INTRODUCTION
Inflammatory bowel diseases (IBD), Crohn’s disease and ulcerative colitis, are chronic inflammatory disorders of the gastrointestinal tract which withstand strong influence from both genetic and environmental factors[1]. A dysfunctional mucosal barrier combined with an aberrant mucosal tolerance to pathogens and/or components of the endogenous intestinal flora, is suggested to be an important cause of IBD.
The chronic inflammation that is a hallmark of IBD results from the recruitment and activation of immune cells from the circulation. These in turn release pro-inflammatory cytokines locally in the submucosa, including members of the interleukin (IL) family and tumour necrosis factor-alpha (TNFα), which play a key role in the pathogenesis of IBD[2,3]. Cytokine signalling leads to the activation of two major inflammatory signalling pathways, the nuclear factor kappa B (NF-κB) and mitogen activated protein kinase (MAPK) pathways, which both have been found to be active in mucosal biopsy specimens from patients with IBD[4,5]. As expected, these signalling pathways are important down-stream targets for current therapies including steroids and immunomodulating drugs such as azathioprine and 6-mercaptopurine. However, these current therapies are also associated with side effects and accordingly, there is a great need for alternative remedies.
Dietary fibres are complex carbohydrates, which serve as substrates for anaerobic fermentation by bacteria in the colon. Fermentation gives rise to three major luminal Short-Chain Fatty Acids (SCFAs): acetate, propionate, and butyrate[6]. SCFAs readily reach millimolar concentrations in the colonic lumen[7,8] from which they are absorbed by passive and active transport over the epithelium[7]. Butyrate is considered to be the preferred source of energy for colonocytes[9], and is to a major extent metabolized by these cells[7,10,11]. Butyrate is, by far, the most extensively studied SCFA, and its anti-inflammatory capacity is well documented both in vitro and in vivo. Importantly, several clinical studies demonstrate beneficial effects of butyrate in IBD[12-14]. The exact mechanism of action of butyrate on inflammation is only partially understood. Recently, several reports have been published describing inhibitory effects of butyrate on NF-κB, one of the key transcription factors regulating genes implicated in innate immunity, cell cycle control and apoptosis[15,16].
The production of acetate, propionate and butyrate occurs in the molar ratio of approximately 60: 20: 20[17]. Thus, acetate can be considered the predominant SCFA in the colon. However, with regard to inflammation, little is known about acetate and propionate relative to the well-established anti-inflammatory properties of butyrate. The purpose of this study was therefore to investigate and compare the in vitro anti-inflammatory properties of SCFAs in model systems relevant to IBD.
MATERIALS AND METHODS
Cell culture
Human neutrophils were isolated from whole blood of healthy donors through density gradient separation with PolymorphprepTM according to the manufacturer's instructions (AXIS-SHIELD PoC AS, Oslo, Norway). Briefly, fresh citrate-anticoagulated blood was layered over an equal volume of PolymorphprepTM and centrifuged at 500 ×g for 30 min. The granulocyte fraction was selected and washed with PBS (Sigma) followed by removal of erythrocytes through hypotonic lysis. Immunophenotyping of the isolated cells was done with a FACSCalibur flow cytometer (BectonDickinson, Erembodegem, Belgium). Greater than 95% of the cells were identified as neutrophils, confirmed by side and forward scatter diagrams; CD66b positivity (Acris, Hiddenhausen, Germany) and CD14 negativity (Pharmingen, San Diego, CA, USA). The neutrophils were cultured in RPMI 1640 (Invitrogen) supplemented with 0.5% fetal bovine serum (FBS; Nordic Biolabs AB) and 1% penicillin/streptomycin (PEST; Invitrogen) in 12-well plates at 37°C in 5% CO2. Culture viability was 98.1% determined by a Cedex HiRes Analyzer (Innovatis AG, Bielefeld, Germany).
Colo320DM cells (American Type Culture Collection, Rockville, MD, USA) were cultured in RPMI 1640 supplemented with 10% FBS and 1% PEST.
Transfection and luciferase assay
COLO320DM cells, seeded 24 h prior to transfection, were transiently transfected using FuGENE6 transfection agent (Roche) and 0.5 μg/mL of a luciferase reporter vector containing three tandem copies of the NF-κB binding consensus sequence fused to firefly luciferase. The cells were co-transfected with 25 ng/mL Renilla luciferase-positive control vector (pRL-SV40: Promega) to normalize for transfection efficiency. Five hours post-transfection the cells were seeded into a 96 well plate (white/clear bottom, Wallac) at a density of 6 × 104 cells/well in assay medium (phenol-free RPMI 1640, 10% FBS dextran/charcoal stripped serum and 1% L-Glutamine) containing 10 ng/mL TNFα with or without 1 nmol/L to 100 mmol/L acetate, propionate or butyrate. After 24 h of treatment the cells were lysed and the luciferase activity was measured using the Dual-Luciferase Reporter Assay Systems reagent kit (Promega) in a Viktor luminometer plate reader (Wallac). The firefly luciferase activity was divided by Renilla luciferase activity and presented as per cent inhibition with TNFα treatment alone representing 0% inhibition and assay medium without TNFα as 100% inhibition.
Animals
Female C57BL/6J mice (Harlan Nederlands B.V.) at 7-9 wk of age were used. 5% (w/v) dextran sulphate sodium (DSS, T&D consulting, Uppsala, Sweden) was given in the drinking water for five days in order to induce colonic inflammation. Water was given one day prior to ending the experiment.
All mice were kept under standard conditions of temperature and light, and were fed with standard laboratory chow and water ad libitum. All experiments were approved by the Animal Ethics Committee at Gothenburg University.
Organ culture
Mice were sacrificed and colons were collected and placed in ice-cold Dulbecco's modified Eagle's medium (DMEM). Transverse 1 mm sections were cut longitudinally and placed in 12-well TranswellTM plates (Costar), containing medium with 1% PEST and compounds. Organ cultures were maintained at 37°C in 5% CO2. After six hours the medium was replaced with fresh medium containing compounds. Cultures were then further incubated 18 h, after which medium was collected and frozen until further processing. Tissue was placed in Collection microtubes (Qiagen) containing stainless steel beads (Qiagen) followed by homogenisation in RLT lysis buffer (RNeasy Kit, Qiagen) with a TissueLyser (Qiagen) at 30 Hz for 10 min. Total RNA was isolated according to the manufacturer’s instructions and cDNA was prepared from purified total RNA with the iScript cDNA synthesis kit (Bio-Rad).
Real-time PCR arrays
TaqManTM Low Density mouse immune-arrays (Micro Fluidic Cards, Applied Biosystems) were used to assess the regulation of immune-related genes in organ cultures established from non-inflamed and colitic mouse colon. Samples were assayed in quadruplicate and the expression levels were determined with an ABI Prism 7900HT Sequence Detector (Applied Biosystems). Data was normalized to GAPDH mRNA levels and subsequently expressed as the fold change relative to non-inflamed or colitic vehicle controls.
ELISA
The levels of TNFα, IL-8 or IL-6 in conditioned medium from neutrophil or organ cultures were determined with commercial ELISA kits (human TNFα, BD OptEIA, BD Biosciences; human IL-8, QuantiGlo ELISA, R&D systems; mouse IL-6, Quantikine ELISA, R&D systems), according to the manufacturer’s instructions.
Lactate dehydrogenase activity
Lactate dehydrogenase (LDH) activity was determined with a commercial kit (Cytotoxicity Detection Kit, Roche), according to the manufacturer’s instructions.
Statistical analysis
Values are expressed as mean ± SEM and tested for statistical significance using Student´s t-test.
RESULTS
SCFAs suppress LPS-induced release of TNFα from neutrophils
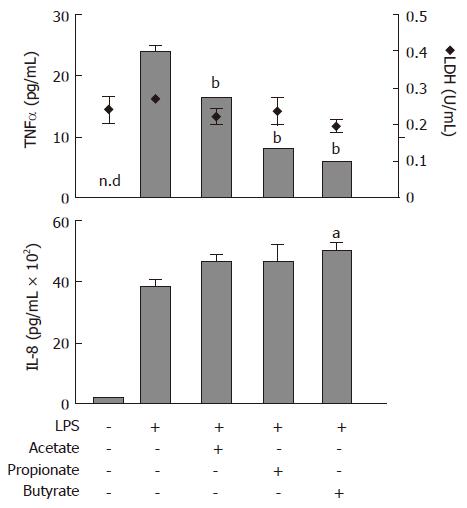
Protein levels of TNFα and IL-8, released from lipopolysaccaride (LPS)-stimulated (1 μg/mL) human neutrophils, were determined with ELISA (Figure 1). Acetate, propionate and butyrate (all 30 mmol/L) decreased LPS-stimulated TNFα release to the culture medium from 24 ± 1 pg/mL to 16 ± 0.1 pg/mL (P < 0.01), 8 ± 0.2 pg/mL (P < 0.01) and 6 ± 0.2 (P < 0.01), respectively (Figure 1; upper panel, bars). The LPS-stimulated increase in IL-8 protein levels from 190 ± 21 pg/mL to 3753 ± 152 pg/mL was not affected by SCFAs (Figure 1; lower panel). Furthermore, SCFAs did not have an effect on LDH activity (Figure 1; upper panel, diamonds).
Figure 1 Effects of Short-Chain Fatty Acids on LPS-stimulated TNFα and IL-8 release, and lactate dehydrogenase (LDH) activity in conditioned medium from neutrophil cultures.
Cells were exposed to LPS (1 μg/mL) concomitant with acetate, propionate or butyrate (all 30 mmol/L) for 6 h followed by analysis of TNFα (upper panel, bars) and IL-8 (lower panel) protein levels and LDH activity (upper panel, diamonds). n.d: not detectable. mean ± SEM (n = 3), aP < 0.05, bP < 0.01.
SCFA-induced inhibition of the NF-κB pathway in Colo320DM cells
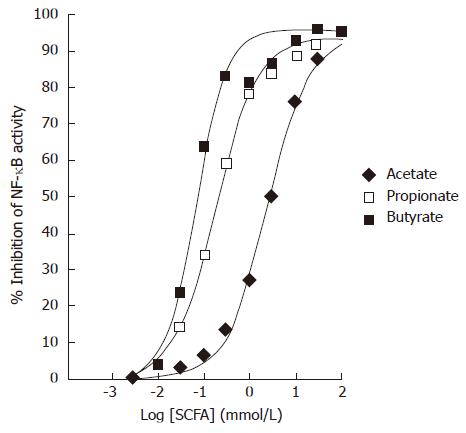
Colo320DM cells were transiently transfected with a NF-κB luciferase reporter. TNFα at 10 ng/mL was used to induce NF-κB activity in the presence of varying concentrations of SCFAs. All SCFAs dose dependently inhibited the TNFα-stimulated NF-κB luciferase activity in Colo320DM cells (Figure 2). The EC50 values for inhibition of NF-κB were as follows: acetate 2.4 mmol/L (diamonds), propionate 120 μmol/L (open squares) and butyrate 64 μmol/L (filled squares). Data points represent the mean of triplicate values.
Figure 2 Effects of Short-Chain Fatty Acids on TNFα-induced NF-κB reporter activity in Colo320DM cells.
Cells were exposed to TNFα (10 ng/mL) along with acetate (diamonds), propionate (open squares) or butyrate (closed squares) for 24 h. Data is presented as the mean % inhibition of NF-κB activity after normalization to Renilla Luciferase (n = 3).
Anti-inflammatory effects of propionate in colon organ cultures
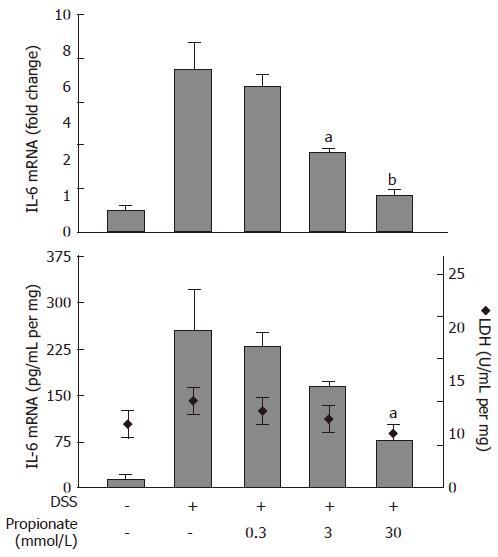
In order to study the anti-inflammatory properties of SCFAs in the context of intestinal inflammation, we established an organ culture protocol where pieces of colitic mouse colon were cultured in vitro in the absence or presence of SCFAs. Exposure of organ cultures to 0.3-30 mmol/L propionate caused a dose dependent decrease in IL-6 mRNA levels from 7.5 ± 1.3 fold (relative to non-inflamed colon) to 1.7 ± 0.2 fold (P < 0.01) at the highest propionate concentration (30 mmol/L) (Figure 3; upper panel). This was paralleled by a decrease in IL-6 protein levels in the culture medium from 253 ± 65 pg/mL per mg to 75 ± 31 pg/mL per mg (30 mmol/L propionate; P < 0.05) (Figure 3; lower panel, bars). Consistent with the results obtained in the previous cell experiments, the effect of propionate was not a result of SCFA-induced cytotoxicity as determined by LDH activity in the culture medium (Figure 3; lower panel, diamonds).
Figure 3 Dose-dependent effects of propionate in organ cultures from inflamed mouse colon.
Dextran sulphate sodium (DSS) was used to induce colonic inflammation. Upper panel: IL-6 mRNA expression (data is shown as changes relative to the expression value obtained in non-inflamed organ cultures). Lower panel: IL-6 protein levels (bars) and lactate dehydrogenase (LDH) activity (diamonds) in culture medium. Organ cultures were exposed to various concentrations of propionate for 24 h. Mean ± SEM (n = 4), aP < 0.05, bP < 0.01.
SCFAs inhibit immune-related gene expression in colon organ cultures
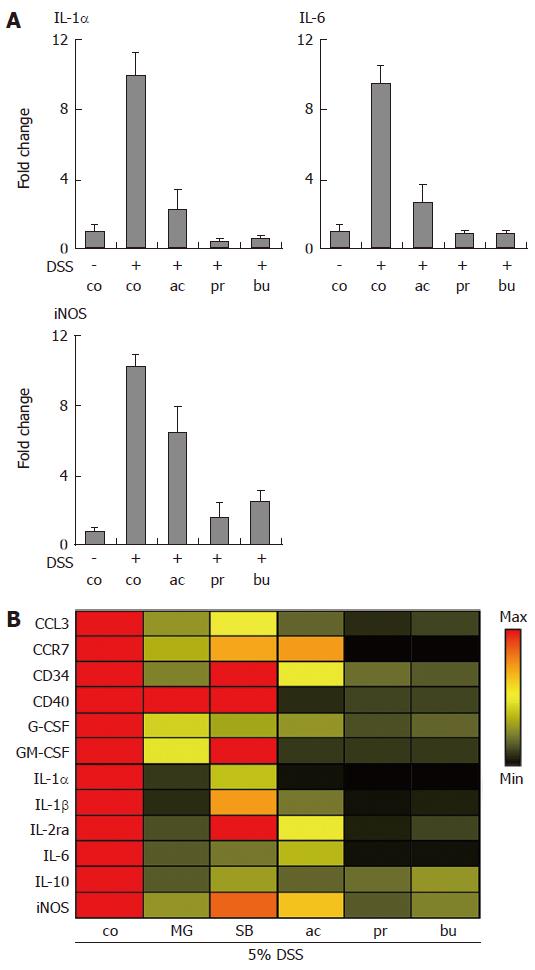
Quantitative Real-time PCR was used to determine the transcriptional regulation of immune-related genes in colon organ cultures (Figure 4). As shown in Figure 4A, IL-1α, IL-6 and inducible nitric oxide synthetase (iNOS) mRNA levels were strongly induced in colon organ cultures established from mice treated with 5% dextran sulphate sodium, compared to non-colitic organ culture. This induction was heavily suppressed by SCFA treatment (all at 30 mmol/L). In order to compare effects of compounds with known anti-inflammatory activity to the immune suppressive effects of SCFAs, the expression levels of twelve selected genes (increased > 3-fold in colitis vs. non-inflamed colon) were compiled as a heat map representation showing the effect of treatments relative to control levels (Figure 4B). MG-132 (a proteasome and NF-κB inhibitor) at 10 μmol/L, acetate, propionate and butyrate (all at 30 mmol/L) potently decreased the mRNA levels of selected gene transcripts. In addition, MG-132 was clearly more potent than SB203580 (a p38 MAPK inhibitor) at suppressing transcription of the selected genes at the same dose (Figure 4B).
Figure 4 Transcriptional profiling of gene expression in Short-Chain Fatty Acid-treated organ cultures.
Dextran sulphate sodium (DSS) was used to induce colonic inflammation. (A) Organ cultures were incubated with acetate, propionate or butyrate (all 30 mmol/L) for 24 h followed by analysis of IL-1α, IL-6 and iNOS mRNA levels. Data was normalized to GAPDH mRNA levels and presented as fold change relative to control levels. Mean ± SEM (n = 4). (B) Heat map representation of gene expression changes induced by MG-132, SB203580 (both 10 μmol/L); acetate, propionate and butyrate (all 30 mmol/L). Data was normalized to GAPDH and expressed as the ratio between treatment and control. control (co), acetate (ac), propionate (pr), butyrate (bu), MG-132 (MG), SB203580 (SB).
SCFAs suppress IL-6 release from colon organ cultures
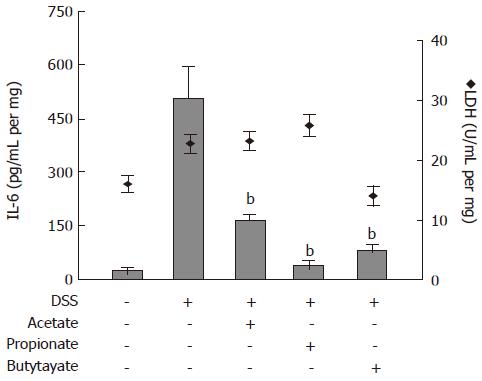
The effects of SCFAs on IL-6 protein release from colon organ cultures were studied (Figure 5). Acetate, propionate and butyrate (all 30 mmol/L) decreased the IL-6 levels in conditioned medium from colon organ cultures from 507 ± 84 pg/mL per mg to 169 ± 16 pg/mL per mg (P < 0.01), 39 ± 14 pg/mL per mg (P < 0.01) and 87 ± 11 pg/mL per mg (P < 0.01), respectively (Figure 5; bars). SCFA-treatment did not cause any significant change in LDH activity (Figure 5; diamonds).
Figure 5 Effects of Short-Chain Fatty Acids on IL-6 release and lactate dehydrogenase (LDH) activity in organ cultures from inflamed mouse colon.
Dextran sulphate sodium (DSS) was used to induce colonic inflammation. Organ cultures were exposed to acetate, propionate or butyrate (all 30 mmol/L) for 24 h followed by analysis of IL-6 level (bars) and LDH activity (diamonds) in culture medium. Mean ± SEM (n = 4), bP < 0.01.
DISCUSSION
SCFAs represent a major constituent of the luminal contents of the colon. Among SCFAs butyrate is believed to play an important role for epithelial homeostasis[18]. Solutions containing butyrate[12-14] or mixtures of SCFAs[19-23] have been used for treatment of active IBD. Unfortunately these studies do not discriminate between actions of individual SCFAs, with the exception of butyrate.
To our knowledge the present study is the first aimed at comparing the effects of acetate, propionate and butyrate on markers of inflammation (i.e., NF-κB activity and cytokine production). The findings of the present study substantiate previous findings, relating to butyrate and its anti-inflammatory properties. For the first time it is also clearly demonstrated that acetate and propionate ameliorate an ongoing inflammatory response at the cellular level and thus acetate and propionate may also contribute to the anti-inflammatory properties of SCFA mixtures in vivo.
Because leukocytes are considered the primary effector cells in IBD, the properties of acetate, propionate and butyrate, were first investigated in human blood-derived neutrophils. Several studies in the past have analysed the effects of SCFAs on neutrophils[24]. Propionic acid has been shown to stimulate superoxide generation in human neutrophils[25] and recently it has been demonstrated that propionate also stimulates neutrophil chemotaxis[26]. However, it is not clear how SCFAs influence the response to inflammatory mediators, such as LPS in neutrophils. We demonstrate that SCFAs strongly inhibit the LPS-stimulated release of TNFα in human neutrophils. Interestingly, our findings also show that SCFAs do not affect LPS-induced release of IL-8, indicating that LPS stimulates TNFα and IL-8 release by separate mechanisms in neutrophils.
The colonic epithelium is considered an important target for the beneficial effects of butyrate[18]. Furthermore, butyrate has anti-inflammatory activity in colon adenocarcinoma cells, which is mediated by inhibition of the NF-κB pathway[15,27]. We therefore next compared the anti-inflammatory actions of acetate, propionate and butyrate on TNFα-induced NF-κB reporter activity in the colon adenocarcinoma cell line Colo320DM. Our results demonstrate that SCFAs inhibit NF-κB activity in Colo320DM cells with the rank order of potency being butyrate > propionate > acetate. Previous studies have suggested that butyrate inhibits the NF-κB pathway by interfering with proteasome-mediated degradation of IκBα in colonocytes[27]. It is possible that acetate and propionate also act via this mechanism.
In animal models of colitis butyrate reduces the ulcer index and myeloperoxidase (MPO) activity[28,29]. The effect of other SCFAs in experimental colitis is less clear. There are, for example, contrasting results of propionate-treatment in trinitrobenzene sulfonic acid (TNBS)-induced colitis, demonstrating either a reduced ulcer index[30], or an aggravated inflammation[31]. In order to compare the effect of different SCFAs on intestinal inflammatory responses in the absence of confounding factors, such as inappropriate uptake or metabolism of SCFAs, we developed an organ culture protocol where colitis is established by administering dextran sulphate sodium to mice in the drinking water. The inflamed colonic tissue is then collected and the effect of anti-inflammatory agents is studied in vitro. A dose finding study with propionate revealed a dose dependent suppression of IL-6 mRNA and protein in colon organ cultures with an IC50 of approximately 1 mmol/L, without any sign of cytotoxicity. Furthermore, acetate and propionate, along with butyrate at 30 mmol/L were shown to reduce the expression levels of selected immune-related genes in colon organ cultures. Acetate was less potent than the two other SCFAs at this dose, a finding in line with the results in neutrophils and in Colo320DM cells. SCFA treatment of organ cultures suppressed a majority of genes decreased also by MG-132, a proteasome and NF-κB inhibitor. Interestingly, at 10 μmol/L MG-132 was far more potent than the p38 MAPK inhibitor SB203580 in suppressing immune-related gene expression, indicating a central role for the NF-κB pathway in DSS-mediated inflammation. Finally, SCFAs potently reduced IL-6 protein release from colon organ cultures with the rank order being propionate > butyrate > acetate.
The mechanism whereby SCFAs mediate their anti-inflammatory effects is currently poorly understood. As discussed above the NF-κB pathway is an important target for butyrate, and possible mechanisms for this could involve inhibition of IκBα kinase activity or proteasome function. Interestingly, our findings in Colo320DM cells suggest a positive correlation between potency and carbon chain length, which may possibly reflect the preferential utilization of larger SCFAs in a specific metabolic pathway. One illustrating example of a component of intermediary metabolism with anti-inflammatory properties is pyruvate which has well-documented immune suppressive activities in vitro and in vivo[32,33]. Thus, it is likely that the metabolism of SCFAs may give rise to metabolites with activity comparable to that of pyruvate. Alternatively, the rank order potency of SCFAs may result from an affinity relationship with a specific receptor. Recently, several independent groups have characterized SCFAs as ligands for the G-protein coupled receptors, GPR41 and GPR43[26,34,35]. The transcript for GPR43 is readily detectable with real-time PCR in neutrophils as well as in the colon (authors unpublished observation), thus it is possible that receptor dependent mechanisms may underlie some of the anti-inflammatory effects of SCFAs described in this study. GPR43 deficient mice may become an important tool for the understanding of SCFA signalling during inflammatory conditions in future studies.
As mentioned before, there are few and inconclusive studies regarding the ameliorating effects of acetate and propionate on inflammation, hence it is difficult to predict the outcome of treatment with these SCFAs in IBD. However, some interesting observations have been published which provide indirect evidence that acetate and propionate may exhibit immune suppressive activity in vivo. Ethanol-intake, which is associated with elevated plasma acetate[36], was recently shown to prevent the development of destructive arthritis in rats[37]. In addition lactulose, a polysaccharide that is rapidly converted into acetate by colonic fermentation[38], ameliorates DSS-induced colitis in mice[39]. Noteworthy is also the observation that elevated systemic levels of propionate, due to mutations in the propionyl-CoA carboxylase enzyme, are associated with progressive immune suppression[40].
In conclusion, the present study demonstrates that acetate and propionate have anti-inflammatory properties, which are comparable to those of butyrate. It is demonstrated for the first time that SCFAs inhibit LPS-induced TNFα release, but not IL-8 secretion, from human blood-derived neutrophils. Furthermore, we show that acetate and propionate, similar to butyrate, inhibit TNFα-mediated activation of the NF-κB pathway in a human colon adenocarcinoma cell line with the rank order of potency being, butyrate > propionate > acetate. Finally anti-inflammatory activity, comparable to that of butyrate, was demonstrated for acetate and propionate in an in vitro model of murine experimental colitis. Taken together these findings suggest that propionate and acetate, in addition to butyrate, could be efficacious in the treatment of inflammatory conditions such as IBD.