Published online Jul 21, 2007. doi: 10.3748/wjg.v13.i27.3705
Revised: November 5, 2006
Accepted: November 11, 2006
Published online: July 21, 2007
AIM: To evaluate the protective activity of allylpyrocatechol (APC), the major antioxidant constituent of Piper betel, against the indomethacin-induced stomach ulceration in the rat model and correlates with its antioxidative and mucin protecting properties.
METHODS: Male Sprague-Dawley rats were divided into five groups. Normal control rats (group I) were given the vehicle oral dose of gum acacia in distilled water (1 mL per rat); ulcerated control and treated rats (groups II-V) were given a single dose of indomethacin (30 mg/kg body wt.); group II rats were sacrificed 4 h after indomethacin administration; groups III-V rats were given the vehicle (1 mL per rat) or APC (2 mg/kg body wt.) or misoprostol (1.43 μg/kg body wt.) once daily by oral intubation for 7 d starting from 4 h after the indomethacin administration. After 7 d, the stomach tissues were excised for histological examination and biochemical analysis.
RESULTS: Treatment with APC (2 mg/kg body wt per day) and misoprostol (1.43 μg/kg body wt per day) for 7 d could effectively heal the stomach ulceration as revealed from the ulcer index and histopathological studies. Compared to the zero day ulcerated group, treatment with APC and misoprostol reduced the ulcer index by 93.4% and 85.4% respectively (P < 0.05). Both APC and misoprostol accelerated ulcer healing observed in natural recovery (P < 0.05), their respective healing capacities not being significantly different. The healing capacities of APC and misoprostol could be attributed to their antioxidant activity as well as the ability to enhance the mucin content of the gastric tissues. Compared to the ulcerated untreated rats, those treated with APC and misoprostol showed near normal MDA levels, while the protein levels were 86% and 78% of the normal value respectively (P < 0.05). Likewise, both APC and misoprostol increased the SOD, catalase, and mucin levels significantly (P < 0.05), the effect of APC being better.
CONCLUSION: APC can protect indomethacin-induced gastric ulceration due to its antioxidative and mucin protecting properties.
-
Citation: Bhattacharya S, Banerjee D, Bauri A, Chattopadhyay S, Bandyopadhyay S. Healing property of the
Piper betel phenol, allylpyrocatechol against indomethacin-induced stomach ulceration and mechanism of action. World J Gastroenterol 2007; 13(27): 3705-3713 - URL: https://www.wjgnet.com/1007-9327/full/v13/i27/3705.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i27.3705
Gastrointestinal toxicity associated with nonsteroidal anti-inflammatory drugs (NSAIDs) is an important medical problem despite recent pharmaceutical advances[1]. Besides being used as pain-killers, the NSAIDs are being increasingly used for prevention of malignancies, stroke, pre-eclampsia, Alzheimer’s disease, and many other illnesses[2-4]. The percentage of gastric ulcer cases induced by NSAIDs is emerging day by day, accounting for approximately 25% of gastric ulcers[5]. In addition, various factors such as stress, hunger, H pylori invasion etc are also known to cause gastric ulcer. Consequently, prevention of gastrointestinal disorder continues to be of concern for both medical professionals and researchers. Various synthetic anti-ulcer drugs are presently available, and some of these like misoprostol are specifically used to prevent or treat the NSAID induced gastric ulcer. However, each of these drugs confers simpler to severe side effects such as diarrhea, itching, skin rash, dizziness, and inactivation of some antifungal drugs (proton pump inhibitors), confusion in elderly patients, headache and antiandrogenic effect (H-2 receptor blockers), constipation, vomiting, indigestion, back pain, and dizziness (sucralfate), bleeding diathesis and abortion for pregnant women (misoprostol)[6]. Thus, there is a growing interest on non-toxic, antiulcer formulations from medicinal plants, and many taxa of medicinal plants have been assessed worldwide for their antiulcerogenic effects[7,8]. In the developing nations, this turn of events has also been prompted, in part, by the high cost of the modern antiulcer medication.
The Piper betel plant is found widely growing in the tropical humid climate of South East Asia, and its leaves, with a strong pungent and aromatic flavor, are widely consumed as a mouth freshener. The Indian traditional system of medicine has identified the P. betel leaves with digestive and pancreatic lipase stimulant activities[9,10]. Earlier, we also reported gastrocytoprotective properties of the leaf extract on experimentally induced gastric lesions[11].
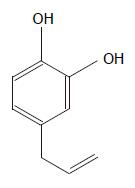
The major problem with the herbal drugs is the lack of their quality control and improper authentication. Identifying the major active principle(s), and ensuring their presence in optimum quantity in the herbal preparations can minimize problems associated with their questionable quality. Very recently we have found that the extraordinary antioxidant activity of the P. betel leaf extract is due to its major phenolic constituent, allylpyrocatechol (APC, Figure 1)[12]. Consequently, the aim of the present study was to evaluate the healing effect of APC on indomethacin-induced acute gastric ulceration of rats and compare the activity with that of the drug, misoprostol.
The P. betel leaves were collected from the local market and identified (collection no. 2610) by taxonomy by the Botanical Survey of India, Indian Botanical Garden, West Bengal. 2-Thiobarbituric acid (TBA), Tris, ethylenediaminetetraacetic acid (EDTA), acetic acid, methanol and ethanol were procured from E Merck (India), while trichloroacetic acid (TCA) was from Thomas Baker, India. Alcian blue, indomethacin, dimethylaminobenzaldehyde, epinephrine, Cu (II) sulphate, bovine serum albumin (BSA), hematoxylin, alum, mercuric oxide, eosin, Schiff’s reagent and periodic acid were procured from Sigma Chemicals, St. Louis, MO (USA). Other reagents used were H2O2 (35%, Lancaster, England), and perchloric acid, 2,4-dinitrophenyl hydrazine (DNPH), K2HPO4, KH2PO4, KOH and HCl (all from SRL, India). Stock solutions of EDTA and H2O2 were prepared in triply distilled deaerated water just prior to use. Stock solutions (1% w/v) of TBA were prepared in 50 mmol/L NaOH solution and used within a week.
APC was isolated from P. betel ethanol extract as in the reported procedure[12]. The air-dried leaves of the P. betel (250 g) were chopped into fine pieces, soaked in 95% ethanol (1 L) for two days and the supernatant decanted. The entire process was repeated three times, the combined extracts were filtered through a nylon mesh, and evaporated in vaccuo. The extract (8.0 g) was dissolved in methanol (50 mL), treated with activated charcoal (0.2 g), and the mixture warmed at -60°C. After filtration, the extract was concentrated under vacuum, and finally lyophilized to obtain a chlorophyll-free amorphous yellowish brown solid (yield: 1.23% w/w) that was stored in a vacuum dessicator.
The extract (4.7 g) was subjected to column chromatography over silica gel using gradient elution. The column was eluted with 0, 2%, 5% and 10% ethyl acetate/hexane followed by 0, 5%, 10%, 15%, 50% and 100% methanol/chloroform (500 mL each), and 50 mL fractions were collected. The fraction eluting with 10% ethyl acetate/hexane furnished APC (yield: 0.9% w/w of the extract) as a light yellow oil, which was characterized using IR and 1H NMR spectroscopic data. The chemical structure of APC is shown in Figure 1.
3,4-Dihydroxyallylbenzene (Allylpyrocatechol, APC): 1H NMR (CDCl3): δ 3.28 (d, J = 6.5 Hz, 2H, ArCH2), 4.99-5.08 (m, 2H, olefin), 5.36 (broad, 2H, 2 × Ar-OH), 5.80-6.01 (m, 1H, olefin), 6.59-6.79 (m, 3H, ArH).
A specific dry weight of APC or misoprostol was macerated with a mortar and pestle in double distilled water containing gum acacia 2% (w/w) to provide the drugs, which were administered po.
The rats were bred at Dr. B. C. Roy Post Graduate Institute of Basic Medical Sciences, Kolkata, India and BARC Laboratory Animal House Facility, Mumbai, India. These were procured after obtaining clearance from the respective Animal Ethics Committee of the two centers and were handled following international Animal Ethics Committee guidelines. Male Sprague-Dawley rats (weighing 180-200 g) were reared on a standard laboratory diet (Ralston Purina, Chicago, Illinois, USA) and given tap water. They were kept in a room where temperature (20 ± 2°C), humidity (65%-70%), and day/night cycle (12/12 h) were controlled.
Ulceration in the rats was induced with a single dose of indomethacin (30 mg/kg body wt., oral intubation) dissolved in distilled water. The rats were deprived of food but had free access to tap water 24 h before ulcer induction.
For the standardization of drug dose, APC (0.5, 1, 2, 5, and 10 mg/kg body wt.) was given once daily by oral intubation for 7 d starting from 4 h after the indomethacin administration. Five rats were taken for each dose and each experiment was repeated three times. The extent of healing was assessed from the macroscopic damage scores. The effect of the treatment with APC (2 mg/kg body wt.) for 10 d was also studied.
Based on the results of dose optimization, the histopathological, and biochemical parameters were assessed in separate experiments. For this, a total of 25 rats were randomly divided into 5 groups in each set of experiment, which was replicated three times. GroupIrats serving as the normal control received only the vehicle oral dose of gum acacia in distilled water (1 mL per rat). Ulceration was induced in the groups II-V rats. Group II rats serving as the experimental control received only indomethacin and were sacrificed 4 h after indomethacin administration. Group III-V rats were given the vehicle (1 mL per rat) or APC (2 mg/kg body wt.) or misoprostol (1.43 μg/kg body wt.) once daily by oral intubation for 7 d starting from 4 h after the indomethacin administration. After 7 d, the rats of groupIand groups III-V were sacrificed under ether anesthesia, followed by cutting off the aorta abdominalis.
The stomach from the normal and treated groups were removed rapidly, opened along the greater curvature, and thoroughly rinsed with normal saline. After recording the ulcers produced in the stomach, a longitudinal section of the gastric tissue was taken from the anterior part of the stomach and fixed in a 10% formalin solution. After 24 h of fixation followed by embedding in a paraffin block, it was cut into sections of 5 micron onto a glass slide and stained with hematoxylin-eosin for histological assessment of the gastric mucosa. For biochemical studies, the stomach was opened along the greater curvature, and the gastric antral portion was used. The wet weight of the portion was also recorded.
Area of glandular portion comprising of the fundic and corpus region of each stomach was measured in square millimeters. The total eroded gastric mucosal areas (lesions) were also measured (mm2) with a dissecting microscope under × 20 magnification. The percentages of the whole glandular area contributing to the damaged part are referred to as the ulcer indices according to a reported procedure[13]. The ulcer indices for the cold stress rats were also calculated in the same manner.
The stomach tissue was homogenized in a 50 mmol/L phosphate saline buffer (PBS) pH 7.2 under cold condition, using a glass-teflon homogenizing tube. The homogenate was centrifuged at 2500 r/min for 10 min and the supernatant was carefully removed from the pellet and used for biochemical analyses.
The lipid peroxidation products were estimated[14] in terms of malondialdehyde (MDA) formation. The amounts of protein carbonyls and DNA contents were estimated following reported methods[15,16].
The specific activity of catalase (CAT) in the tissue was estimated according to a reported method[17]. The tissue homogenate (20 μL) was added to a H2O2-phosphate buffer mixture (3 mL), maintaining the optical density at 240 nm to 0.500 ± 0.010 (d = 1 cm) against buffer. The rate of change of optical density at 240 nm with time was recorded for the calculation of the catalase activity.
Following a reported method[18], the tissue superoxide dismutase (SOD) activity was measured. This involves assaying the SOD-mediated inhibition of epinephrine autooxidation at an alkaline medium. For this, the absorbances of the samples at 480 nm were noted at an interval of 30 s. The enzyme activity was measured in arbitrary units, considering 50% inhibition as 1 unit of enzyme activity.
Following a reported method[19], the free mucin in the gastric tissues was estimated. Briefly, the gastric tissues were incubated with a 1% buffered solution of Ab in 3% aqueous acetic acid (0.5 mL) at 37°C for 30 min. After centrifuging, the concentration of Ab in the solution was measured from the absorbance at 615 nm.
The hexosamine concentrations in gastric tissues were assayed according to a reported method[20] with a minor modification. The gastric tissues were hydrolyzed in acidic medium, the hydrolysate neutralized with 3 mol/L NaOH (litmus) and diluted to 10 mL with double distilled water. Acetyl acetone solution (1 mL, prepared by dissolving 1 mL of acetyl acetone in 50 mL 0.5 mol/L sodium carbonate) was added to the above solution (1 mL), which was mixed well, and heated on a boiling water bath for 15 min avoiding evaporation. After cooling, ethanol (5 mL, 95%) was added to the mixture followed by Ehrlich’s reagent (1 mL, prepared by dissolving 0.8 g para-dimethylaminobenzaldehyde in 30 mL methanol and 30 mL conc. HCl). The mixture was diluted to 10 mL with 95% ethanol, allowed to stand for 30 min, and its absorbance at 530 nm was read.
All the results were expressed as mean ± SE. Student’s paired t-test for comparison between groups and one way analysis of variance (ANOVA) for multi-sample groups at P < 0.05 were used to assess statistical significance in various groups of animals.
Oral administration of APC at different doses accelerated the rate of healing of gastric lesion induced by indomethacin. As shown in Table 1, for a 7-d treatment, increasing the dose of APC from 1 to 2 mg/kg body wt. led to a significantly improved ulcer healing. Increasing the doses of APC further did not provide any additional benefit. Even when the treatment with APC (2 mg/kg body wt.) was continued for 10 d, the extent of ulcer healing remained almost the same. Thus, administration of APC at a dose of 2 mg/kg body wt. for 7 d after ulcer induction was found to be optimal for effective ulcer healing. Hence all subsequent experiments were carried out with the same protocol. The dose of the positive control, misoprostol (1.43 μg/kg body wt.) was decided based on its recommended therapeutic dose for humans. Earlier misoprostol has been used at significantly higher doses with the rat models[21]. However, our studies were primarily aimed at developing anti-ulcer drugs for humans. For this it was essential to compare the ulcer-healing efficacy of APC with that of the established drug, misoprostol at the dose recommended for humans.
Treatment of rats with indomethacin (30 mg/kg body wt.) by oral intubation produced typical time-dependent acute lesions in the gastric mucosa, while the rats receiving vehicle only showed no visible mucosal lesions. Compared to the zero day ulcerated group (group II) for which an ulcer index was taken as 100%, treatment with APC and misoprostol reduced the ulcer index by 93.4% and 85.4% respectively. The ulcer healing data of the treated groups (APC and misoprostol) were significantly different from those of both 0 and 7 d untreated experimental control rats (groups II and III) (P < 0.05). However, the difference in healing between the two treated groups (groups IV and V) was insignificant (Table 2).
The visible morphological features of the ulcerated rat stomachs of the treated and untreated groups were compared to those with normal rats. This revealed that the zero-day ulcerated stomach had a number of blood clots in the ulcer spots and perforations. The stomach of the untreated experimental control rats (group III) showed lesser spots, but the tissues were hyaline in nature. The result with misoprostol treatment was marginally better than that of the group III rats. In comparison, stomachs of the APC treated rats (groups IV) were healthy, and equivalent to those of the normal control rats (group I).
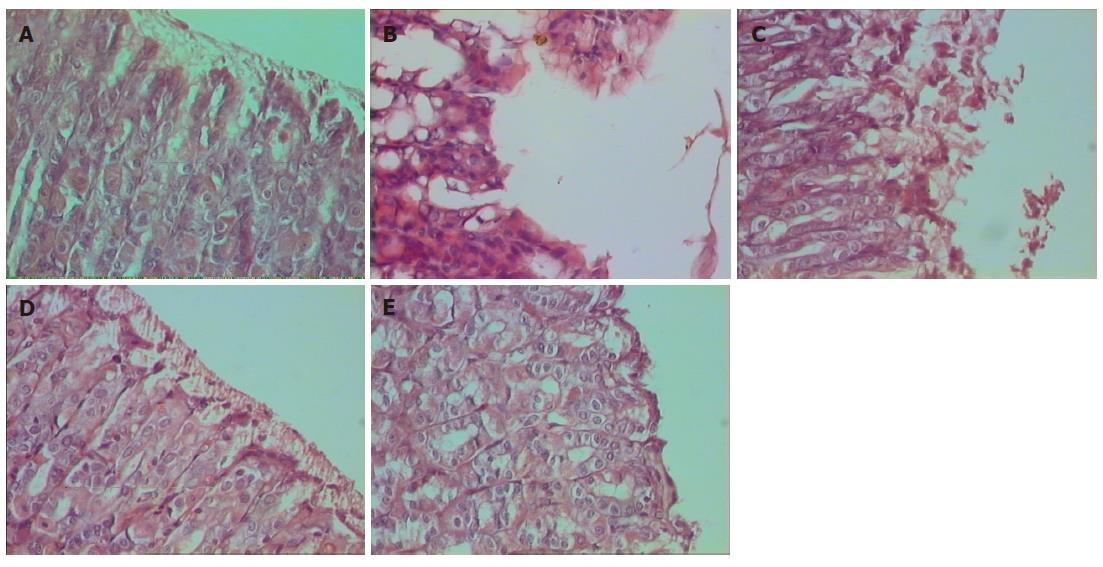
The photomicrographs of rat stomachs belonging to groups I-V shown in Figure 2A-E gives a better comparison of the gastric lesion and its healing. Significant acute ulceration in rats was developed within 4 h of indomethacin (30 mg/kg body weight) administration as revealed from Figure 2A (normal rats) and Figure 2B (rats after 4 h of indomethacin administration). Indomethacin intake induced severe and extensive macroscopic gastric mucosal damage in the irrigated starved rats, characterized by injury in the epithelial layer of the mucosa. The lamina propria also were greatly damaged along with elongated hemorrhagic lesions confined mainly to the gastric corpus and running parallel to the long axis of the stomach that had the highest ulcer scoring rate. The disruption in the gastric mucosa was partially restored after 7 d even without any treatment, revealing autohealing. These observations matched well with the ulcer index parameter. Histology of the rat stomach of the untreated ulcerated group showed some damage in the mucosal layer with moderate infiltration in the mucous membrane (Figure 2C). However, the gastric mucosal tissues of the APC treated group (Figure 2D) showed almost normal and continuous mucosal layer and formation of the epithelial layer. The efficacy of APC was better than that of misoprostol as revealed from the Figures 2D and E.
The effects of indomethacin intake alone, and following administration of APC on the extent of lipid peroxidation (measured in terms of MDA), protein oxidation (measured in terms of total proteins and protein carbonyls), and DNA damage in the gastric tissues of rats are shown in Table 3. Indomethacin administration markedly stimulated lipid peroxidation in gastric tissues, and the MDA content was elevated by about 14 times compared to the normal rats. This was reduced by 11% after 7 d due to autohealing for the untreated control rats (group III), although the MDA content remained significantly high (13 times) compared to that in the normal rats. Administration of APC for 7 d significantly reduced the MDA level (2.02 nmol/mg prot.) in gastric tissues almost to that of the normal rats (1.89 nmol/mg prot.). The effect of misoprostol was marginally less (MDA level 2.62 nmol/mg prot.) to that of APC. The data for the APC and misoprostol treatment are significant compared to that of the indomethacin induced untreated ulcerated control rats of groups II and III (P < 0.05).
| Parameters | Group I | Group II2 | Group III | Group IV | Group V |
| MDA (nmol/mg protein) | 1.89 ± 0.01 | 27.04 ± 0.84 | 24.28 ± 1.12 | 2.02 ± 0.03 | 2.62 ± 0.02 |
| Proteins (mg/mL) | 16.52 ± 6.05 | 9.03 ± 1.28 | 12.55 ± 1.18 | 14.21 ± 1.8 | 12.89 ± 0.87 |
| CO (mg/mg protein) | 5.26 ± 1.34 | 17.29 ± 1.83 | 9.46 ± 2.15 | 6.03 ± 1.51 | 6.54 ± 1.12 |
| DNA (mg/g tissue) | 1.82 ± 0.22 | 0.81 ± 0.06 | 1.12 ± 0.21 | 3.56 ± 0.05 | 1.68 ± 0.23 |
| SOD (U/min/mg protein) | 22.2 ± 2.34 | 5.76 ± 2.32 | 11.58 ± 1.32 | 21.14 ± 1.24 | 16.71 ± 2.57 |
| CAT (U/min/mg protein) | 21.2 ± 1.12 | 9.02 ± 2.54 | 14.20 ± 2.84 | 19.04 ± 2.15 | 15.84 ± 2.09 |
Compared to the protein contents in the normal animals, the ulcerated group showed poor protein content (54% reduction), which improved for the untreated group on the 7 d and became 76% of the normal value. Treatment with APC and misoprostol increased the protein levels respectively to 86% and 78% of the normal value. The increase of the protein contents by the APC was significantly different (P < 0.05) compared to both ulcerated and untreated ulcerated controls (groups II and III). However, the data of misoprostol treatment group (group V) was not significantly different from that due to autohealing (group III).
The oxidative damage to tissue proteins was also evaluated by assessing the contents of protein carbonyls. The amount of protein carbonyls that increased significantly (329% compared to normal rats) due to indomethacin administration, was reduced by 45.3% due to autohealing. APC treatment restored the level of protein carbonyls to normalcy (6.03 μg/mg vs 5.26 μg/mg protein in normal rats), while the effect of misoprostol was less (6.54 μg/mg protein). The reduction of protein carbonyls by APC was significant (P < 0.05) as compared to untreated ulcerated controls (group III).
The tissue DNA concentration was significantly (56%) reduced by indomethacin administration. During autohealing, the DNA level increased to 61.5% of the normal value. Surprisingly the APC treatment increased the DNA level by 196% compared to the normal rats, while treatment with misoprostol restored the level of DNA to normalcy (1.68 mg/g tissue for treated vs 1.82 mg/g tissue for normal rats). The augmentation of DNA by APC was significant (P < 0.05) compared to the ulcerated control (group II) as well as the misoprostol group.
The effect of indomethacin intake alone, and following administration of APC on the tissue levels of SOD and CAT in gastric tissues of rats are also presented in Table 3. Following indomethacin administration, the levels of these enzymes in gastric tissue were also depleted. The SOD activity decreased by 74%, while that of CAT was reduced by about 57% compared to those in normal control rats. Treatment with APC increased the SOD activity (P < 0.05) to near normalcy (21.1 U/min vs 22.2 U/min per mg protein in normal rats), while the CAT level showed 90% recovery (P < 0.05). The effect of misoprostol was significantly less than that of APC (P < 0.05) restoring the SOD and CAT levels to 75% and 74% of the normal values. In comparison, the autohealing restored the SOD and CAT levels to 52% and 67% of the normal values only. The data for the APC-treated rats were significantly different (P < 0.05) from those of the untreated 0 and 7 d groups.
Alcian blue assay: Indomethacin administration to rats significantly decreased the secretion of mucin (43.5%, P < 0.05) and mucosal glycoproteins (76.4% decrease, P < 0.05) in the ulcerated rats of group II compared to those in unulcerated rats (group I). Treatment with APC enhanced the tissue mucin level to that in normal unulcerated rats, while the mucosal glycoprotein content was also increased to 65.4% of the normal value. The augmentation of mucin level by APC was significant (P < 0.05) as compared to ulcerated controls (group II). Misoprostol also increased the mucin secretion and mucosal glycoproteins in the ulcerated rats to 94.2% and 89.9% of the respective normal values. The results are summarized in Table 4.
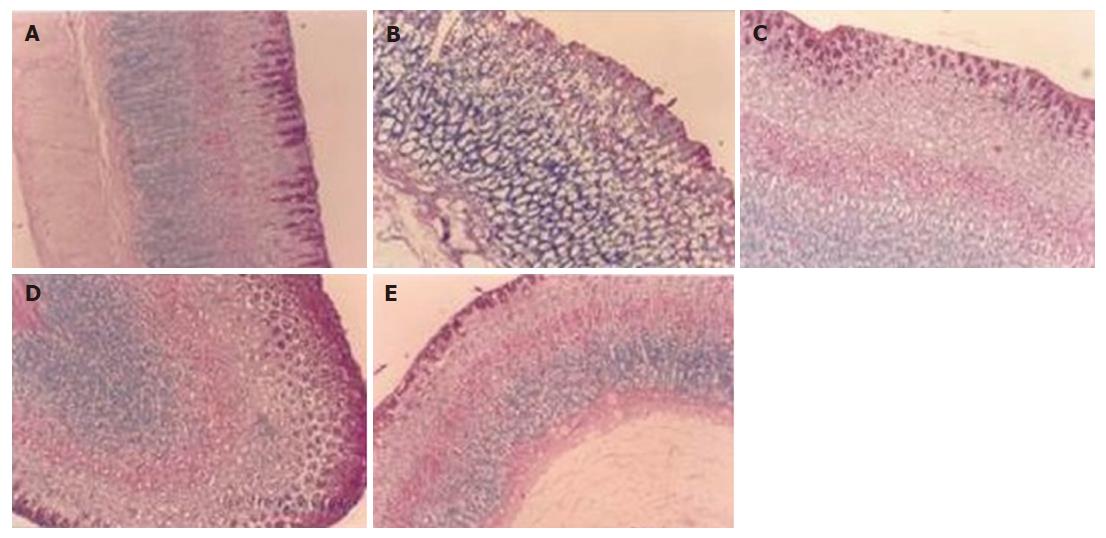
PAS staining: The photomicrographs of groups I-V rat stomachs after PAS staining are shown in Figure 3A-E. The indomethacin-induced stomach ulceration led to a drastic reduction in the mucin content that was partially restored due to autohealing. However, treatment with APC improved the mucin content in the stomach tissues (Figure 3D) further, bringing to a normal level (Figure 3A) along with considerable restoration of stomach morpho-logy and epithelium lining. In comparison, the effect of misoprostol was not significantly different from that due to autohealing.
Oxygen free radicals are known to play a role in the induction and pathogenesis of gastroduodenal injury[22]. Extensive research has proven that antioxidants might be effective not only in protecting against gastric mucosal injury, but also inhibiting progression of a gastric ulcer. Ulcer progression is caused by free radical-induced chain processes. Consequently, its arrest by radical scavengers helps in faster healing. Indomethacin is known to induce the reactive oxygen metabolites in animal models, which may contribute to mucosal injury[23]. The cytoprotective role of antioxidants in the prevention and healing of gastric lesions has been widely investigated in a number of studies[24,25].
The notion that APC has shown a powerful antioxidant potential in various in vitro models[26] warranted our attention to address its possible protective effects against indomethacin-induced gastric lesions in rats. Earlier, the cytoprotective and healing properties of P. betel crude extract against indomethacin as the gastric mucosal irritant was established by us[11]. The aim of the present study was, therefore, to study the healing effect of its principal antioxidant constituent, APC on indomethacin-induced acute stomach ulceration of rats.
Administration of indomethacin to rats induced marked damage to the gastric mucosa as evident by macroscopic and histopathological examinations. This led to elongated hemorrhagic lesions, confined to the glandular portion, with the highest subjective ulcer-scoring rate.
In the ulcerated control animals that were given the vehicle only during the seven-day period of treatment, the ulcer craters also receded through the process of autohealing, reducing the ulcer index by 36.2% for the group III rats even without any drug treatment. The present study demonstrated that APC had a potent healing effect on indomethacin-induced gastric lesions in rats. The rate of healing in the APC-treated animals was significantly faster than that found in the case of autohealing. The histopathological observations revealed that the ulceration was acute explaining the autohealing observed in the untreated control rats.
Tissue damage is always associated with the loss/reduction of DNA content and loss or impairment of protein synthesis[27] due to excess generation of free radicals. These free radicals also damage the cellular antioxidant enzymes such as CAT, SOD and others, acting as the first line of cellular defense against oxidative injury. This might lead to aggravated tissue damage during stomach ulceration[28]. Our results revealed that the indomethacin-induced stomach ulceration was accompanied with a severe oxidative stress in gastric tissue causing damages to key biomolecules such as lipids, proteins and DNA. This was apparent from the stimulated lipid and protein oxidation leading to increased accumulation of MDA and protein carbonyls, as well as reduction in the tissue protein and DNA contents. These findings are in tune with many previous reports. APC provided a marked suppression of oxidative damage due to its excellent radical scavenging capacity and brought most of these parameters to normal levels, than observed in natural recovery.
Accumulation of the reactive products is known to markedly alter the antioxidant enzymes leading to enhanced oxidative damage during stomach ulceration[28]. In the present study, the gastric activities of SOD and CAT were found to be reduced notably following indomethacin intake. Treatment with APC reversed these oxidative changes with concomitant increase in SOD and CAT levels, thereby suppressing most of the biochemical adverse effects induced by indomethacin. This might decrease the ulcer progression and promote healing of gastric lesions induced by acute intake of indomethacin.
Ulcer-healing is a complex process involving a com-bination of wound retraction and re-epithelization[29]. Release of preformed mucus also plays a role in promoting epithelial recovery after acute injury by forming a mucoid cap beneath which re-epithelization occurs[30]. Besides providing significant buffering capacity for the neutralization of luminal acid, the mucus can offer protection against the endogenous aggressors like pepsin and oxidants produced in the gastric lumen, as well as against exogenous damaging agents, such as NSAIDs.
The macromolecular glycoprotein, hexosamine constitutes the major fraction of gastric mucin and is accepted to be an ideal index of gastric mucus production[31]. The NSAID-produced mucosal hemorrhagic ulcer may be due to a decrease of gastric mucus production[32]. Thus, drugs that arrest ulcer progression by antioxidant action, and also increase the synthesis and secretion of gastric mucus would accelerate gastric ulcer healing.
In this study, the decreased mucin secretion in the indomethacin-administered rats indicated reduced ability of the mucosal membrane to protect the mucosa from physical damage and back diffusion of hydrogen ions. The decrease in the glycoprotein content of the gastric mucosa further proved the decreased ability of the gastric mucosa to withstand the offensive onslaught. Mucosal damage can be easily produced by the generation of exogenous and endogenous active oxygen and free radicals[33]. An increase in mucus production usually assist the healing process by protecting the ulcer crater against irritant stomach secretions (HCl and pepsin) thereby enhancing the rate of the local healing process. Treatment with APC and misoprostol significantly accelerated the ulcer healing process, which is associated with an increase in the mucus layer in the gastric mucosa. Apparently, the free radicals scavenging property of APC might be contributing in protecting the oxidative damage to gastric mucosa.
It is well known that PAS stain is the best method to identify gastric metaplasia of duodenal mucosa. Metaplastic gastric type epithelium in the duodenum has been regarded as an adaptive defensive response to mucosal injury of any kind, including acid and/or pepsin[34]. The presence and extent of gastric metaplasia have been correlated with acid secretory capacity in men[35], and they have been induced in experimental animals by stimulation of excessive acid secretion. In contrast to the untreated controls in which the autohealing process can be attributed to other mechanisms, the healing action of APC is evidently related to its ability to improve mucus production. All these indicated that enhancement of the mucus modulation by APC play a significant role in its ulcer healing effect.
Given that some drugs can show mild-to-severe side effects even after short-term intake, we also evaluated the possible toxic effects of APC up to a dose of 25 mg/kg body wt. with both mice and rats. There was no observable physical sign change and the animals had normal food and water as well as stool during the experimental period. These findings suggested that APC given at the current dose does not have any potential side effects in the animals. The non-toxicity of APC was expected considering that it is the major constituent of P. betel leaves that is freely consumed in India and South-East Asian countries.
In conclusion, the present study established that the APC, the major constituent of P. betel leaves can heal indomethacin-induced stomach ulceration in rats by its antioxidant action and ability to form mucus. Apparently, by scavenging free radicals, APC might be protecting the gastric mucosa, and in turn, the stomach epithelium from the oxidative damage. This accelerates healing of gastric ulcers. Earlier, our group has also reported the cytoprotective activity of the crude ethanol extract of P. betel leaves against indomethacin-induced stomach ulceration. These results, taken together along with the non-toxicity of APC indicate its potential as an anti-ulcerogenic drug for further investigations. Comparison of its efficacy with that of misoprostol further confirmed the findings.
Ulceration due to NSAID is also believed to occur because of non-selective inhibition of cyclooxygenases that hampers the release of mucus due to reduction in prostaglandin synthesis. The relationship between prostaglandins and leukotrienes, the products of prostaglandin H synthase (PGHS) and 5-lipoxygenase, respectively, seems to be an important factor in gastric ulcers. Many phenolic compounds stimulate prostaglandin synthesis by acting as reducing substrates for the oxidized intermediates of PGHS, thereby accelerating the peroxidase cycle and by functioning as electron-donating co-substrates for the peroxidase component of PGHS. In this way, they can modulate the PGHS and 5-lipoxygenase pathways of arachidonic acid[36]. Thus, it would be of interest to study the effect of APC on the PG-dependent pathway of healing gastric ulcer. Investigation in this regard is currently in progress in our laboratory and the results will be reported later.
The authors thank the Board of Research in Nuclear Science, Department of Atomic Energy, Government of India for generous support in providing the financial grant.
| 1. | Hawkins C, Hanks GW. The gastroduodenal toxicity of nonsteroidal anti-inflammatory drugs: a review of the literature. J Pain Symptom Manage. 2000;20:140-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Pace V. Use of nonsteroidal anti-inflammatory drugs in cancer. Palliat Med. 1995;9:273-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Coruzzi G, Menozzi A, Dobrilla G. Novel non-steroidal anti-inflammatory drugs: what we have learned from animal studies. Curr Drug Targets Inflamm Allergy. 2004;3:43-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Garner A. Adaptation in the pharmaceutical industry, with particular reference to gastrointestinal drugs and diseases. Scand J Gastroenterol Suppl. 1992;193:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Dhikav V, Singh S, Pande S, Chawla A, Anand KS. Non-steroidal drug-induced gastrointestinal toxicity: Mechanisms and management. J Indian Clin Med. 2003;4:315-322. |
| 6. | Miederer SE. Will anti-ulcer drugs soon differ only in their side effects? Fortschr Med. 1986;104:918-920. [PubMed] |
| 7. | Borrelli F, Izzo AA. The plant kingdom as a source of anti-ulcer remedies. Phytother Res. 2000;14:581-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 8. | Yesilada E, Gurbuz I. A compilation of the studies on the antiulcerogenic effects of medicinal plants. II: Phytochemistry and pharmacology. Singh S, Singh VK, Govil JN, editors. Houston: SCI Tech Publishing LLC 2003; 111-174. |
| 9. | Chatterjee A, Pakrashi SC. Treatise of Indian Medicinal Plants. New Delhi: CSIR Publication 1995; 26. |
| 10. | Prabhu MS, Platel K, Saraswathi G, Srinivasan K. Effect of orally administered betel leaf (Piper betle Linn.) on digestive enzymes of pancreas and intestinal mucosa and on bile production in rats. Indian J Exp Biol. 1995;33:752-756. [PubMed] |
| 11. | Majumdar B, Ray Chaudhuri SG, Ray A, Bandyopadhyay SK. Effect of ethanol extract of Piper betle Linn leaf on healing of NSAID-induced experimental ulcer--a novel role of free radical scavenging action. Indian J Exp Biol. 2003;41:311-315. [PubMed] |
| 12. | Rathee JS, Patro BS, Mula S, Gamre S, Chattopadhyay S. Antioxidant activity of piper betel leaf extract and its constituents. J Agric Food Chem. 2006;54:9046-9054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Szabo S, Trier JS, Brown A, Schnoor J, Homan HD, Bradford JC. A quantitative method for assessing the extent of experimental gastric erosions and ulcers. J Pharmacol Methods. 1985;13:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2154] [Cited by in RCA: 2472] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 15. | Palamanda JR, Kehrer JP. Inhibition of protein carbonyl formation and lipid peroxidation by glutathione in rat liver microsomes. Arch Biochem Biophys. 1992;293:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Burton K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956;62:315-323. [PubMed] |
| 17. | Luck H. Catalase. Bergmeyer HU, editor. New York: Verlag Chemical and Academic Press 1963; 885-888. |
| 18. | McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969;244:6049-6055. [PubMed] |
| 19. | Corne SJ, Morrissey SM, Woods RJ. Proceedings: A method for the quantitative estimation of gastric barrier mucus. J Physiol. 1974;242:116P-117P. [PubMed] |
| 20. | Winzler RJ. Determination of serum glycoproteins. Methods Biochem Anal. 1955;2:279-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 442] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 21. | Chang FY, Chen TS, Lee SD, Doong ML, Wang PS. Misoprostol-inhibited rat gastric emptying is independent of gastric inhibitory polypeptide release. Pharmacology. 1999;58:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Biswas K, Bandyopadhyay U, Chattopadhyay I, Varadaraj A, Ali E, Banerjee RK. A novel antioxidant and antiapoptotic role of omeprazole to block gastric ulcer through scavenging of hydroxyl radical. J Biol Chem. 2003;278:10993-11001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 213] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 23. | McAlindon ME, Muller AF, Filipowicz B, Hawkey CJ. Effect of allopurinol, sulphasalazine, and vitamin C on aspirin induced gastroduodenal injury in human volunteers. Gut. 1996;38:518-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Santos FA, Rao VS. 1,8-cineol, a food flavoring agent, prevents ethanol-induced gastric injury in rats. Dig Dis Sci. 2001;46:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Bilici D, Süleyman H, Banoğlu ZN, Kiziltunç A, Avci B, Ciftçioğlu A, Bilici S. Melatonin prevents ethanol-induced gastric mucosal damage possibly due to its antioxidant effect. Dig Dis Sci. 2002;47:856-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Bhattacharya S, Subramanian M, Roychowdhury S, Bauri AK, Kamat JP, Chattopadhyay S, Bandyopadhyay SK. Radioprotective property of the ethanolic extract of Piper betel Leaf. J Radiat Res. 2005;46:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Marotta F, Tajiri H, Safran P, Fesce E, Ideo G. Ethanol-related gastric mucosal damage: evidence of a free radical-mediated mechanism and beneficial effect of oral supplementation with bionormalizer, a novel natural antioxidant. Digestion. 1999;60:538-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | El-Missiry MA, El-Sayed IH, Othman AI. Protection by metal complexes with SOD-mimetic activity against oxidative gastric injury induced by indomethacin and ethanol in rats. Ann Clin Biochem. 2001;38:694-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Szabo S, Hollander D. Pathways of gastrointestinal protection and repair: mechanisms of action of sucralfate. Am J Med. 1989;86:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 105] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Tabor CW, Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2655] [Cited by in RCA: 2591] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 31. | Wang JY, Hsieh JS, Huang TJ. Changes in rat gastric mucosal glycoproteins in portal hypertension. Eur Surg Res. 1997;29:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 32. | Rainsford KD. The effects of aspirin and other non-steroid anti-inflammatory/analgesic drugs on gastro-intestinal mucus glycoprotein biosynthesis in vivo: relationship to ulcerogenic actions. Biochem Pharmacol. 1978;27:877-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 74] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Naito Y, Yoshikawa T, Matsuyama K, Yagi N, Arai M, Nakamura Y, Nishimura S, Yoshida N, Kondo M. Effects of oxygen radical scavengers on the quality of gastric ulcer healing in rats. J Clin Gastroenterol. 1995;21 Suppl 1:S82-S86. [PubMed] |
| 34. | James AH. Gastric epithelium in the duodenum. Gut. 1964;5:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 91] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Chang CC, Pan S, Lien GS, Liao CH, Chen SH, Cheng YS. Deformity of duodenal bulb, gastric metaplasia of duodenal regenerating mucosa and recurrence of duodenal ulcer: a correlated study. World J Gastroenterol. 2005;11:1802-1805. [PubMed] |
| 36. | Alanko J, Riutta A, Holm P, Mucha I, Vapaatalo H, Metsä-Ketelä T. Modulation of arachidonic acid metabolism by phenols: relation to their structure and antioxidant/prooxidant properties. Free Radic Biol Med. 1999;26:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
S- Editor Zhu LH L- Editor Alpini GD E- Editor Liu Y