Published online Jul 28, 2007. doi: 10.3748/wjg.v13.i28.3829
Revised: April 23, 2007
Accepted: April 26, 2007
Published online: July 28, 2007
AIM: To clarify the usefulness of immunohistochemical molecular markers in predicting lymph node metastasis of submucosal colorectal cancer.
METHODS: We examined microvessel density, lymphatic vessel density, the Ki-67 labeling index, expression of MUC1 and Matrix metalloproteinase-7 (MMP-7) in tumor cells, and expression of cathepsin D in stromal cells at the invasive front by immunostaining of samples resected from 214 patients with submucosal colorectal cancer. Pathologic features were assessed on hematoxylin-eosin-stained samples. We evaluated the relations between clinicopathologic/immunohistochemical features and lymph node metastasis.
RESULTS: Lesions of the superficial type, with an unfavorable histologic grade, budding, lymphatic involvement, high microvessel density (≥ 40), high lymphatic vessel density (≥ 9), high Ki-67 labeling index (≥ 42), and positivity of MUC1, cathepsin D, and MMP-7 showed a significantly high incidence of lymph node metastasis. Multivariate analysis revealed that high microvessel density, unfavorable histologic grade, cathepsin D positivity, high lymphatic vessel density, superficial type, budding, and MUC1 positivity were independent risk factors for lymph node metastasis. A combined examination with four independent immunohistochemical markers (microvessel density, cathepsin D, lymphatic vessel density, and MUC1) revealed that all lesions that were negative for all markers or positive for only one marker were negative for lymph node metastasis.
CONCLUSION: Analysis of a combination of immuno-histochemical molecular markers in endoscopically resected specimens of submucosal colorectal cancer allows prediction of curability regardless of the pathologic features visible of hematoxylin-eosin-stained sections.
- Citation: Kaneko I, Tanaka S, Oka S, Yoshida S, Hiyama T, Arihiro K, Shimamoto F, Chayama K. Immunohistochemical molecular markers as predictors of curability of endoscopically resected submucosal colorectal cancer. World J Gastroenterol 2007; 13(28): 3829-3835
- URL: https://www.wjgnet.com/1007-9327/full/v13/i28/3829.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i28.3829
Endoscopic mucosal resection (EMR) has become a major curative treatment for early colorectal cancer (CRC) without lymph node (LN) metastasis[1,2]. As previously reported, the depth of submucosal invasion, histologic grade at the deepest invasive portion, and vascular involvement are significant predictive markers for LN metastasis of submucosal CRC[3-7]. According to the Japanese guidelines for treatment of CRC, curative endoscopic resection of submucosal CRC must satisfy all of the following criteria: (1) achievement of complete resection, (2) diagnosis of the carcinoma as well- or moderately differentiated, (3) a depth of invasion of less than 1000 μm, and (4) no evidence of vessel involvement[4,7]. If, after endoscopic treatment, the specimens do not meet these criteria, additional surgery is recommended. However, it has been reported that submucosal CRC shows LN metastasis with probability of 3.6% to 16.2%[3,4,8-11], and even if additional surgical treatment is performed, LN metastasis is not found in many patients. Therefore a marker that predicts LN metastasis more effectively than evaluation with hematoxylin-eosin (HE)-staining of endoscopically resected specimens is needed.
The proliferation, infiltration, and metastasis of cancer involve several steps, each of which is associated with many molecular factors. It was reported that microvessel density (MVD), expression of Ki-67, MUC1 and matrix metalloproteinase-7 (MMP-7) in tumor cells and cathepsin D expression in stromal cells at the invasive front correlated significantly with LN metastasis from submucosal CRC[12-15]. Recently, a novel marker for lymphatic vessels, an antibody recognizing podoplanin that selectively stains lymphatic vessels, has become available[16]. It has been reported that lymphatic vessel density (LVD) is significantly related to LN metastasis of some mali-gnancies[17-22], and we have also reported that LVD correlates significantly with LN metastasis of submucosal CRC[23].
It has been clarified that CRC cells at the site of deepest invasion have higher malignant and metastatic potentials than do cells in other parts of the tumor[3,4,12-14,24-29]; therefore, for predicting LN metastasis of submucosal CRC, various kinds of markers should be evaluated at the site of deepest penetration.
In this study, our purpose was to evaluate the significance of several immunohistochemical markers for prediction of LN metastasis and to examine whether these markers are clinically applicable.
We studied 214 submucosal CRCs that were resected surgically or endoscopically from 214 patients at Hiroshima University Hospital or an affiliated hospital during the period January 1992 through December 2004. All patients gave informed consent to this study. No patients had a history of inflammatory bowel disease. No lesion satisfied the criteria for curative endoscopic resection according to the Japanese guidelines for the treatment of submucosal CRC [4,7]. Additional surgical treatment was performed for patients who had undergone endoscopic resection, and the presence of LN metastasis was evaluated. The entire tumor was cut into parallel 2 to 3-mm-thick sections, and the portion of deepest submucosal invasion was selected for microscopic examination. All regional LNs at the para-colorectal artery and along the main artery supplying the primary tumor were resected and cut into two or three sections.
Tumor size, location, macroscopic type, depth of submucosal invasion, histologic grade, vessel involvement, budding, number of LNs examined per patient and LN metastasis were determined on the basis of histologic reports according to the World Health Organization classification system[30]. Lymphatic vessel involvement in all specimens was assessed on podoplanin-immunostained specimens. Macroscopic type was described as protruded or superficial[12]. The depth of submucosal invasion was measured as reported previously[4] and classified as less or greater than 1000 μm[7]. Histologic grade was assessed at the deepest portion of the tumor according to the most unfavorable histologic features and was classified as favorable or unfavorable as previously reported [5]. Budding was defined as an isolated single cancer cell or a cluster composed of fewer than five cancer cells as previously reported [5]. After selection of one field (× 200) where budding was most prevalent, a bud count was performed. A field with five or more buds was regarded as positive. The number of LNs examined per patient was classified as less or greater than 15. The cut off level was defined based on minimum number of LNs to analyze recommended in clinical practice guidelines for colorectal cancers[31].
Consecutive 4-μm-thick sections were cut from each paraffin-embedded sample and immunolabeled. Immunohistochemical labeling was done with the immunoperoxidase method following antigen retrieval with 0.1% trypsin (37°C, 30 min). The primary antibodies were anti-CD34 monoclonal antibody (Nichirei, Tokyo, Japan)[12], a mouse monoclonal antibody against podoplanin (AngioBio, Del Mar, CA)[18], anti-Ki-67 monoclonal antibody MIB-1 (Immunotech SA, Marseille, France)[13], anti-MUC1 monoclonal antibody (KL-6)[13], anti-cathepsin D monoclonal antibody (NCL-CDm, Novocastra Laboratories, Newcastle, UK)[14], and anti-human MMP-7 monoclonal antibody (141-7B2, Daiichi Fine Chemicals, Toyama, Japan)[15]. Immunohistochemistry was carried out with an LSAB kit (DAKO, Glostrup, Denmark) as reported previously [18].
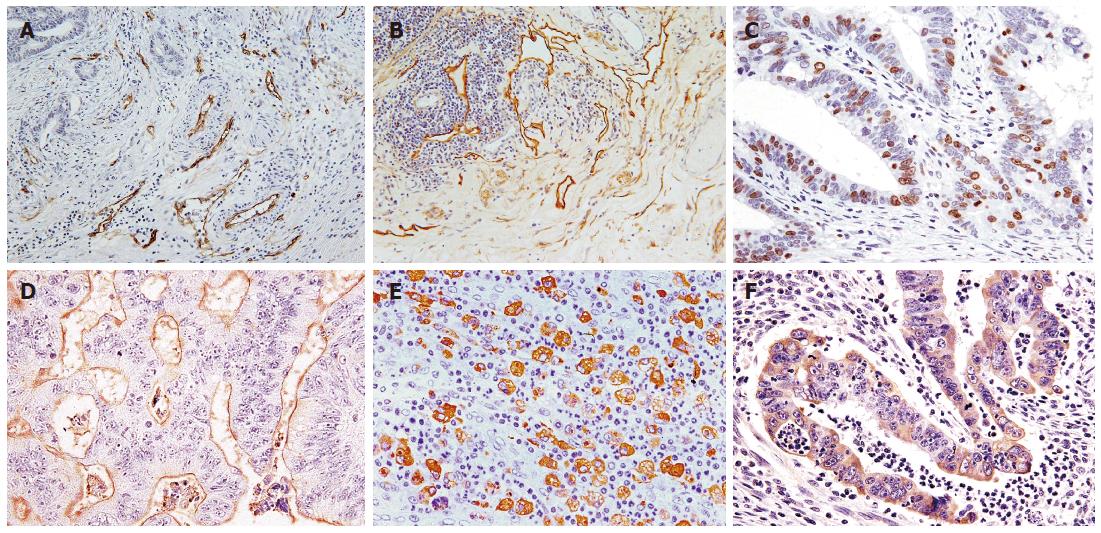
Microvessels and lymphatic vessels were identified as CD34- and podoplanin-positive capillaries, respectively (Figure 1A and B). Vessel density was assessed on immunolabeled specimens by light microscopy in areas of tumor containing the greatest numbers of capillaries and small venules (so-called hot spots) at the site of deepest submucosal penetration as described previously[32]. After three hot spots were identified at low magnification, a vessel count was performed at × 400 for MVD and at × 200 for LVD. The mean count of the three fields was calculated. Carcinoma cells were considered positive for Ki-67 when unequivocal diffuse or dot-like brown nuclear staining with MIB-1 was visible (Figure 1C). The Ki-67 LI was defined as the mean percentage of Ki-67-positive tumor cells per 1000 cancer cells counted in three randomly chosen microscopic fields at the site of deepest invasion. MVD, LVD, and Ki-67LI were classified as high or low with the cut off level defined based on the mean of results, respectively. Expression of MUC1 was considered positive if distinct staining of the luminal surfaces or cytoplasm was seen in at least 10% of the tumor cells (Figure 1D). Cathepsin D expression in stromal cells was examined at the border of the invasive front and was defined as the mean percentage of stromal cells showing cathepsin D-positive staining per 1000 stromal cells counted in three randomly chosen microscopic fields (Figure 1E). Cathepsin D expression in stromal cells was considered positive when at least 15% of stromal cells were stained. MMP-7 expression was considered positive when cytoplasmic staining was visible in at least 10% of tumor cells at the invasive front (Figure 1F).
Two investigators blinded to patient status examined all samples. In cases of disagreement, the slides were reviewed, and consensus was reached.
Data were evaluated by Chi-square or Fisher’s exact test. To clarify the relative importance of each risk factor for LN metastasis, multivariate analysis with logistic regression using stepwise method in selecting variables was performed. Furthermore, we analyzed a combination of the significant immunohistochemical markers by multivariate analysis to identify cases without LN metastasis. All statistical analyses were performed with JMP software (SAS Institute, Cary, NC). P values of less than 0.05 were considered significant.
LN metastasis was detected in 28 (13.1%) of 214 patients. The incidence of LN metastasis in relation to clinicopathologic features is shown in Table 1. The incidence of LN metastasis was significantly high in cases with superficial type lesions (P = 0.0311), unfavorable histologic grade (P = 0.0026), budding (P < 0.0001), and lymphatic vessel involvement (P = 0.0012). Tumor size, location, depth of invasion, venous vessel involvement and number of LNs examined were not associated with LN metastasis.
| Clinicopathologic | features | n | LN positive (%) | P value |
| Size (mm) | ≤ 20 | 159 | 20 (12.6) | NS |
| > 20 | 55 | 8 (14.6) | ||
| Location | Rectum | 61 | 8 (13.1) | NS |
| Colon | 153 | 20 (13.1) | ||
| Macroscopic type | Protruded type | 145 | 14 (9.7) | 0.0311 |
| Superficial type | 69 | 14 (20.3) | ||
| Depth of invasion (μm) | < 1000 | 25 | 1 (4.0) | NS |
| ≥ 1000 | 189 | 27 (14.3) | ||
| Histologic grade | Favorable | 198 | 22 (11.1) | 0.0026 |
| Unfavorable | 16 | 6 (37.5) | ||
| Budding | Negative | 175 | 13 (7.4) | < 0.0001 |
| Positive | 39 | 15 (38.5) | ||
| Lymphatic | Negative | 148 | 12 (8.1) | 0.0012 |
| involvement1 | Positive | 66 | 16 (24.2) | |
| Venous involvement | Negative | 166 | 20 (12.1) | NS |
| Positive | 48 | 8 (16.7) | ||
| Number of LNs | ≤ 15 | 128 | 16 (12.5) | NS |
| examined | > 15 | 86 | 12 (14.0) | |
| Total | 214 | 28 (13.1) |
The incidence of LN metastasis in relation to immuno-histochemical molecular markers is shown in Table 2. MVD ranged from 12 to 102 per field, with a mean of 39.4 ± 19.2. We split patients into two groups, a high MVD (≥ 40) group and a low MVD (< 40) group. LVD ranged from 2 to 26 per field, with a mean of 9.06 ± 4.79. Patients were assigned to a high LVD (≥ 9) group or a low LVD (< 9) group. The Ki-67 LI ranged form 12.4 to 88.7, with a mean of 41.8 ± 15.8. The Ki-67 LI was classified as high (≥ 42) or low (< 42). The incidence of LN metastasis was significantly high in cases with high MVD (P < 0.0001), high LVD (P = 0.0002), high Ki-67 LI (P = 0.0499), MUC1 positivity (P = 0.0012), cathepsin D positivity (P = 0.0008), or MMP-7 positivity (P = 0.0263).
| Immunohistochemical | markers | n | LN positive(%) | P value |
| MVD | Low | 88 | 2 (2.3) | < 0.0001 |
| High | 126 | 26 (20.6) | ||
| LVD | Low | 109 | 5 (3.8) | 0.0002 |
| High | 105 | 23 (18.9) | ||
| Ki-67 LI | Low | 90 | 7 (7.8) | 0.0499 |
| High | 124 | 21 (16.9) | ||
| MUC1 | Negative | 108 | 5 (4.6) | 0.0002 |
| Positive | 106 | 23 (21.7) | ||
| Cathepsin D | Negative | 85 | 3 (3.5) | 0.0008 |
| Positive | 129 | 25 (19.4) | ||
| MMP-7 | Negative | 103 | 8 (7.8) | 0.0263 |
| Positive | 111 | 20 (18.0) | ||
| Total | 214 | 28 (13.1) |
Factors predictive of LN metastasis are shown with odds ratios in Table 3. Multivariate analysis showed high MVD, unfavorable histologic grade, cathepsin D positivity, high LVD, superficial type, budding, and MUC1 positivity to be independent risk factors for LN metastasis.
| Risk factors | P value | Odds ratio |
| MVD-High | 0.0008 | 16.67 |
| Histologic grade-Unfavorable | 0.0116 | 11.73 |
| Cathepsin D-Positive | 0.0071 | 8.41 |
| LVD-High | 0.0027 | 7.65 |
| Macroscopic type-Superficial | 0.0029 | 7.27 |
| Budding-Positive | 0.0160 | 4.36 |
| MUC1-Positive | 0.0266 | 3.96 |
| Lymphatic involvement Positive | NS | - |
| Ki-67 LI-High | NS | - |
| MMP-7-Positive | NS | - |
We examined the value of combining four independent immunohistochemically-determined molecular markers (MVD, cathepsin D, LVD and MUC1) for prediction of LN metastasis. Pairing of the following immunohistochemical markers allowed recognition of cases without LN metastasis: cathepsin D negativity and MUC1 negativity (Table 4), low MVD and MUC1 negativity (Table 5), and low MVD and cathepsin D negativity (Table 6). No other combinations were predictive for absence of LN metastasis (data not shown). The incidence of LN metastasis is shown in relation to the number of positive immunohistochemical markers in Table 7. All lesions that were negative for all markers or positive for only one marker were without LN metastasis. As the number of positive immunohistochemical markers increased, the incidence of LN metastasis increased.
| Cathepsin D expression | MUC1 expression | |
| Negative | Positive | |
| Negative | 0/49 (0) | 3/36 (8.3) |
| Positive | 5/59 (8.5) | 20/70 (28.6)b |
| MVD | MUC1 expression | |
| Negative | Positive | |
| Low | 0/51 (0) | 2/37 (5.4) |
| High | 5/57 (8.8) | 21/69 (30.4)b |
| MVD | MUC1 expression | |
| Negative | Positive | |
| Low | 0/33 (0) | 2/55 (3.6) |
| High | 3/52 (5.8) | 23/74 (31.1)b |
MVD is a measure of angiogenesis and has strong prognostic value in patients with CRC[33-35]. We reported previously that angiogenesis plays an important role in metastasis and that MVD correlates significantly with LN metastasis of submucosal CRC[12]. It has also been reported that lymphangiogenesis is an important factor in the metastasis of various cancers and that LVD is significantly related to LN metastasis of some malignancies[17-22]. We have also reported that LVD is significantly related to LN metastasis of submucosal CRC[23]. Ki-67 is a proliferation antigen and is reported to have prognostic significance in various types of cancers[36-39]. MUC1 is reported to inhibit cell-cell adhesion among epithelial cells[40,41], and its expression correlates significantly with malignant potential of advanced CRC[26,42]. It was reported that Ki-67 LI and MUC1 expression correlated significantly with LN metastasis of submucosal CRC[13]. Cathepsin D, a member of the aspartic protease family, is an acid lysosomal enzyme that correlates directly with the prognosis of patients with cancer of various organs[43-46]. Cathepsin D expression in both cancer cells and stromal cells was reported to correlate significantly with LN metastasis of submucosal CRC[14]. In that study, multivariate analysis revealed that cathepsin D expression in stromal cells was an independent risk factor for LN metastasis but that expression in cancer cells was not[14]. MMP-7 has proteolytic activity against a wide spectrum of extracellular matrix components and plays an important role in carcinoma invasion and metastasis through degradation of the extracellular matrix[15,47,48]. Previous studies have shown that MMP-7 expression correlates significantly with LN metastasis and distant metastasis of submucosal CRC[15,49]. Our present study also indicated that MMP-7 expression correlates with LN metastasis of submucosal CRC.
Although there have been reports regarding the relation between molecular markers and LN metastasis of submucosal CRC, it is still not clear which marker is most useful. Moreover, molecular markers in this study are useful for predicting the possibility of LN metastasis, but we must be able to accurately predict the presence of LN metastasis in individual patients. Our present study revealed the importance of four molecular markers and showed that immunohistochemical analysis of these markers in combination allows identification of patients without LN metastasis regardless of other clinicopathologic risk factors.
Endoscopic treatment is indicated for scanty invasive submucosal CRC because of the very low incidence of LN metastasis[3-7,10]. According to the Japanese guidelines for treatment of submucosal CRC[4,7], for a lesion that does not satisfy the criteria for curative EMR, we have to consider additional surgical treatment. In the present study, we excluded cases that satisfied the criteria of the Japanese guidelines for treatment of CRC for curative endoscopic treatment and tried to identify cases without LN metastasis. The incidence of LN metastasis was only 13.1% in the present study. To reduce the frequency of unnecessary additional surgeries after endoscopic treatment, predictors of LN metastasis regardless of pathologic features of HE-stained sections are needed. The immunohistochemical staining method used in the present study may be applicable in clinical practice because it is convenient and very stable. To apply this methodology to endoscopically resected submucosal CRCs, complete resection, including the submucosa around the deepest invasive portion, is required because evaluation at the site of deepest penetration is needed. By providing a total biopsy specimen, EMR can serve as an important method for diagnosis.
In conclusion, immunohistochemical analysis of molecular markers individually and in combination at the site of deepest penetration of submucosal CRC can allows prediction of the existence of LN metastasis regardless of the pathologic risk factors identified with HE staining. Our results may broaden the indication for curative endoscopic treatment of submucosal CRC and may reduce the number of unnecessary additional surgeries performed after endoscopic treatment.
| 1. | Karita M, Tada M, Okita K, Kodama T. Endoscopic therapy for early colon cancer: the strip biopsy resection technique. Gastrointest Endosc. 1991;37:128-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 140] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Kudo S. Endoscopic mucosal resection of flat and depressed types of early colorectal cancer. Endoscopy. 1993;25:455-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 562] [Article Influence: 17.0] [Reference Citation Analysis (11)] |
| 3. | Tanaka S, Haruma K, Teixeira CR, Tatsuta S, Ohtsu N, Hiraga Y, Yoshihara M, Sumii K, Kajiyama G, Shimamoto F. Endoscopic treatment of submucosal invasive colorectal carcinoma with special reference to risk factors for lymph node metastasis. J Gastroenterol. 1995;30:710-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 107] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Tanaka S, Haruma K, Oh-E H, Nagata S, Hirota Y, Furudoi A, Amioka T, Kitadai Y, Yoshihara M, Shimamoto F. Conditions of curability after endoscopic resection for colorectal carcinoma with submucosally massive invasion. Oncol Rep. 2000;7:783-788. [PubMed] |
| 5. | Ueno H, Mochizuki H, Hashiguchi Y, Shimazaki H, Aida S, Hase K, Matsukuma S, Kanai T, Kurihara H, Ozawa K. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology. 2004;127:385-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 551] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 6. | Egashira Y, Yoshida T, Hirata I, Hamamoto N, Akutagawa H, Takeshita A, Noda N, Kurisu Y, Shibayama Y. Analysis of pathological risk factors for lymph node metastasis of submucosal invasive colon cancer. Mod Pathol. 2004;17:503-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 105] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Kitajima K, Fujimori T, Fujii S, Takeda J, Ohkura Y, Kawamata H, Kumamoto T, Ishiguro S, Kato Y, Shimoda T. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol. 2004;39:534-543. [PubMed] |
| 8. | Kyzer S, Bégin LR, Gordon PH, Mitmaker B. The care of patients with colorectal polyps that contain invasive adenocarcinoma. Endoscopic polypectomy or colectomy? Cancer. 1992;70:2044-2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Minamoto T, Mai M, Ogino T, Sawaguchi K, Ohta T, Fujimoto T, Takahashi Y. Early invasive colorectal carcinomas metastatic to the lymph node with attention to their nonpolypoid development. Am J Gastroenterol. 1993;88:1035-1039. [PubMed] |
| 10. | Tanaka S, Yokota T, Saito D, Okamoto S, Oguro Y, Yoshida S. Clinicopathologic features of early rectal carcinoma and indications for endoscopic treatment. Dis Colon Rectum. 1995;38:959-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Tanaka S, Haruma K, Tatsuta S, Hiraga Y, Teixeira CR, Shimamoto F, Yoshihara M, Sumii K, Kajiyama G. Proliferating cell nuclear antigen expression correlates with the metastatic potential of submucosal invasive colorectal carcinoma. Oncology. 1995;52:134-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Oh-e H, Tanaka S, Kitadai Y, Shimamoto F, Yoshihara M, Haruma K. Angiogenesis at the site of deepest penetration predicts lymph node metastasis of submucosal colorectal cancer. Dis Colon Rectum. 2001;44:1129-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Aoki R, Tanaka S, Haruma K, Yoshihara M, Sumii K, Kajiyama G, Shimamoto F, Kohno N. MUC-1 expression as a predictor of the curative endoscopic treatment of submucosally invasive colorectal carcinoma. Dis Colon Rectum. 1998;41:1262-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Oh-e H, Tanaka S, Kitadai Y, Shimamoto F, Yoshihara M, Haruma K. Cathepsin D expression as a possible predictor of lymph node metastasis in submucosal colorectal cancer. Eur J Cancer. 2001;37:180-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Kurokawa S, Arimura Y, Yamamoto H, Adachi Y, Endo T, Sato T, Suga T, Hosokawa M, Shinomura Y, Imai K. Tumour matrilysin expression predicts metastatic potential of stage I (pT1) colon and rectal cancers. Gut. 2005;54:1751-1758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 823] [Cited by in RCA: 839] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 17. | Shields JD, Borsetti M, Rigby H, Harper SJ, Mortimer PS, Levick JR, Orlando A, Bates DO. Lymphatic density and metastatic spread in human malignant melanoma. Br J Cancer. 2004;90:693-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 122] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Kitadai Y, Kodama M, Cho S, Kuroda T, Ochiumi T, Kimura S, Tanaka S, Matsumura S, Yasui W, Chayama K. Quantitative analysis of lymphangiogenic markers for predicting metastasis of human gastric carcinoma to lymph nodes. Int J Cancer. 2005;115:388-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Sedivy R, Beck-Mannagetta J, Haverkampf C, Battistutti W, Hönigschnabl S. Expression of vascular endothelial growth factor-C correlates with the lymphatic microvessel density and the nodal status in oral squamous cell cancer. J Oral Pathol Med. 2003;32:455-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Nakamura Y, Yasuoka H, Tsujimoto M, Imabun S, Nakahara M, Nakao K, Nakamura M, Mori I, Kakudo K. Lymph vessel density correlates with nodal status, VEGF-C expression, and prognosis in breast cancer. Breast Cancer Res Treat. 2005;91:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Renyi-Vamos F, Tovari J, Fillinger J, Timar J, Paku S, Kenessey I, Ostoros G, Agocs L, Soltesz I, Dome B. Lymphangiogenesis correlates with lymph node metastasis, prognosis, and angiogenic phenotype in human non-small cell lung cancer. Clin Cancer Res. 2005;11:7344-7353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 147] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 22. | Zeng Y, Opeskin K, Horvath LG, Sutherland RL, Williams ED. Lymphatic vessel density and lymph node metastasis in prostate cancer. Prostate. 2005;65:222-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Kaneko I, Tanaka S, Oka S, Kawamura T, Hiyama T, Ito M, Yoshihara M, Shimamoto F, Chayama K. Lymphatic vessel density at the site of deepest penetration as a predictor of lymph node metastasis in submucosal colorectal cancer. Dis Colon Rectum. 2007;50:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Teixeira CR, Tanaka S, Haruma K, Yoshihara M, Sumii K, Kajiyama G, Shimamoto F. The clinical significance of the histologic subclassification of colorectal carcinoma. Oncology. 1993;50:495-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Teixeira CR, Tanaka S, Haruma K, Yoshihara M, Sumii K, Kajiyama G. Proliferating cell nuclear antigen expression at the invasive tumor margin predicts malignant potential of colorectal carcinomas. Cancer. 1994;73:575-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Hiraga Y, Tanaka S, Haruma K, Yoshihara M, Sumii K, Kajiyama G, Shimamoto F, Kohno N. Immunoreactive MUC1 expression at the deepest invasive portion correlates with prognosis of colorectal cancer. Oncology. 1998;55:307-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Furudoi A, Tanaka S, Haruma K, Yoshihara M, Sumii K, Kajiyama G, Shimamoto F. Clinical significance of human erythrocyte glucose transporter 1 expression at the deepest invasive site of advanced colorectal carcinoma. Oncology. 2001;60:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Furudoi A, Tanaka S, Haruma K, Kitadai Y, Yoshihara M, Chayama K, Shimamoto F. Clinical significance of vascular endothelial growth factor C expression and angiogenesis at the deepest invasive site of advanced colorectal carcinoma. Oncology. 2002;62:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Kaio E, Tanaka S, Kitadai Y, Sumii M, Yoshihara M, Haruma K, Chayama K. Clinical significance of angiogenic factor expression at the deepest invasive site of advanced colorectal carcinoma. Oncology. 2003;64:61-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Hamilton SR, Aaltonen LA, editors . World Health Organi-zation classification of tumours: pathology and genetics of tumours of the digestive system. Lyon (France): IARC Press 2000; 104-119. |
| 31. | Otchy D, Hyman NH, Simmang C, Anthony T, Buie WD, Cataldo P, Church J, Cohen J, Dentsman F, Ellis CN. Practice parameters for colon cancer. Dis Colon Rectum. 2004;47:1269-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 32. | Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4014] [Cited by in RCA: 4111] [Article Influence: 117.5] [Reference Citation Analysis (0)] |
| 33. | Galindo-Gallego M, Fernández-Aceñero MJ, Sanz-Ortega J, Aljama A, López-Elzaurdia C. Prognostic significance of microvascular counts in rectal carcinoma. Pathol Res Pract. 2000;196:607-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Galindo Gallego M, Fernández Aceñero MJ, Sanz Ortega J, Aljama A. Vascular enumeration as a prognosticator for colorectal carcinoma. Eur J Cancer. 2000;36:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Matsuura T, Fukuda Y, Fujitaka T, Nishisaka T, Sakatani T, Ito H. Preoperative treatment with tegafur suppositories enhances apoptosis and reduces the intratumoral microvessel density of human colorectal carcinoma. Cancer. 2000;88:1007-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 36. | Kirla R, Salminen E, Huhtala S, Nuutinen J, Talve L, Haapasalo H, Kalimo H. Prognostic value of the expression of tumor suppressor genes p53, p21, p16 and prb, and Ki-67 labelling in high grade astrocytomas treated with radiotherapy. J Neurooncol. 2000;46:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Pollack A, DeSilvio M, Khor LY, Li R, Al-Saleem TI, Hammond ME, Venkatesan V, Lawton CA, Roach M, Shipley WU. Ki-67 staining is a strong predictor of distant metastasis and mortality for men with prostate cancer treated with radiotherapy plus androgen deprivation: Radiation Therapy Oncology Group Trial 92-02. J Clin Oncol. 2004;22:2133-2140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 121] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 38. | Bui MH, Visapaa H, Seligson D, Kim H, Han KR, Huang Y, Horvath S, Stanbridge EJ, Palotie A, Figlin RA. Prognostic value of carbonic anhydrase IX and KI67 as predictors of survival for renal clear cell carcinoma. J Urol. 2004;171:2461-2466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 39. | Tisell LE, Oden A, Muth A, Altiparmak G, Mõlne J, Ahlman H, Nilsson O. The Ki67 index a prognostic marker in medullary thyroid carcinoma. Br J Cancer. 2003;89:2093-2097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Dahiya R, Kwak KS, Byrd JC, Ho S, Yoon WH, Kim YS. Mucin synthesis and secretion in various human epithelial cancer cell lines that express the MUC-1 mucin gene. Cancer Res. 1993;53:1437-1443. [PubMed] |
| 41. | Gendler SJ, Spicer AP, Lalani EN, Duhig T, Peat N, Burchell J, Pemberton L, Boshell M, Taylor-Papadimitriou J. Structure and biology of a carcinoma-associated mucin, MUC1. Am Rev Respir Dis. 1991;144:S42-S47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 141] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 42. | Nakamori S, Ota DM, Cleary KR, Shirotani K, Irimura T. MUC1 mucin expression as a marker of progression and metastasis of human colorectal carcinoma. Gastroenterology. 1994;106:353-361. [PubMed] |
| 43. | Tandon AK, Clark GM, Chamness GC, Chirgwin JM, McGuire WL. Cathepsin D and prognosis in breast cancer. N Engl J Med. 1990;322:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 330] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 44. | Otto FJ, Goldmann T, Biess B, Lippold A, Suter L, Westhoff U. Prognostic classification of malignant melanomas by combining clinical, histological, and immunohistochemical parameters. Oncology. 1999;56:208-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 45. | Allgayer H, Babic R, Grützner KU, Beyer BC, Tarabichi A, Wilhelm Schildberg F, Heiss MM. An immunohistochemical assessment of cathepsin D in gastric carcinoma: its impact on clinical prognosis. Cancer. 1997;80:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 46. | Arao J, Fukui H, Ono Y, Ueda Y, Chiba T, Fujimori T. Immunohistochemical localization of cathepsin D in colorectal tumors. Dis Colon Rectum. 2000;43:396-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Woessner JF, Taplin CJ. Purification and properties of a small latent matrix metalloproteinase of the rat uterus. J Biol Chem. 1988;263:16918-16925. [PubMed] |
| 48. | Miyazaki K, Hattori Y, Umenishi F, Yasumitsu H, Umeda M. Purification and characterization of extracellular matrix-degrading metalloproteinase, matrin (pump-1), secreted from human rectal carcinoma cell line. Cancer Res. 1990;50:7758-7764. [PubMed] |
| 49. | Masaki T, Matsuoka H, Sugiyama M, Abe N, Goto A, Sakamoto A, Atomi Y. Matrilysin (MMP-7) as a significant determinant of malignant potential of early invasive colorectal carcinomas. Br J Cancer. 2001;84:1317-1321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
S- Editor Zhu LH L- Editor Alpini GD E- Editor Ma WH