Published online Jul 28, 2007. doi: 10.3748/wjg.v13.i28.3886
Revised: January 5, 2007
Accepted: January 14, 2007
Published online: July 28, 2007
AIM: To investigate the selective tropism of liver stem cells to hepatocellular carcinoma (HCC) in an animal model and its feasibility as a vector to deliver therapeutic genes for targeted therapy of HCC.
METHODS: WB-F344, a kind of rat liver stem cell, was infected with recombinant virus to establish a cell line with stable, high-level expressing enhanced green fluorescent protein (EGFP). An animal model of HCC in Wistar rats was established by implanting HCC cells (CBRH7919) combined with an immunosuppressive drug. EGFP labeled liver stem cells were injected into caudal veins of the animals and distribution was observed at different time points after injection. SDF-1 and c-kit expression in non-tumor liver and tumor tissue were analysed by immunohistochemistry for the relationshiop between the expression and migration of liver stem cells. Furthermore, hepatic stem cells were injected via the portal vein, hepatic artery, caudal vein, or directly into the pericancerous liver tissue, respectively, and effects on migration, localization, and proliferation of the hepatic stem cells within the tumor tissue were observed and analyzed.
RESULTS: Recombinant adenovirus could deliver the EGFP gene to hepatic stem cells. A new stem cell line, named WB-EGFP, was established that stably expressed EGFP. WB-EGFP cells still showed selective tropism towards HCC and EGFP expression was stable in vivo. According to immunohistochemistry results, SDF-1 may not be related to the mechanisms of tropism of hepatic stem cells. Different application sites affected the distribution of liver stem cells. Injection via the portal vein was superior with regard to selective migration, localization, and proliferation of the hepatic stem cells within the tumor tissue.
CONCLUSION: Liver stem cells have the biological behavior of selective migration to HCC in vivo and they could localize and proliferate within HCC tissue stably expressing the target gene. Liver stem cells are a potential tool for a targeted gene therapy of HCC.
-
Citation: Zhong XG, He S, Yin W, Deng JY, Cheng B. Selective tropism of liver stem cells to hepatocellular carcinoma
in vivo . World J Gastroenterol 2007; 13(28): 3886-3891 - URL: https://www.wjgnet.com/1007-9327/full/v13/i28/3886.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i28.3886
Hepatocellular carcinoma (HCC) patients have a poor prognosis even in the case of extensive surgical excision, adjuvant radio- and chemotherapy, or liver transplantation[1,2]. Treatment of HCC still requires the development of new therapeutic approaches and could largely benefit from gene therapy strategies[3]. Gene therapy relies on the transfer of a therapeutic protein into a selected cell population. At present, gene transfer vectors for HCC target cells have low transduction efficiency and unstable transgene expression[4,5]. Thus, development of a gene transfer method for HCC therapy should be envisaged as a very promising effort for patients. It is necessary that research focuses on the selection and design of the most efficient gene transfer strategy. It has been reported that neural stem cells (NSC) migrate to the intracerebral gliomas and display an extensive tropism. The NSC-based gene therapy of gliomas has shown promising results in animal experiments[6-8]. However, whether stem cells have selective tropism toward HCC lesion like NSC is still unclear. External liver stem cells have the capacity of tropism towards injured liver[9], and selective migration of liver stem cells to HCC cells in a coculture system has been observed in vitro. It has been reported early[10], but whether external liver stem cells can maintain the capacity of targeting the HCC lesion in vivo is unclear. EGFP is a good marker gene. After being transferred into liver stem cells, it can be expressed stably and used as a marker for these cells. EGFP can indicate the migration of hepatic stem cells in vivo. This study was undertaken to explore the tropism of external liver stem cells to HCC and its feasibility as vectors to deliver therapeutic genes in vivo. It provided the primary theoretical supports for the use of migratory liver stem cells to deliver therapeutic genes for a targeted gene therapy of HCC.
The rat liver stem cell line (WB-F344) was obtained from the Institute of Materia Medica of Chinese Academy of Medical Sciences. This cell line was established by Grisham[11]. The cells were cultured in DMEM/F-12 (1:1) supplemented with 100 mL/L FBS(GibcoBRL, USA). HCC cells (CBRH7919) from the Shanghai Institute of Cell Biology, Chinese Academy of Sciences, with the cell line established from rat liver cancer by DH Zhu, were cultured in RPMI-1640 supplemented with 10% FBS (GibcoBRL,USA)[12]. Plasmid pAdEasy-1 and pAdTrack-EGFP were provided by Dr. TC He from Johns Hopkins Oncology center. Lipofectamine was provided by Invitrogen Co.USA. Antibodies directed against c-kit, SDF-1, C-kit, ck19, AFP, and α1-ATT were purchased from Boster Co.Ltd, China.
Plasmid pAdEasy-1 and pAdTrack-EGFP were constructed to produce the vector pAd-CMV-EGFP by homologous recombination in E.coli BJ 5183, and the recombinant adenovirus was generated in the HEK 293 packaging cell line (Pathology Laboratory of Huaxi Hospital)[13,14]. WB-F344, a kind of rat liver stem cell, was cultured in vitro and infected with recombinant virus. Infection efficiency and expression of EGFP were assessed by a fluorescent microscopic survey. A new cell line (WB-EGFP) which had stable, high-level expression of EGFP was chosen and established through cloning of selective culture[15], and the biological characteristics of the cell line were analyzed by fluorescent microscopy, flow cytometry, immunocytochemical staining (C-kit, ck19, AFP, α1-ATT), and experimentally by inducing differentiation via sodium butyrate, which was performed as previously reported[16].
HCC cells CBRH7919 were cultured and harvested. To establish an HCC tumor, CBRH7919 cells were implanted into the liver tissue of adult rats. Wistar rats (n = 65) were used to make the animal model of HCC. After anesthesia, each animal received an implantation of 4 × 106 tumor cells suspended in culture medium without serum and daily cyclosporin injections (10 μg/g) intraperitoneally[17]. When the tumors were grown, animals were used for the further experiments. All animal studies were conducted under protocols approved by the Animal Care and Use Committee in accordance with National Institute of Health Guidelines.
Experimental Wistar rats were divided into two groups: (1) non-tumor bearing healthy rats (n = 14); and (2) rats which had developed HCC (n = 15). On d 0, recipients received an injection of liver stem cells (4 × 106 WB-EGFP cells in 200 μL media without serum) through the rat’s caudal vein, using identical coordinates. All rats received daily cyclosporin injections (10 μg/kg) at the same time each day. Recipients were sacrificed on d 3-5 or 7-9 after liver stem cell implantation. The number and distribution of cells positive for the EGFP marker were analysed in ice-frozen sections of tumor, peritumor, liver, kidney, spleen, and lung tissue by fluorescence microscopy. Hemotoxylin and eosin stainings were used for histopathological analysis.
Immunocytochemical staining of EGFP expressing liver stem cells was carried out according to standard protocols. Antibodies against C-kit, ck19, AFP, α1-ATT were detected in differentiated and undifferentiated cells.
Immunohistochemical staining of formalin-fixed, paraffin-embedded tissue from tumor and liver tissue of both groups was performed using antibodies against c-kit and SDF-1 by SP methods[18,19]. According to the intensity and proportion of stained cells, the results of immunohistochemistry were judged. The negative control was obtained with PBS replacing the primary antibody.
HCC tumor-bearing rats were divided into four groups. Liver stem cells with EGFP label were implanted via the portal vein (group A, n = 9), hepatic artery (group B, n = 9), caudal vein (group C, n = 8), or directly into pericancerous liver tissue (group D, n = 9). On day 0, recipients received injection of liver stem cells (4 × 106 WB-EGFP cells in 200 μL media without serum ), at identical coordinates. All rats received daily cyclosporin injections (10 μg/kg) at the same time each day. Rats were sacrificed on d 7-9 after implantation of the liver stem cells. The same observations and analysis were performed as in the previous protocol.
Biostatistical analyses were done using the SPSS11.5 software package. The non-parametric Kruskal-Wallis rank test was used to detect differences among different experimental groups. Some findings were statistically significant and compared using the Fisher test for evaluation in a two-group experiment. P < 0.05 was regarded as statistically significant.
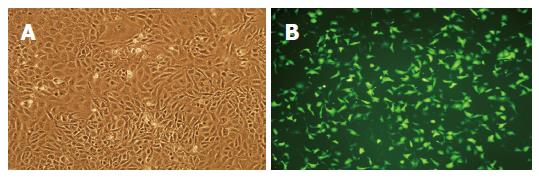
The recombinant adenovirus Ad-EGFP was generated in the HEK 293 packaging cell line by homologous recombination. Liver stem cells (WB-F344) were infected with different amounts of recombinant viruses. After selective culture cloning, a cell line that stably expressed high levels of EGFP was established (Figure 1). This new cell line, WB-EGFP, displayed a similar proliferation and cell cycle distribution profile. Differentiation characteristics of the new stem cells were not affected. WB-EGFP expressed C-kit(+), ck19(+), AFP(+), and α1-ATT(-). After induction with sodium butyrate, they could differentiate into hepatic cells, expressing the marker of liver cell, C-kit(-), ck19(-), AFP(-), but α1-ATT(+). EGFP expression in WB-EGFP cells could be maintained for 8-9 generations in vitro.
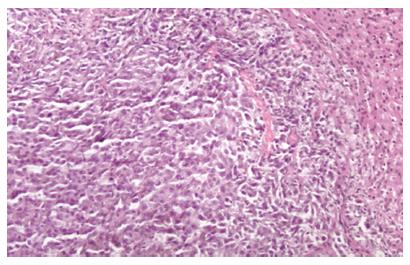
After the CBRH7919 cells were implanted into the liver tissue of adult rats, daily cyclosporin injections (10 μg/g) were given intraperitoneally, and the rats were observed every day. About two weeks later, a tumor model was established for further experiments. Tumor lesions about 0.3-0.7 cm in diameter were found in the implanted site of the liver, with tumors still displaying the same pathological morphology characteristics as hepatocellular carcinoma in hemotoxylin and eosin staining (Figure 2).
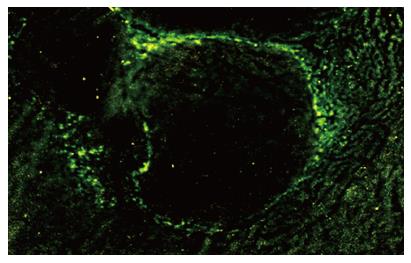
Liver stem cells were injected into the tail vein of Wistar rats with HCC liver tumors. At different time points, tissues specimens from tumor, peritumor, liver, kidney, spleen, and lung were sampled. Ice-frozen tissues sections were examined by fluorescent microscopy to identify EGFP positive stem cells. Four days after injection, EGFP positive cells were found around the tumor lesions and a few cells had infiltrated the tumor inner walls (Figure 3). Eight days after injection, the labeled cells were found in the central area of the tumors. The cells with EGFP were still identically anti-c-kit (+), but such cells were not found in the liver tissue of the control group, in surrounding normal-appearing liver tissue, or in other organs, such as kidney, lung, or spleen.
Expression of c-kit was analyzed as follows: Slides were examined at low-power magnification (100 ×) to identify areas with high density of c-kit positive cells. Three areas with highest density were selected, and positive cells were counted at 400 × magnification. The proportion of c-kit(+) cells less than 5% was taken as background, higher levels were considered positive. The result of SDF-1 positive staining was obtained according to the accepted standard of the staining intensity and area. C-kit positive cells were localized around the margin of the tumor lesion as are EGFP positive cells. Expression of c-kit was negative in liver tissue of the control animals. The chemokine SDF-1 could be localized in the cytoplasm of tumor and normal hepatic cells. There was no difference in the expression of SDF-1 between the control and experimental groups, while the quantity of cells with c-kit(+) increased in tissues with HCC (P < 0.01, Table 1).
Different sites of injection affected the localization and proliferation of liver stem cells. Liver stem cells were injected via four different sites: portal vein, hepatic artery, caudal vein, and pericancerous liver tissue. On d 8 after the liver stem cell implantation, in most of the cases, donor liver stem cells migrated and targeted the tumor. Liver stem cells were found within the main tumor bed, with very few liver stem cells in other locations. Migrated cells had formed cell “islands” with EGFP expression in the tumor tissue. Distribution could be ranked according to the localization of liver stem cells. No cells at the tumor bed were (-), cells in the margin of the tumor bed were (+), cells between the margin and central area of tumor were (++), and cells in the central area of the tumor were (+++). These liver stem cells’ behaviors appeared to show more selective tropism for the HCC lesion and proliferation capacity when injected from portal vein (P < 0.05) (Table 2).
Gene therapy researchers have employed two major strategies for delivering therapeutic transgenes into human recipients[20,21]. Direct infusion of a gene into a person is a fairly imprecise method and is limited to specific types of human cells that the viral vehicle can infect[22]. The use of living cells to deliver therapeutic transgenes into the body is another important strategy. Gene modified cells[23,24], such as stem cells, lymphocytes, or fibroblasts, are allowed to grow and proliferate and are then infused back into the patient. Stem cells are self-renewing[25,26] and thus may reduce or eliminate the need for gene therapy. Stem cells thus have a high potential as a platform for gene therapy[27,28].
External liver stem cells have the capacity of tropism towards injured liver. But whether liver stem cells have selective tropism has still not been clarified. Liver cells may have the ability of tropism to HCC just as neural stem cells do, liver stem cells may have the ability of tropism to HCC. It was hypothesized that the pathology of HCC promotes directed liver stem cell migration towards HCC lesions in vivo. The liver stem cell may be a potential target vector for HCC gene therapy.
Firstly, liver stem cells were engineered to express EGFP as a label for in vivo experiments. For effective gene therapy, it is necessary to achieve high levels of sustained expression of the therapeutic gene[29,30]. As we know, a major limitation to gene therapy is a low efficiency of transduction when targeting nondividing cells[31]. We developed an adenovirus system for liver gene therapy in which we could transduce liver stem cells ex vivo with expression of a reporter gene in a high proportion of the cells and amplify transgene expression in the host by maintaining expression of the introduced gene after multiple rounds of cell division. In ex vivo transduced liver stem cells expressing EGFP the capacity of proliferation and differentiation were not affected, so EGFP could be taken as a marker for liver stem cells in the experiment. Transgene expressing cells could be applied to gene therapy to treat many forms of liver disease.
The in vitro study using the coculture system with HCC cells showed that the relative direct migratory capability towards HCC cells of liver stem cells was comparable with fibroblasts[10]. HCC cells may have some factors that promoted the movement of liver stem cells towards the HCC cells. To determine the behavior of liver stem cells in vivo, the cells were injected via the tail vein of HCC-bearing rats. Four days after injection, EGFP expressing cells migrated to surround the tumor lesions, and the labeled cells gradually infiltrated into the central area of tumors, but normal liver tissue of the control group didn’t provide such a permissive migratory environment to liver stem cells. In other organs, such as kidney, lung, or spleen, significantly fewer numbers of liver stem cells were observed. Fluorescent staining of EGFP and positive expression of c-kit noticeably increased in lesions of tumors or around the tumor (P < 0.01), which suggested that liver stem cells had the capability of tracing the lesions of hepatocellular carcinoma, and stably expressing the transferred gene. Liver stem cells may become an ideal gene vector for targeted therapy.
There are no reports about the mechanism of the selective tropism towards HCC tissue. Stromal cell derived factor-1 (SDF-1) is one of the chemokine members. Interaction between SDF-1 and CXCR4 play an important role in the regulation of hemotopoietic stem cell mobilization[32], and according the report, the mechanism of neural stem cells (NSC) migrating to the intracerebral gliomas involved mediators as SDF-1. Meanwhile, some researchers found that SDF-1 and other growth factors are related to the migration of stem cells from bone marrow into liver and their hepatocellular differentiation[33]. In this experiment, expression of SDF-1 showed no difference between the normal liver tissue and HCC tissue (P > 0.05), which implied that SDF-1 may have no effect on the tropism of liver stem cells to HCC. Thus, it is necessary to further understand the mechanism.
In 1992, Wilson performed gene therapy by transplanting hepatocellular cells from the portal vein, which was an early model to treat liver disease. How the liver stem cells localize and proliferate is not known, especially when they are transplanted from different approaches. On the basis of this experiment, liver stem cells still migrated towards the tumor from different implant locations, although more cells distributed and proliferated in the tumor lesion via portal vein implantation. The migratory cells in tumor beds survived and proliferated to form some cell “islands”, and the reporter gene was expressed stably by surviving liver stem cells in tumor beds. These findings suggest that external liver stem cells can target HCC and possess the ability of a selective tropism to HCC in vivo. Combined with the results of early research in vitro[10], it could be concluded that liver stem cells could deliver the therapeutic gene protein into HCC lesions selectively. Meanwhile, aside from the self-renewing character of stem cells, liver stem cells, being a kind of adult stem cell, have a lot of superiorities compared with embryonic stem cells. They are easy to acquire and have no ethical controversies. Therefore, liver stem cells show a potential as a promising important vector for cell-based targeted gene therapy of HCC. However, the mechanism of the tropism of liver stem cells still needs clarification, and whether these cells are an ideal vector for targeted gene therapy of HCC still needs to be explored.
We are grateful to Dr. Qiao Zhou and Sheng-Fu Li for technical assistance. We thank the Pathological Central Laboratory of Sichuan University.
Liver stem cells are an ideal vector for gene therapy of some benign liver diseases. Whether the liver stem cell can be used to treat the hepatic cellular carcinoma is not yet clear. For gene therapy to be effective in cancers, it is necessary to deliver therapeutic genes into cells with high specificity and efficiency. It has been reported that neural stem cells (NSC) migrate to the intracerebral gliomas and display extensive tropism. The NSC-based gene therapy of gliomas has yielded promising results in animal experiments. External liver stem cells still have the capacity of tropism for injured liver. Whether the hepatic stem cell has the selective tropism toward an HCC lesion like NSC has not yet been clarified and whether the liver stem cell is a potential targeted vector for HCC gene therapy needs further study.
The development of more effective therapeutic tools and strategies for hepatic cellular carcinoma is much needed. The current focus of research is in HCC gene therapy. To find and modify an ideal targeted gene vector is very important in improving the efficacy. Liver stem cells are an important vector which have had good effects on the treatment of benign liver diseases. How to apply the cell is a burgeoning area of research. However, fewer studies have observed or revealed the mechanism of the tropism trait of the liver stem cell in injured liver and malignant disease.
Through engineering a liver progenitor cell line expressing EGFP and tracing the labeled cells in an animal model with liver cancer established as an allograft into rat liver, the selective tropism of liver stem cells for an HCC lesion in vivo has been observed. Liver stem cells migrated into the liver and proliferated around or in the tumor lesion, expressing the reporter gene, which suggested the use of migratory liver stem cells to deliver therapeutic genes. It may be promising in delivering therapeutic genes to target HCC cells.
According to the characteristic tropism of liver stem cells, the stem cell vector can deliver the therapeutic gene directly to cancer cells to improve the efficacy and decrease the damage to normal liver cells. Stem cells can self-renew, and the effect can be maintained for a long time. This may reduce or eliminate the need for gene therapy. Stem cells are the most promising potential platform for targeted gene therapy.
Liver stem cells are an adult stem cell of liver or a liver progenitor cell characterized by their capacity for self-renewal and ability to give rise to multiple differentiated cellular populations.
The authors have engineered a liver progenitor cell line expressing EGFP and suggest that these cells have an in vivo tropism to liver cancer cells established as an allograft into rat liver. This is a potentially interesting finding in the context of gene therapy of liver cancer.
| 1. | Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 669] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 2. | Carr BI. Hepatocellular carcinoma: current management and future trends. Gastroenterology. 2004;127:S218-S224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 187] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Farmer DG, Seu P, Swenson K, Economou J, Busuttil RW. Current and future treatment modalities for hepatocellular carcinoma. Clin Liver Dis. 1997;1:361-396, ix. [PubMed] |
| 4. | Ruiz J, Mazzolini G, Sangro B, Qian C, Prieto J. Gene therapy of hepatocellular carcinoma. Dig Dis. 2001;19:324-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Mohr L, Geissler M, Blum HE. Gene therapy for malignant liver disease. Expert Opin Biol Ther. 2002;2:163-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, Small JE, Herrlinger U, Ourednik V, Black PM. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA. 2000;97:12846-12851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 888] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 7. | Ehtesham M, Kabos P, Kabosova A, Neuman T, Black KL, Yu JS. The use of interleukin 12-secreting neural stem cells for the treatment of intracranial glioma. Cancer Res. 2002;62:5657-5663. [PubMed] |
| 8. | Brown AB, Yang W, Schmidt NO, Carroll R, Leishear KK, Rainov NG, Black PM, Breakefield XO, Aboody KS. Intravascular delivery of neural stem cell lines to target intracranial and extracranial tumors of neural and non-neural origin. Hum Gene Ther. 2003;14:1777-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 122] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Chen JZ, Hong H, Xiang J, Xue L, Zhao GQ. A selective tropism of transfused oval cells for liver. World J Gastroenterol. 2003;9:544-546. [PubMed] |
| 10. | Zhong XG, He S, Yin W, Deng JY, Chen B. Tropism of adult liver stem cells toward hepatocellular carcinoma cells in vitro. Zhonghua GanZangBing Za Zhi. 2005;13:644-647. [PubMed] |
| 11. | Muller-Borer BJ, Cascio WE, Anderson PA, Snowwaert JN, Frye JR, Desai N, Esch GL, Brackham JA, Bagnell CR, Coleman WB. Adult-derived liver stem cells acquire a cardiomyocyte structural and functional phenotype ex vivo. Am J Pathol. 2004;165:135-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Zou W, Li ZY, Li YL, Ma KL, Tsui ZC. Overexpression of PEMT2 downregulates the PI3K/Akt signaling pathway in rat hepatoma cells. Biochim Biophys Acta. 2002;1581:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Pan X, Li ZS, Xu GM, Cui L, Tu ZX. Adenovirus-mediated gene transfer in the treatment of pancreatic cancer. Pancreas. 2003;26:274-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509-2514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2861] [Cited by in RCA: 3058] [Article Influence: 109.2] [Reference Citation Analysis (0)] |
| 15. | Masamune A, Satoh M, Kikuta K, Suzuki N, Shimosegawa T. Establishment and characterization of a rat pancreatic stellate cell line by spontaneous immortalization. World J Gastroenterol. 2003;9:2751-2758. [PubMed] |
| 16. | Zhong XG, He S, Yin W, Deng JY, Chen B. Adenoviral-mediated efficiency expression of enhanced green fluorescence protein in adult liver stem cells of rats. Shijie Huaren Xiaohua Zazhi. 2004;12:2341-2344. |
| 17. | Hoogenhout J, Kazem I, Jerusalem CR, Bakkeren JA, de Jong J, Kal HB, van Munster PJ. Growth pattern of tumor xenografts in Wistar rats after treatment with cyclophosphamide, total lymphoid irradiation and/or cyclosporin A. Int J Radiat Oncol Biol Phys. 1983;9:871-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Fang CH, Zhang W, Zhu XY, Gong JQ, Zhang GQ. The expression of c-kit and proliferating cell nuclear antigen in oval cells of rats with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2003;2:537-544. [PubMed] |
| 19. | Tanaka S, Yamamoto T, Tanaka H, Kodai S, Ogawa M, Ichikawa T, Hai S, Sakabe K, Uenishi T, Shuto T. Potentiality of combined hepatocellular and intrahepatic cholangiocellular carcinoma originating from a hepatic precursor cell: Immunohistochemical evidence. Hepatol Res. 2005;32:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Roemer K, Friedmann T. Concepts and strategies for human gene therapy. Eur J Biochem. 1992;208:211-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 51] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Crystal RG. In vivo and ex vivo gene therapy strategies to treat tumors using adenovirus gene transfer vectors. Cancer Chemother Pharmacol. 1999;43 Suppl:S90-S99. [PubMed] |
| 22. | Strayer DS. Viral vectors for gene therapy: past, present and future. Drug News Perspect. 1998;11:277-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Deng W, Bivalacqua TJ, Chattergoon NN, Jeter JR, Kadowitz PJ. Engineering ex vivo-expanded marrow stromal cells to secrete calcitonin gene-related peptide using adenoviral vector. Stem Cells. 2004;22:1279-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Melo LG, Pachori AS, Kong D, Gnecchi M, Wang K, Pratt RE, Dzau VJ. Gene and cell-based therapies for heart disease. FASEB J. 2004;18:648-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Prockop DJ, Sekiya I, Colter DC. Isolation and characterization of rapidly self-renewing stem cells from cultures of human marrow stromal cells. Cytotherapy. 2001;3:393-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 149] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci USA. 2001;98:7841-7845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 701] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 27. | Song S, Witek RP, Lu Y, Choi YK, Zheng D, Jorgensen M, Li C, Flotte TR, Petersen BE. Ex vivo transduced liver progenitor cells as a platform for gene therapy in mice. Hepatology. 2004;40:918-924. [PubMed] [DOI] [Full Text] |
| 28. | Turgeman G, Pittman DD, Müller R, Kurkalli BG, Zhou S, Pelled G, Peyser A, Zilberman Y, Moutsatsos IK, Gazit D. Engineered human mesenchymal stem cells: a novel platform for skeletal cell mediated gene therapy. J Gene Med. 2001;3:240-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 29. | Palmer TD, Rosman GJ, Osborne WR, Miller AD. Genetically modified skin fibroblasts persist long after transplantation but gradually inactivate introduced genes. Proc Natl Acad Sci USA. 1991;88:1330-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 346] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 30. | Davidoff AM, Nathwani AC. Antiangiogenic gene therapy for cancer treatment. Curr Hematol Rep. 2004;3:267-273. [PubMed] |
| 31. | Takahashi K, Luo T, Saishin Y, Saishin Y, Sung J, Hackett S, Brazzell RK, Kaleko M, Campochiaro PA. Sustained transduction of ocular cells with a bovine immunodeficiency viral vector. Hum Gene Ther. 2002;13:1305-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Hattori K, Heissig B, Rafii S. The regulation of hematopoietic stem cell and progenitor mobilization by chemokine SDF-1. Leuk Lymphoma. 2003;44:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 89] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Kollet O, Shivtiel S, Chen YQ, Suriawinata J, Thung SN, Dabeva MD, Kahn J, Spiegel A, Dar A, Samira S. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112:160-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 449] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
S- Editor Zhu LH L- Editor Mihm S E- Editor Liu Y