Published online Jul 21, 2007. doi: 10.3748/wjg.v13.i27.3773
Revised: March 23, 2007
Accepted: March 31, 2007
Published online: July 21, 2007
This is a case report of a patient who presented with acute pancreatitis without the common causes. A pancreatic biopsy revealed large B cell lymphoma. Spleen lymphoma with pancreatic involvement inducing acute pancreatitis, which is a rare disorder, was diagnosed. Here we also review the few similar cases reported in the literature.
- Citation: Wu CM, Cheng LC, Lo GH, Lai KH, Cheng CL, Pan WC. Malignant lymphoma of spleen presenting as acute pancreatitis: A case report. World J Gastroenterol 2007; 13(27): 3773-3775
- URL: https://www.wjgnet.com/1007-9327/full/v13/i27/3773.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i27.3773
The spleen is involved in 30%-40% of non-Hodgkin’s lymphoma cases and primary spleen lymphoma (PSL) is a rare disorder, with an incidence of less than 1%[1]. Most patients have only nonspecific symptoms, such as fatigue, weight loss, and fever of unknown source, so it is therefore difficult to diagnose at an early stage. The prognosis for patients with PSL is related to the stage of the disease and pathological cell type involved. Here we report a case of malignant lymphoma of the spleen with invasion to the pancreatic tail with presentation as acute pancreatitis. We also review the literature on PSL.
A 68-year-old female patient presented with complaints of abdominal pain for 3 d and weight loss of about 6 kilograms in one month. Symptoms also included anorexia, nausea, and postprandial vomiting. Pain was localized to the right upper quadrant and was not related to meal or bowel habit changes. The pain could be relieved by assuming a knee-chest position and a left side decubitus posture. The symptoms did not include cough and fever. The patient had a history of type 2 diabetes mellitus with oral hypoglycemic agent control for more than 5 years. She denied any other systemic disease and did not have a habit of smoking or alcohol abuse. The patient was admitted to the hospital after being seen in the emergency room.
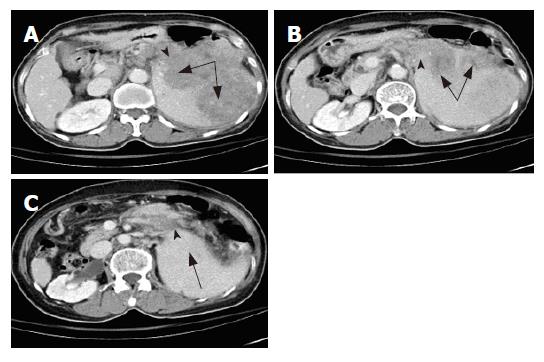
Physical examination showed anemic conjunctiva, no icteric sclera, and mild right upper quadrant tenderness. Neither a mass nor lymph nodes were palpable. The spleen was palpable about 8 cm below the costal margin. Laboratory tests showed the following: white blood cell count, 6300/cumm; hemoglobin, 12.0 g%; platelets, 101 000/cumm; segment, 60%; lymphocytes, 30%; monocytes, 10%; sugar, 155 mg/dL. Liver panel results, including total bilirubin, alanine aminotransferase, asparate aminotransferase, gamma glutamyltransferase, and lactic dehydrogenase, were within the normal range. Amylase was 410 U/L (< 180 U/L) and lipase was 715 U/L (< 160 U/L). An abdominal computed tomography (CT) scan showed a mass lesion of about 11.2 cm at the pancreatic tail and the spleen with multiple lymphadenopathy around the tumor. Splenic vein obliteration was also noted as well as edematous change over the peripancreatic area (Figure 1A-C). A panendoscopy examination showed esophageal and gastric varices. Our initial impression was tumor invasion resulting in acute pancreatitis.
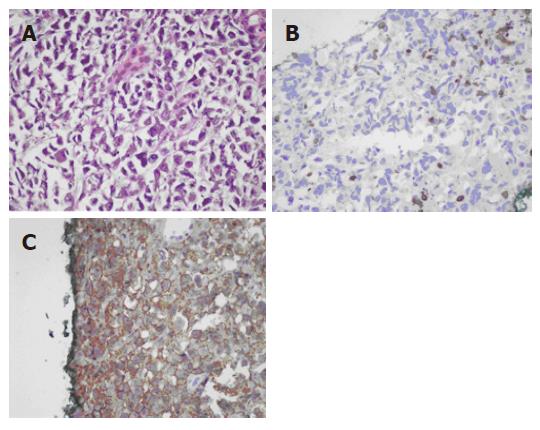
Tumor markers, including carcinoembryonic antigen (CEA), alfafetal protein (AFP), and C19-9, were within the normal range. Sono-guided biopsy over the pancreatic tumor was carried out. Pathological examination showed diffuse large B-cell lymphoma. Cytokeratin was negative, LCA was positive, CD20 was positive, and CD3 was negative (Figure 2A-C). The brain CT scan was negative. Chest imaging showed right pleural effusion and a pig-tail tube was inserted. Analysis of pleural fluid showed no malignant cells.
The patient fasted with fluid hydration and electrolytes for 3 d. Subsequently, the abdominal pain subsided and a biopsy of the pancreas was performed. After the procedure, the patient’s condition remained stable. Our initial treatment plan was chemotherapy. Unfortunately, fever and a urinary tract infection developed on the 7th day after admission. Cefazolin, 1 gram every 8 h, and Gentamycin, 80 mg every 12 h, were administered. However, sepsis with respiratory failure developed, the chemotherapy was put on hold and the patient died after 12 d of hospitalization.
Acute pancreatitis is a disorder with numerous causes and an obscure pathogenesis. Bile duct stones and alcohol abuse together account for about 80% of acute pancreatitis. Other causes are various toxins and drugs, obstructions, such as malignancy, fibrotic sphinctor of Oddi, metabolic abnormalities, trauma, ischemia, infection, and autoimmune diseases. In 10% of the cases of acute pancreatitis no underlying cause can be identified, and these cases are described as idiopathic pancreatitis. Occult microlithiasis may be the cause of two-thirds of the cases of idiopathic acute pancreatitis[2].
Acute pancreatitis related to tumor obstruction is not unusual and primary pancreatic carcinoma (about 3% of all patients) and common bile duct cancer are the main etiologies. A case of carcinoid tumor of the pancreas with obstructive pancreatitis has been reported. Acute pancreatitis caused by metastatic carcinoma is uncommon, with broncogenic carcinoma as the main metastasis-induced pancreatitis tumor[3,4]. Metastasis-induced acute pancreatitis typically has occurred in patients known to have advanced brochogenic carcinoma[5]. Our patient presented with typical abdominal pain and high levels of amylase and lipase. We excluded other etiologies of pancreatitis, and therefore spleen lymphoma with pancreatic involvement was the main etiology of the observed acute pancreatitis.
Das Guta et al stated that clinical presentation of these symptoms must indicate splenomegaly without any evidence of disease elsewhere. They emphasized that the liver biopsy specimen, as well as para-aortic and mesenteric lymph nodes, should be free of malignant lymphoma[5]. Sharin et al reported on splenomegaly without significant lymphoadenopathy and no hepatomegaly or peripheral blood involvement[6]. Catherian et al defined malignant lymphoma with primary presentation in the spleen as splenomegaly without peripheral lymphadenopathy, pathological involvement of spleen with or without involvement of regional lymph nodes, bone marrow or liver[8]. Therefore, our patient could be diagnosed as having primary malignant spleen lymphoma due to the main involvement of the spleen without peripheral blood involvement, according to the definition of Catherian et al.
The spleen is involved in 30%-40% of the cases of non-Hodgkin’s lymphoma and PSL has a reported incidence of less than 1%. In published reports, the incidence of diffuse large cell lymphoma in PSL varies from 22.4% to 33.3%[1]. Large B cell lymphoma, presenting with a tumor mass, is associated with a relatively favorable clinical course and the clinical presentation of a tumor confined to the spleen and the hilar lymph node is associated with lower aggravates[7]. The most common presenting symptoms in malignant lymphoma of the spleen are fever, malaise and weight loss.
There are some reports that revealed large B cell lymphoma in the spleen in patients with hepatitis C virus infection[5]. The prevalence of HCV infection (51.7%) in the examined splenic diffuse large B cell lymphoma cases was significant (P < 0.05)[1,10,11]. Saadoun et al demonstrated that treatment with interferon and ribavirin led to a complete virological response and hematological remission as well as the disappearance of its clinical symptoms. However, there were no studies indicating whether aggressive treatment of HCV infection by pegylated interferon is effective in treating or preventing lymphoma.
The spleen can accommodate different types of large B-cell lymphomas, which need to be distinguished to establish a precise prognosis and the most suitable treatment[5]. Large B-cell lymphoma in the spleen as a tumor mass has a relatively favorable clinical course[12]. Only a few case reports have been published that included pancreas involvement.
In the case discussed in this report, the patient presented with non-specific symptoms, such as body weight loss and fatigue, followed by acute abdomen pain and symptoms of pancreatitis. The tumor was large enough to detect in the imaging studies, such as sonography or CT scan. A main problem is detecting the tumor as soon as possible because prognosis is related to tumor stage. Therefore acute pancreatitis with unknown etiology needs more detailed study to exclude primary pancreatic or metastasis, especially with those showing previous warning symptoms, such as fatigue, malaise or unknown cause of fever. If we could detect tumors earlier, prognoses would be better. Even if tumors are hard to remove surgically, chemotherapy should be effective.
The experience of our patient also warned us that these patients have weakened immunity and sepsis may be a major cause of mortality. If there is clinical suspicion of sepsis, antibiotics are indicated.
| 1. | Grosskreutz C, Troy K, Cuttner J. Primary splenic lymphoma: report of 10 cases using the REAL classification. Cancer Invest. 2002;20:749-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Sakorafas GH, Tsiotou AG. Etiology and pathogenesis of acute pancreatitis: current concepts. J Clin Gastroenterol. 2000;30:343-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 125] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 3. | Stewart KC, Dickout WJ, Urschel JD. Metastasis-induced acute pancreatitis as the initial manifestation of bronchogenic carcinoma. Chest. 1993;104:98-100. [PubMed] |
| 4. | Chowhan NM, Madajewicz S. Management of metastases-induced acute pancreatitis in small cell carcinoma of the lung. Cancer. 1990;65:1445-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Das Gupta E, Coombes B, Brasfield RD. Primary malignant neoplasms of the spleen. Surg Gynecol Obstet. 1965;120:947-960. [PubMed] |
| 6. | Skarin AT, Davey FR, Moloney WC. Lymphosarcoma of the spleen. Results of diagnostic splenectomy in 11 patients. Arch Intern Med. 1971;127:259-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Mollejo M, Algara P, Mateo MS, Menárguez J, Pascual E, Fresno MF, Camacho FI, Piris MA. Large B-cell lymphoma presenting in the spleen: identification of different clinicopathologic conditions. Am J Surg Pathol. 2003;27:895-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Spier CM, Kjeldsberg CR, Eyre HJ, Behm FG. Malignant lymphoma with primary presentation in the spleen. A study of 20 patients. Arch Pathol Lab Med. 1985;109:1076-1080. [PubMed] |
| 9. | Takeshita M, Sakai H, Okamura S, Oshiro Y, Higaki K, Nakashima O, Uike N, Yamamoto I, Kinjo M, Matsubara F. Splenic large B-cell lymphoma in patients with hepatitis C virus infection. Hum Pathol. 2005;36:878-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | De Vita S, Sacco C, Sansonno D, Gloghini A, Dammacco F, Crovatto M, Santini G, Dolcetti R, Boiocchi M, Carbone A. Characterization of overt B-cell lymphomas in patients with hepatitis C virus infection. Blood. 1997;90:776-782. [PubMed] |
| 11. | Satoh T, Yamada T, Nakano S, Tokunaga O, Kuramochi S, Kanai T, Ishikawa H, Ogihara T. The relationship between primary splenic malignant lymphoma and chronic liver disease associated with hepatitis C virus infection. Cancer. 1997;80:1981-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Brox A, Shustik C. Non-Hodgkin's lymphoma of the spleen. Leuk Lymphoma. 1993;11:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
S- Editor Liu Y L- Editor Lutze M E- Editor Wang HF