finally, he agreed for operation and restorative procto-colectomy with ileal pouch anal anastomosis was performed.
Figure 2 Ileo-colonoscopy showed severe pancolitis.
Figure 3 Colonic biopsy showed histological features of active ulcerative colitis.
DISCUSSION
Glomerulonephritis (GN) and nephrotic syndrome have been reported as rare extra-intestinal manifestations of inflammatory bowel disease. So far, less than 30 cases had been reported in the literature[6]. Different types of lesion that have been described, including IgA nephropathy[7-9], IgM nephropathy[10], membranoproliferative glomerulonephritis[11], mesangial proliferative glome-rulonephritis[12,13], membranous nephropathy[14,15] , and antiglomerular basement membrane glomerulonephritis[6]. The renal disease occurs in a setting of active IBD in many of these cases. The proposed mechanism of association between IBD and GN is the release of intestinal antigens secondary to bowel inflammation. The subsequent antibody production and immune complex formation cause glomerular injury[12]. However, the exact mechanism and the type of immune complex remain to be elucidated. At least in some cases, the renal disease may not concur with active bowel disease at the same time[12,16].
MCD, also known as lipoid nephrosis or foot process disease, accounts for majority of nephrotic syndrome in children and 10%-15% of cases in adults. It is characterized by normal glomeruli as visualized by light microscopy and the non-detectability of immune complexes using immunofluorescence assays. The pathogenesis of MCD is thought to be related to the T-lymphocyte production of an abnormal cytokine which only affect glomerular permeability but do not promote sclerogenic mechanisms[5]. In the past, only 2 cases of MCD associated with Crohn’s disease (CD) has been reported (1 in English literature)[17]. Perard L et al[16] reported a case of T-cell acute lymphoblastic leukemia (ALL) which developed in a 17-year old boy with a history of MCD and CD. In their case, the development of MCD preceded that of CD for 4 years. They proposed that T-cell dependent dysfunction with abnormal cytokine production could be the common pathologic link between the 3 diseases: glomerular lesion, inflammatory bowel disease, and proliferation of ALL blast cells. Similarly, the pathogenesis of psoriasis is also attributed to the immune dysregulation by T-lymphocytes[18]. An epidemiological study shows a higher prevalence of psoriasis in patients with Crohn’s disease[4]. The findings of high tumour necrosis factor- alpha (TNF) level and effective treatment with infliximab (a TNF inhibitor) for both diseases suggest a relationship between IBD and psoriasis[19]. Thus, abnormal cytokine production by dysfunctional T-lymphocytes could be a common pathogenetic mechanism of all 3 diseases in our patient. To our knowledge, this is the first reported case of ulcerative colitis associated with minimal change disease and psoriasis.
We believe that there is true association between UC, MCD, and psoriasis rather than just a coincidence in our patient. Scrapa R et al[20] found that microscopic colitis occurred in patients with active psoriasis or psoriatic arthritis even they did not have any bowel symptoms. During therapy for MCD, our patient did not complain of significant intestinal symptoms. We speculated that his colitis activity could be suppressed by the treatment of steroid and immunosuppressive agents. Abrupt withdrawal of immunosuppressive treatment might contribute to his fulminant presentation of ulcerative colitis. Previous studies also demonstrated that withdrawal of azathioprine could lead to a relapse of IBD activity[21,22].
In conclusion, we report the first case with rare association of UC, MCD, and psoriasis. The pathologic link between these diseases with presumed autoimmune mechanisms could be due to abnormal cytokine production in dysregulated T-cells, causing damage to intestine, kidneys, and skin tissues. Further studies are required to identify that cytokine and delineate the exact pathogenesis.