Published online Jun 7, 2007. doi: 10.3748/wjg.v13.i21.3016
Revised: March 16, 2007
Accepted: March 26, 2007
Published online: June 7, 2007
Yttrium-90 (Y-90) radioembolization, also known as selective internal radiation therapy (SIRT), is a regional hepatic therapy used in the treatment of unresectable colorectal cancer (CRC) liver metastases. In SIRT, Y-90 impregnated microspheres are injected into the VASCULAR SUPPLY of hepatic tumor, leading to selective irradiation and necrosis of tumor TISSUE. While several studies demonstrate improved local control and survival with SIRT, the specific indications for this therapy have yet to be defined. Typically, SIRT is given in combination with chemotherapy as multimodal treatment for unresectable hepatic CRC. However, it HAS ALSO FOUND INCREASING USE as a salvage therapy in chemo-refractory patients. Herein, the authors describe their experience with SIRT as “stand alone” therapy in a surgically-prohibitive, chemotherapy naive patient with hepatic CRC metastasis. The results suggest that Y-90 SIRT may have potential applications beyond its usual role as a palliative or salvage therapy for unresectable hepatic CRC.
- Citation: Garrean S, Muhs A, Bui JT, Blend MJ, Owens C, Helton WS, Espat NJ. Complete eradication of hepatic metastasis from colorectal cancer by Yttrium-90 SIRT. World J Gastroenterol 2007; 13(21): 3016-3019
- URL: https://www.wjgnet.com/1007-9327/full/v13/i21/3016.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i21.3016
Surgical resection is the only potentially curative strategy in the treatment OF PATIENTS WITH HEPATIC COLORECTAL CANCER (CRC) METASTASIS. Unfortunately, due to advanced liver disease or widespread extrahepatic metastases, only about 10%-15% of patients are candidates for resection. FURTHER, of those who are deemed appropriate surgical candidates and undergo hepatectomy, the overwhelming majority develop recurrent DISEASE that IS not amenable to re-resection[1]. Clearly, other liver-directed therapies are needed to treat unresectable malignancy and to improve the overall response to surgery.
Recently, data have emerged supporting the use of Yttrium-90 selective internal radiation therapy (SIRT) in the treatment of unresectable CRC liver metastases[2-4]. SIRT is a regional, liver-directed therapy based on the principle that hepatic tumors derive their arterial blood supply predominantly from the systemic circulation rather than the portal vein. In SIRT, the pure beta-emitting isotope Y-90 is compounded onto millions of microspheres that are injected into the hepatic artery or one of its branches. The radioactive microspheres deposit in the feeding vasculature of the tumor, resulting in the delivery of intense local radiation to tumor but relative sparing of normal liver parenchyma.
Y-90 SIRT is a safe and effective regional therapy for single or multiple unresectable hepatic colorectal metastases. The principal limitation to SIRT is excessive hepatopulmonary shunt (> 18%), though this occurs rarely in metastatic hepatic disease. In general, SIRT is well-tolerated by patients, with limited duration (24-96 h) side effects inclusive of fatigue, anorexia, nausea, and vomiting. Major complications occur occasionally, and are not SIRT-treatment dependent but RATHER associated with the percutaneous arterial access as with ANY other selective hepatic embolization.
SIRT has been evaluated AS an adjuvant to systemic or hepatic artery chemotherapy and AS salvage therapy in chemo-refractory patients with unresectable hepatic CRC[5,6]. While the data show improved response rates and prolonged survival, there is CURRENTLY no consensus currently on the exact place of Y-90 SIRT in the treatment algorithm for CRC hepatic metastases. The following report highlights the novel use of Y-90 SIRT as “stand alone” therapy in a chemotherapy naive patient with hepatic CRC metastasis.
A 68-year-old man with previously resected hepatic flexure colorectal cancer presented with a single 1.5 cm lesion in medial segment VII of the liver. The lesion was detected on PET scan performed during routine oncologic follow-up 9 mo after primary colon resection. The patient was referred to the hepatobiliary surgery clinic for consideration of possible resection or ablative therapy. Due to the lesion location and the patient’s explicit desire not to receive systemic chemotherapy, treatment options were limited to right hemihepatectomy or RFA ablation. However, the patient had undergone three previous laparotomies and had multiple medical co-morbidities (obesity with BMI > 40, hypertension, and cane-assisted ambulation). Furthermore, his tumor was located in the medial aspect of the right hemi-liver, necessitating a right hemihepatectomy. As the patient did not wish to proceed with this procedure, resection was not an option. Further limit of the potential treatment options was the poor ultrasound visualization of the lesion due to the patients’ body habitus, which precluded the use of percutaneous RFA. Consequently, Y-90 SIRT was offered as an alternate treatment strategy, with the recommendation that the patient receive adjuvant systemic chemotherapy after SIRT. The patient agreed to the treatment plan and subsequently underwent pre-therapy imaging.
Celiac and mesenteric angiography was performed from a standard femoral artery approach using a 5 Fr catheter and 3 Fr coaxial microcatheter (Renegade High-Flow; Boston Scientific, Natick, MA). Normal hepatic arterial anatomy was noted and coil embolization of the right gastric and gastroduodenal arteries was performed in order to prevent reflux of microspheres into the gastrointestinal circulation. Following angiography, the patient underwent a technetium-99m labeled macroaggregated albumin (MAA) scanning, which showed an acceptable hepato-pulmonary shunt fraction of only 2.4%.
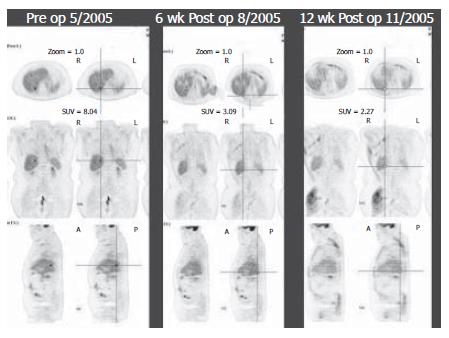
After completion of pre-therapy planning, the patient underwent Y-90 SIRT. In July 2005, he received a single dose of 1 GBq (about 27 mCi) Y-90 resin microspheres into the right hepatic artery. He tolerated the procedure without complication and was discharged from the hospital the following day. Initial pre-therapy PET scan showed a single 1.5cm focus in the right hepatic lobe with an SUV of 8.04. At 6 wk after treatment, repeated PET scan demonstrated a reduction of SUV to 3.09. PET scan at 12 wk showed complete disappearance of the lesion (Figure 1). The patient remains alive and well at 18 mo of follow-up, with no evidence of hepatic recurrence on repeated PET scan.
Surgical resection is the treatment of choice in SELECT patients with favorable CRC hepatic tumors. Unfortunately, only 5-10% of patients qualify for resection; moreover, recurrence rates are high among those who undergo surgery[7]. As TRANSPLANTATION is not considered an option in metastatic liver DISEASE, other modalities must be considered.
Neoadjuvant chemotherapy is currently employed in some institutions to downsize hepatic CRC tumors and facilitate subsequent resection[8]. In addition, ablative modalities like RFA are occasionally used to expand the limits of resection in SELECT tumors that are too large to be encompassed by a surgical approach alone[9]. For patients in whom resection is precluded despite the aforementioned strategies, adjuvant systemic chemotherapy has become the mainstay of treatment. Likewise, systemic chemotherapy has become standard treatment for prevention of post-resection recurrence after hepatic metastectomy. Despite the development of new chemotherapeutic regimens, response and survival following systemic treatment ALONE remain dismal.
Recently, hepatic arterial chemotherapy (HAC) has been introduced into the algorithm of hepatic CRC tumor management. Several lines of evidence support the use of HAC alone or in combination with systemic chemotherapy as primary or second-line treatment for unresectable hepatic CRC. A randomized clinical trial comparing HAC to systemic chemotherapy in a previously untreated cohort of patients demonstrated significantly better response, survival, and time to hepatic progression with HAC[10]. Similarly, phaseItrials show favorable response rates when HAC is used in combination with systemic chemotherapy in the second-line setting[11,12]. Additionally, combined HAC and systemic chemotherapy results in superior overall and progression-free survival in patients who have previously undergone hepatic metastectomy[13].
Despite these advances, the prognosis of patients with unresectable hepatic CRC remains grim at best. The introduction of Y-90 SIRT, however, represents a significant improvement in the management of unresectable hepatic CRC. Y-90 SIRT is a versatile modality that may be used as both an adjunct to potentiate the effects of chemotherapy and as a stand alone option in chemotherapy-refractory disease. Van Hazel et al[2] published a randomized trial in 2004 in which they evaluated the COMBINATION OF SIRT AND THE THEN CURRENT STANDARD OF CHEMOTHERAPY, 5-FU and leucovorin. Twenty-one patients with previously untreated CRC hepatic metastases underwent SIRT with systemic chemotherapy (5-FU/leucovorin) or chemotherapy alone. The combination group had higher response rates and longer time to disease progression than the chemotherapy alone group.
SIRT can also potentiate the effect of regional hepatic arterial chemotherapy. In a randomized trial by Gray et al[3], 74 patients with bilobar hepatic CRC metastases received either hepatic arterial chemotherapy with FUDR alone or HAC with a single injection of SIRT. Response rate, time to disease progression, and survival were all significantly increased in the SIRT plus HAC group. While the studies by Van Hazel and Gray employed chemotherapy regimens that are now outdated, their results are still informative as they illustrate the overall efficacy of SIRT as an adjunct to systemic and regional chemotherapy in hepatic CRC. New studies are already in progress to assess the potential of SIRT with modern chemotherapeutic drugs (i.e. oxaliplatin and irinotecan) and biological agents (i.e. Cetuximab, antiepidermal growth factor receptor monoclonal antibody)[6].
Not only does SIRT play a role in multimodal therapy, it may also be used as a salvage modality for chemorefractory metastases[4,6]. Kennedy et al[4] recently published the results of a large, multi-institutional series of patients with chemorefractory liver metastases. They noted encouraging tumor response rates based on CT, PET, and CEA levels. Interestingly, the maximum response occurred at approximately 3 mo after treatment; in our patient, complete regression of lesion was also noted at 3 mo of follow-up PET scan.
As SIRT burns no bridges with other modalities, it may actually be effective at multiple points in the algorithm of CRC hepatic tumor management. In addition to palliative and salvage therapy, SIRT may have applicability as a “neoadjuvant” or stand alone treatment in highly selected patients with hepatic CRC. INDEED Y-90 internal radiation has already been explored as a bridge to ablation, resection, or transplantation in patients with primary hepatocellular cancer[14-17]. The limited but promising experience in HCC suggests a need for further investigation of this indication in hepatic metastases.
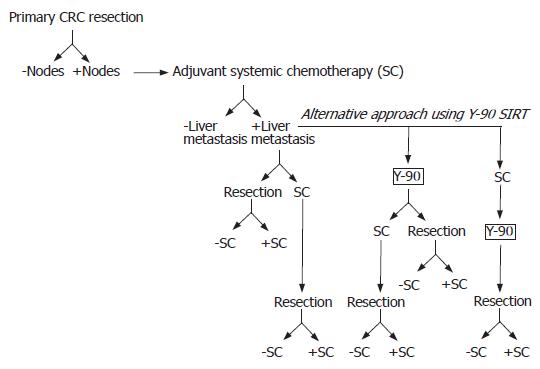
In this report, treatment of metastatic hepatic CRC with Y-90 radioembolization alone resulted in resolution of a single 1.5 cm lesion in the right hepatic lobe on PET imaging. While only anecdotal, these results support a broader role for SIRT in the management of unresectable hepatic CRC. Y-90 could be considered as a possible stand alone therapy in patients with a small, single hepatic focus of metastatic CRC who are not surgical candidates and do not wish to undergo standard systemic chemotherapy. Alternatively, Y-90 could serve as an effective adjuvant therapy to decrease or stabilize tumor bulk before undergoing standard systemic chemotherapy, lesion ablation, or liver resection (Figure 2). Larger series and formal clinical trials are needed to define the optimal indications for SIRT in hepatic CRC management.
S- Editor Wang J L- Editor Ma JY E- Editor Zhou T
| 1. | McCarter MD, Fong Y. Metastatic liver tumors. Semin Surg Oncol. 2000;19:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Van Hazel G, Blackwell A, Anderson J, Price D, Moroz P, Bower G, Cardaci G, Gray B. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol. 2004;88:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 311] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 3. | Gray B, Van Hazel G, Hope M, Burton M, Moroz P, Anderson J, Gebski V. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol. 2001;12:1711-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 370] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 4. | Kennedy AS, Coldwell D, Nutting C, Murthy R, Wertman DE, Loehr SP, Overton C, Meranze S, Niedzwiecki J, Sailer S. Resin 90Y-microsphere brachytherapy for unresectable colorectal liver metastases: modern USA experience. Int J Radiat Oncol Biol Phys. 2006;65:412-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 268] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 5. | Garrean S, Joseph Espat N. Yttrium-90 internal radiation therapy for hepatic malignancy. Surg Oncol. 2005;14:179-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Welsh JS, Kennedy AS, Thomadsen B. Selective Internal Radiation Therapy (SIRT) for liver metastases secondary to colorectal adenocarcinoma. Int J Radiat Oncol Biol Phys. 2006;66:S62-S73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | D'Angelica M, Fong Y. The Liver. Sabiston textbook of surgery: the biological basis of modern surgical practice, 7th edition. Philadelphia: Elsevier Saunders 2004; 1513-1573. |
| 8. | Bismuth H, Adam R, Lévi F, Farabos C, Waechter F, Castaing D, Majno P, Engerran L. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224:509-520; discussion 520-5222. [PubMed] |
| 9. | Curley SA, Izzo F, Delrio P, Ellis LM, Granchi J, Vallone P, Fiore F, Pignata S, Daniele B, Cremona F. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 886] [Cited by in RCA: 805] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 10. | Kemeny NE, Niedzwiecki D, Hollis DR, Lenz HJ, Warren RS, Naughton MJ, Weeks JC, Sigurdson ER, Herndon JE, Zhang C. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol. 2006;24:1395-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 295] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 11. | Kemeny N, Gonen M, Sullivan D, Schwartz L, Benedetti F, Saltz L, Stockman J, Fong Y, Jarnagin W, Bertino J. Phase I study of hepatic arterial infusion of floxuridine and dexamethasone with systemic irinotecan for unresectable hepatic metastases from colorectal cancer. J Clin Oncol. 2001;19:2687-2695. [PubMed] |
| 12. | Kemeny N, Jarnagin W, Paty P, Gönen M, Schwartz L, Morse M, Leonard G, D'Angelica M, DeMatteo R, Blumgart L. Phase I trial of systemic oxaliplatin combination chemotherapy with hepatic arterial infusion in patients with unresectable liver metastases from colorectal cancer. J Clin Oncol. 2005;23:4888-4896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 162] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Kemeny N, Huang Y, Cohen AM, Shi W, Conti JA, Brennan MF, Bertino JR, Turnbull AD, Sullivan D, Stockman J. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999;341:2039-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 646] [Article Influence: 23.9] [Reference Citation Analysis (10)] |
| 14. | Lau WY, Ho SK, Yu SC, Lai EC, Liew CT, Leung TW. Salvage surgery following downstaging of unresectable hepatocellular carcinoma. Ann Surg. 2004;240:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Kulik LM, Atassi B, van Holsbeeck L, Souman T, Lewandowski RJ, Mulcahy MF, Hunter RD, Nemcek AA, Abecassis MM, Haines KG. Yttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J Surg Oncol. 2006;94:572-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 254] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 16. | Kim DY, Kwon DS, Salem R, Ma CK, Abouljoud MS. Successful embolization of hepatocelluar carcinoma with yttrium-90 glass microspheres prior to liver transplantation. J Gastrointest Surg. 2006;10:413-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Kulik LM, Mulcahy MF, Hunter RD, Nemcek AA, Abecassis MM, Salem R. Use of yttrium-90 microspheres (TheraSphere) in a patient with unresectable hepatocellular carcinoma leading to liver transplantation: a case report. Liver Transpl. 2005;11:1127-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |