Published online Jul 7, 2007. doi: 10.3748/wjg.v13.i25.3508
Revised: February 23, 2007
Accepted: March 28, 2007
Published online: July 7, 2007
AIM: To correlate the C/T-13910 variant, associated with lactase persistence/non-persistence (adult-type hypolactasia) trait, with intestinal disaccharidase activities in different age groups of the adult population.
METHODS: Intestinal biopsies were obtained from 222 adults aged 18 to 83 years undergoing upper gastrointestinal endoscopy because of unspecified abdominal complaints. The biopsies were assayed for lactase, sucrase and maltase activities and genotyped for the C/T-13910 variant using PCR-minisequencing.
RESULTS: There was a significant correlation between lactase activity and the C/T-13910 variant (P < 0.00001). The mean level of lactase activity among subjects with C/C-13910 genotype was 6.86 ± 0.35 U/g, with C/T-13910 genotype 37.8 ± 1.4 U/g, and with T/T-13910 genotype 57.6 ± 2.4 U/g protein, showing a trimodal distribution of this enzyme activity. Significant differences were also observed in maltase activities among individuals with different C/T-13910 genotypes (P = 0.005). In contrast, in sucrase activity, no significant differences emerged between the C/T-13910 genotypes (P = 0.14). There were no statistical differences in lactase (P = 0.84), sucrase (P = 0.18), or maltase activity (P = 0.24) among different age groups. In the majority (> 84%) of the patients with the C/C-13910 genotype associated with lactase non-persistence, the lactase activity was less than 10 U/g protein.
CONCLUSION: Our study demonstrates a statistically significant correlation between the C/T-13910 genotype and lactase activity and this correlation is not affected by age in adults but the cut-off value of 20 U/g protein used for the diagnosis of lactase non-persistence might be too high.
- Citation: Enattah NS, Kuokkanen M, Forsblom C, Natah S, Oksanen A, Järvelä I, Peltonen L, Savilahti E. Correlation of intestinal disaccharidase activities with the C/T-13910 variant and age. World J Gastroenterol 2007; 13(25): 3508-3512
- URL: https://www.wjgnet.com/1007-9327/full/v13/i25/3508.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i25.3508
Lactase non-persistence (adult-type hypolactasia) is an autosomal recessive condition resulting from the physiological decline of the lactase-phlorizin hydrolase (LPH) enzyme activity in intestinal cells which occurs in a significant proportion of the global population[1,2]. The age of onset for this down-regulation of the lactase enzyme varies between populations, ranging from 1-2 years of age among the Thais to 10-20 years of age among the Finns[3-5]. However, among Northern Europeans and a few other ethnic groups, LPH activity persists throughout life in the majority of adults, a condition known as lactase persistence. The phenotype lactase persistence/non-persistence is genetically determined, the persistent status being dominant over the non-persistent status[1,3,6].
A single nucleotide polymorphism (SNP), C/T-13910, 14 kb upstream from LCT has been shown to co-segregate with lactase persistence/non-persistence in Finnish pedigrees and in a sample set of 236 individuals from several populations[7]. The genotype C/C-13910 in a homozygous form associates with lactase non-persistence and genotypes C/T-13910 and T/T-13910 with lactase persistence[7]. Importantly, the three genotypes correlate with the level of the lactase activity and lactase/sucrase (L/S) ratio in intestinal biopsy sample specimen[8-11] as well as with the results obtained from the lactose tolerance test (LTT)[12]. A cut point of 20 U/g protein for lactase activity and 0.3 for L/S ratios was the criteria used for the diagnosis of lactase non-persistence in different population studies[17,19,20]. Evidence for the functional role of the C/T-13910 polymorphism as an enhancer has recently been provided giving a support for using the C/T-13910 as a molecular tool for the diagnosis of lactase non-persistence[9-11].
We have recently reported the correlation of the lactase activity and the C/T-13910 genotypes in a pediatric population[13]. However, data regarding the effect of age on disaccharidase activities in adults are still controversial. In animal models (e.g., rats), some studies showed a decline of both lactase and sucrase activities with aging[14,15] while others showed an increase of intestinal lactase activity with aging[16]. The aim of the present study was to investigate the disaccharidase activities in different age groups of adult population and its correlations to the C/T-13910 genetic variant and whether or not the two requirements used for the diagnosis of lactase non-persistence need to be modified based on the molecular findings. A trimodal distribution of lactase activity was observed in the study sample of 222 intestinal biopsies genotyped for the C/T-13910 variant. No statistical differences in disaccharidase activities in relation to age could be found.
We analyzed disaccharidase activities (DSA) of 222 endoscopically obtained intestinal mucosal specimen from adults with abdominal complaints aged between 18-83 years during a 2-year period at the Laboratory of Hospital for Children and Adolescents, Helsinki. Part of the data had been previously reported by Kuokkanen et al[9]. Samples showing sucrase activities < 40 U/g and maltase < 150 U/g, when lactase activity was < 20 U/g, suggesting secondary causes of hypolactasia, were excluded from the study. Only patients with normal mucosal histology with villous height to crypt depth ratio of more than 2:1 were accepted. The study samples were divided into three different age groups, namely 18-39, 40-59 and 60-83 years.
Lactase, maltase and sucrase activities were determined using the method of Dahlqvist[17,18] and were expressed as units of micro-moles of disaccharide hydrolyzed per minute. The assay was carried out as follows: first, the intestinal biopsies were weighted, solubilized in cold NaCL (100 μL/mg tissue) and homogenized on ice for 45 s and centrifuged for 15 min (4500 r/min). Second, supernatants were diluted in cold NaCL, pipetted into a deep well plate, mixed by vortexing and incubated at 37°C for 60 min. Thereafter 300 μL of ice cold glucose oxidase (GO) reagent was added to each well and incubated again at 37°C for 60 min. Third, the absorbance of the samples was measured at the wavelength of 450 nm on a Multiscan spectrophotometer and the activity of the disaccharidases was calculated as units of enzyme activity (U). One unit is defined as the activity of a disaccharidase needed to hydrolyze 1 μmol of dissachride per minute. A cut-off point of 20 U/g protein for lactase activity and 0.3 for L/S ratios was used for adult-type hypolactasia[17,19,20]. Activities of maltase and sucrase were determined in order to exclude patients with secondary lactase deficiency. Low activities of all disaccharidase enzymes are seen when mucosa is injured, in cases such as celiac disease or inflammation, or if the biopsy sample is obtained from a too proximal part of the intestine[21].
DNA was isolated from intestinal biopsy specimens by phenol-chloroform extraction according to standard procedures[22]. The DNA fragment spanning the C/T-13910 variant was amplified using a biotinylated and an unbiotinylated primer (primers available upon request). A 10-μL aliquot of the PCR product was captured in a streptavidin-coated microtitre well (Labsystems, Finland). The wells were washed, and the bound DNA was denatured as previously described[23]. Typically, 50 μL of the minisequencing reaction mixture contained 10 pmoles of the minisequencing primers for C/T-13910 and 0.1 μL of either 3H-dCTP corresponding to the lactase non-persistence allele (Amersham, UK) or 3H-dTTP corresponding to the lactase persistence allele, and 0.05 units of DNA polymerase (Dynazyme II, Finnzymes). The microtitre plates were incubated for 20 min at 50°C, and the wells were washed. The detection primer was eluted, and the eluted radioactivity was measured in a liquid scintillation counter (Rackbeta 1209, Wallac, Finland). Two parallel minisequencing reactions were carried out for each PCR product.
Frequency differences were analyzed with Pearson's chi-squared test. All statistical analysis was performed using the BMDP statistical package (BMDP Statistical Software, Los Angeles, California, USA). Differences between groups were assessed by the non-parametric Kruskal-Wallis test. P values less than 0.05 were considered statistically significant. All variables were expressed as mean ± standard errors (SE).
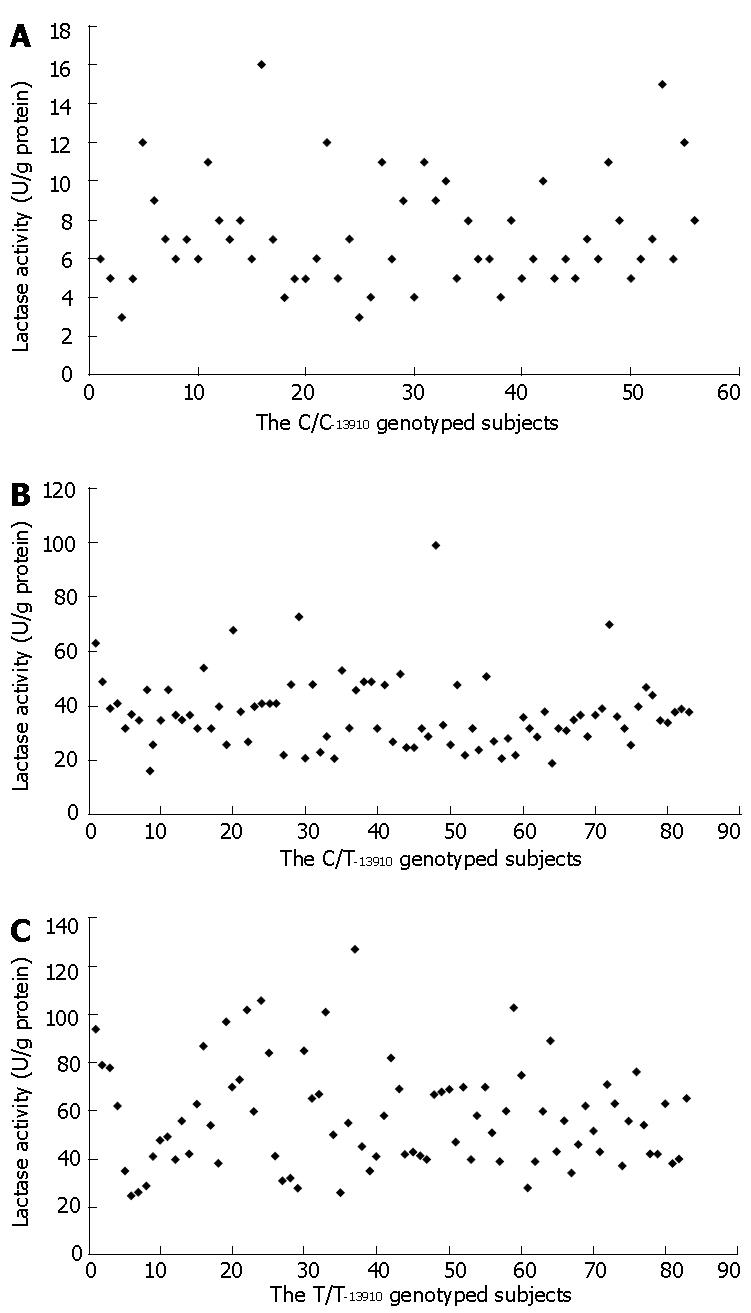
A clear trimodal distribution of the lactase activity was demonstrated in a total of 222 intestinal biopsy sample specimens analyzed for the C/T-13910 genotype (Table 1). There was a statistically significant correlation between lactase activity and the three genotypes: T/T-13910 homozygosity was associated with the highest lactase activity, whereas the heterozygous C/T-13910 genotype was associated with an intermediate activity and the C/C-13910 genotype with the lowest activity (P value for differences between the groups < 0.00001). The C/C-13910 genotype was associated with the very low lactase activity (< 10 U/g protein) in the distinct majority of adults tested. Only 14% of the patients with the C/C-13910 genotype had lactase activities between 11-15 U/g protein, and in only one sample, the lactase activity was 16 U/g protein with L/S ratio of 0.13 (Figure 1A). In all the patients with the C/C-13910 genotype with the lactase activities above 10 U/g protein, the L/S ratio was between 0.09-0.18.
| Disaccharidase | n | Genotype | Total 222 | P | ||
| C/C-13910 | C/T-13910 | T/T-13910 | ||||
| 56 | 83 | 83 | ||||
| Lactase | 6.86 ± 0.35 (3-16) | 37.8 ± 1.4 (21-99) | 57.6 ± 2.4 (20-127) | 37.4 ± 1.7 (3-127) | < 0.00001 | |
| Sucrase | 77.2 ± 3.7 (40-172) | 87.2 ± 3.8 (35-240) | 83.2 ± 4,3 (24-260) | 83.2 ± 2.4 (24-260) | 0.14 | |
| Maltase | 287.7 ± 13.2 (159-599) | 342.0 ± 13.5 (161-868) | 305.5 ± 14.5 (140-887) | 314.6 ± 8.2 (140-887) | 0.005 | |
| L/S ratio | 0.09 ± 0.005 (0.03-0.2) | 0.46 ± 0.02 (0.2-1.6) | 0.73 ± 0.02 (0.25-1.35) | 0.47 ± 0.02 (0.03-1.6) | < 0.00001 | |
No significant correlation between sucrase activity and the three genotypes was detected (P = 0.14). For maltase activities, there was a statistically significant difference according to the genotypes whereby the C/T-13910 genotype was associated with the highest level (342 ± 13.5 U/g), the C/C-13910 genotype with the lowest level (287 ± 13.2 U/g) and the T/T-13910 genotype with the intermediate level (305 ± 14.5 U/g) of maltase activity (P value for differences between the groups = 0.005). Although the C/C-13910 genotype was associated with the lowest level among the three disaccharidases, the C/T-13910 genotype was associated with the highest sucrase and maltase activities (Table 1).
No significant difference in the activities of any of the disaccharidases in relation to age could be demonstrated among 222 samples (Table 2). The mean lactase activity among hypolactasic subjects (C/C-13910) was 6.86 ± 0.35 U/g (range, 1-16 U/g protein), the mean sucrase activity was 77.2 ± 3.7 U/g (versus 84.2 ± 1.5 within lactase persistence subjects), and the mean maltase activity was 287.7 ± 13.2 U/g (Table 1). When analyzing the disaccharidase activities in different age groups within each genotype, no age-related differences in the activities of any of the disaccharidases were found within the three genotypes of the C/T-13910 (Table 3).
| Disaccharidase | n | Age groups (yr) | Total 222 | P | ||
| 18-39 | 40-59 | 60-83 | ||||
| 84 | 94 | 44 | ||||
| Lactase | 35.6 ± 2.4 | 39.3 ± 3 | 37 ± 3.5 | 37.4 ± 1.7 | 0.84 | |
| Sucrase | 77.3 ± 2.8 | 86.6 ± 4.5 | 87.4 ± 4.5 | 83.2 ± 2.4 | 0.18 | |
| Maltase | 298 ± 11 | 326 ± 16 | 324 ± 13 | 315 ± 8 | 0.24 | |
| L/S ratio | 0.48 ± 0.03 | 0.48 ± 0.03 | 0.43 ± 0.04 | 0.47 ± 0.02 | 0.70 | |
| Genotype | Disaccharidase | Age groups (yr) | Total | P | ||
| 18-39 | 40-59 | 60-83 | ||||
| C/C-13910 | n | 20 | 25 | 11 | 56 | |
| Lactase | 7.05 ± 0.49 (6.17-7.55) | 6.84 ± 0.6 (5.6-8.08) | 6.54 ± 0.72 (4.94-8.15) | 6.86 ± 0.35 (6.17-7.55) | 0.74 | |
| Sucrase | 76.6 ± 5.9 (64.2-88.91) | 77.4 ± 6.24 (64.5-90.3) | 78.0 ± 7.72 (60.8-95.2) | 77.2 ± 3.74 (69.7-84.7) | 0.95 | |
| Maltase | 288.2 ± 23.7 (239-338) | 288 ± 20.8 (245-331) | 286.2 ± 23.6 (234-339) | 287.7 ± 13.2 (261-314) | 0.96 | |
| C/T-13910 | n | 32 | 34 | 17 | 83 | |
| Lactase | 34.7 ± 1.7 (31-38) | 39.7 ± 2.9 (34-46) | 39.8 ± 2.2 (35-45) | 37.8 ± 1.4 (35-41) | 0.18 | |
| Sucrase | 80.5 ± 4.3 (72-89) | 87.2 ± 7.3 (72-102) | 99.9 ± 8.1 (83-117) | 87.2 ± 3.8 (80-95) | 0.11 | |
| Maltase | 321 ± 16 (287-354) | 344 ± 27 (290-399) | 377 ± 21 (333-421) | 342 ± 13 (315-369) | 0.08 | |
| T/T-13910 | n | 32 | 35 | 16 | 83 | |
| Lactase | 54.2 ± 3.3 (47.5-60.9) | 62.0 ± 4.2 (53.5-70.5) | 54.9 ± 5.2 (43,8-66) | 57.6 ± 2.4 (52.8-62.4) | 0.42 | |
| Sucrase | 74.5 ± 4.6 (65.1-83,8) | 92.5 ± 8.8 (67.7-93.4) | 80.6 ± 6.03 (67.7-93.4) | 83.2 ± 4.3 (74.6-91.9) | 0.49 | |
| Maltase | 281 ± 17 (246-315) | 334 ± 29 (274-394) | 293 ± 18 (254-331) | 305 ± 15 (277-334) | 0.64 | |
Our data showed that the scattering of the lactase activity values in the T/T-13910 genotype was wider than in the C/T-13910 genotype but the values observed overlapped for these genotypes (Figures 1B and C). However, when a large study sample was assessed as a whole, a tendency toward intermediate lactase activity for the heterozygous genotype C/T-13910 was observed (Table 1).
Disaccharidases are intestinal microvilli membrane hydrolases that play an important role in the carbohydrate digestion. The relationship and the pattern of intestinal disaccharidase activities with aging are poorly understood[14,16,24]. It has been claimed that the prevalence of lactose malabsorption, based on the breath hydrogen test, increases with age secondary to hypochlorhydria[25,26]. Thus, an abnormal result on a breath hydrogen test might actually not reflect carbohydrate malabsorption but rather simply an exposure of a carbohydrate load to the bacteria residing in the small intestine, where fermentation and gas formation take place under the influence of the normal lactase activity. Here, we also observed a tendency for higher disaccharidase activities among those humans > 70 years of age when compared to younger age groups. This could result from a higher turnover rate of intestinal epithelial cells with increasing age.
Our study suggests that the cut-off value of 20 U/g used for the diagnosis of hypolactasia might be too high. The C/C-13910 genotype was associated with a very low lactase activity (< 10 U/g protein) in the distinct majority of adults tested. This is in agreement with our recent study in children in which the majority of the children with C/C-13910 genotype tested after the age of eight years (40/43) had lactase activities below 10 U/g protein[13]. Furthermore, all the samples with the lactase activities above 10 U/g proteins had also L/S ratios below 0.18. This result means that the two requirements which were used for the diagnosis of adult-type hypolactasia (lactase activity below 20 U/g protein and L/S ratios below 0.3) were too conservative. For example, setting the requirement for lactase activity to below 10 U/g protein and keeping the L/S ratios to below 0.3 do not affect the sensitivity of the results and might be used for the diagnosis of adult-type hypolactasia.
Some overlap values of the lactase activity between the genotypes C/T-13910 and T/T-13910 were detected in this study; and as expected, the overlap disappears when a large number of samples analyzed as the regress to the mean feature of the disaccharidase activities become evident giving two separate peaks corresponding to both genotypes. This observation emphasizes the sensitivity of the assay for the activity measurements and the biopsy technique[26]. Inter- and intra-individual variation in the lactase activity was observed in multiple studies and was influenced by a subtle disease status, also by variations in the applied enzyme assay techniques[24,28-31].
The results obtained in this study provide evidence that lactase activity is not dependent on age among the adult population and confirm our previous findings that the C/T-13910 variant is associated with lactase persistence/non-persistence trait[7,9,13]. In addition, we proposed that in the majority of adult population with C/C-13910 genotype associated with lactase non-persistence, the lactase enzyme activity was less than 10 U/g protein, suggesting that the cut-off value of 20 U/g used for the diagnosis of hypolactasia might be too high.
| 1. | Sahi T, Isokoski M, Jussila J, Launiala K, Pyörälä K. Recessive inheritance of adult-type lactose malabsorption. Lancet. 1973;2:823-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 85] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Sahi T. Hypolactasia and lactase persistence. Historical review and the terminology. Scand J Gastroenterol Suppl. 1994;202:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Sahi T. The inheritance of selective adult-type lactose malabsorption. Scand J Gastroenterol Suppl. 1974;30:1-73. [PubMed] |
| 4. | Sahi T, Launiala K. Manifestation and occurrence of selective adult-type lactose malabsorption in Finnish teenagers. A follow-up study. Am J Dig Dis. 1978;23:699-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Wang Y, Harvey CB, Hollox EJ, Phillips AD, Poulter M, Clay P, Walker-Smith JA, Swallow DM. The genetically programmed down-regulation of lactase in children. Gastroenterology. 1998;114:1230-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 74] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Sahi T. Genetics and epidemiology of adult-type hypolactasia. Scand J Gastroenterol Suppl. 1994;202:7-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 163] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Enattah NS, Sahi T, Savilahti E, Terwilliger JD, Peltonen L, Järvelä I. Identification of a variant associated with adult-type hypolactasia. Nat Genet. 2002;30:233-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 740] [Cited by in RCA: 686] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 8. | Enattah NS, Forsblom C, Rasinperä H, Tuomi T, Groop PH, Järvelä I. The genetic variant of lactase persistence C (-13910) T as a risk factor for type I and II diabetes in the Finnish population. Eur J Clin Nutr. 2004;58:1319-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Kuokkanen M, Enattah NS, Oksanen A, Savilahti E, Orpana A, Järvelä I. Transcriptional regulation of the lactase-phlorizin hydrolase gene by polymorphisms associated with adult-type hypolactasia. Gut. 2003;52:647-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 132] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Olds LC, Sibley E. Lactase persistence DNA variant enhances lactase promoter activity in vitro: functional role as a cis regulatory element. Hum Mol Genet. 2003;12:2333-2340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 157] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Troelsen JT, Olsen J, Møller J, Sjöström H. An upstream polymorphism associated with lactase persistence has increased enhancer activity. Gastroenterology. 2003;125:1686-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 128] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Nilsson TK, Johansson CA. A novel method for diagnosis of adult hypolactasia by genotyping of the -13910 C/T polymorphism with Pyrosequencing technology. Scand J Gastroenterol. 2004;39:287-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Rasinperä H, Savilahti E, Enattah NS, Kuokkanen M, Tötterman N, Lindahl H, Järvelä I, Kolho KL. A genetic test which can be used to diagnose adult-type hypolactasia in children. Gut. 2004;53:1571-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Holt PR, Heller TD, Richardson AG. Food restriction retards age-related biochemical changes in rat small intestine. J Gerontol. 1991;46:B89-B94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Bernard A, Caselli C, Blond JP, Carlier H. Diet fatty acid composition, age and rat jejunal microvillus enzyme activities. Comp Biochem Physiol Comp Physiol. 1992;101:607-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Raul F, Gosse F, Doffoel M, Darmenton P, Wessely JY. Age related increase of brush border enzyme activities along the small intestine. Gut. 1988;29:1557-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Dahlqvist A. Assay of intestinal disaccharidases. Scand J Clin Lab Invest. 1984;44:169-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 181] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Messer M, Dahlqvist A. A one-step ultramicro method for the assay of intestinal disaccharidases. Anal Biochem. 1966;14:376-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 310] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Jussila J, Launiala K, Gorbatow O. Lactase deficiency and a lactose-free diet in patients with "unspecific abdominal complaints". Acta Med Scand. 1969;186:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Arola H. Diagnosis of hypolactasia and lactose malabsorption. Scand J Gastroenterol Suppl. 1994;202:26-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 97] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Sambrook J, Maniatis T. Molecular cloning: A laboratory manual. 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 1989;. |
| 22. | Kolho KL, Savilahti E. Ethnic differences in intestinal disaccharidase values in children in Finland. J Pediatr Gastroenterol Nutr. 2000;30:283-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Syvänen AC, Sajantila A, Lukka M. Identification of individuals by analysis of biallelic DNA markers, using PCR and solid-phase minisequencing. Am J Hum Genet. 1993;52:46-59. [PubMed] |
| 24. | Welsh JD, Poley JR, Bhatia M, Stevenson DE. Intestinal disaccharidase activities in relation to age, race, and mucosal damage. Gastroenterology. 1978;75:847-855. [PubMed] |
| 25. | Di Stefano M, Veneto G, Malservisi S, Strocchi A, Corazza GR. Lactose malabsorption and intolerance in the elderly. Scand J Gastroenterol. 2001;36:1274-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Goulding A, Taylor RW, Keil D, Gold E, Lewis-Barned NJ, Williams SM. Lactose malabsorption and rate of bone loss in older women. Age Ageing. 1999;28:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Poulter M, Hollox E, Harvey CB, Mulcare C, Peuhkuri K, Kajander K, Sarner M, Korpela R, Swallow DM. The causal element for the lactase persistence/non-persistence polymorphism is located in a 1 Mb region of linkage disequilibrium in Europeans. Ann Hum Genet. 2003;67:298-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 89] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Duncan A, Park RP, Lee FD, Russell RI. A retrospective assessment of the clinical value of jejunal disaccharidase analysis. Scand J Gastroenterol. 1994;29:1111-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Wallis JL, Lipski PS, Mathers JC, James OF, Hirst BH. Duodenal brush-border mucosal glucose transport and enzyme activities in aging man and effect of bacterial contamination of the small intestine. Dig Dis Sci. 1993;38:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Heitlinger LA, Rossi TM, Lee PC, Lebenthal E. Human intestinal disaccharidase activities: correlations with age, biopsy technique, and degree of villus atrophy. J Pediatr Gastroenterol Nutr. 1991;12:204-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Welsh JD, Stevenson DE, Poley JR, Walker AW. Intestinal alkaline phosphatase activity in relation to age in humans. J Pediatr Gastroenterol Nutr. 1985;4:954-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
S- Editor Liu Y L- Editor Kumar M E- Editor Lu W