Published online May 21, 2007. doi: 10.3748/wjg.v13.i19.2707
Revised: March 14, 2007
Accepted: April 11, 2007
Published online: May 21, 2007
AIM: To accurately and realistically elucidate human stem cell behaviors in vivo and the fundamental mechanisms controlling human stem cell fates in vivo, which is urgently required in regenerative medicine and treatments for some human diseases, a surrogate human-rat chimera model was developed.
METHODS: Human-rat chimeras were achieved by in utero transplanting low-density mononuclear cells from human umbilical cord blood into the fetal rats at 9-11 d of gestation, and subsequently, a variety of methods, including flow cytometry, PCR as well as immunohistochemical assay, were used to test the human donor contribution in the recipients.
RESULTS: Of 29 live-born recipients, 19 had the presence of human CD45+ cells in peripheral blood (PB) detected by flow cytometry, while PCR analysis on genomic DNA from 11 different adult tissues showed that 14 selected from flow cytometry-positive 19 animals possessed of donor-derived human cell engraftment in multiple tissues (i.e. liver, spleen, thymus, heart, kidney, blood, lung, muscle, gut and skin) examined at the time of tissue collection, as confirmed by detecting human β2-microglobulin expression using immunohistochemistry. In this xenogeneic system, the engrafted donor-derived human cells persisted in multiple tissues for at least 6 mo after birth. Moreover, transplanted human donor cells underwent site-specific differentiation into CK18-positive human cells in chimeric liver and CD45-positive human cells in chimeric spleen and thymus of recipients.
CONCLUSION: Taken together, these findings suggest that we successfully developed human-rat chimeras, in which xenogeneic human cells exist up to 6 mo later. This humanized small animal model, which offers an in vivo environment more closely resembling to the situations in human, provides an invaluable and effective approach for in vivo investigating human stem cell behaviors, and further in vivo examining fundamental mechanisms controlling human stem cell fates in the future. The potential for new advances in our better understanding the living biological systems in human provided by investigators in humanized animals will remain promising.
-
Citation: Sun Y, Xiao D, Pan XH, Zhang RS, Cui GH, Chen XG. Generation of human/rat xenograft animal model for the study of human donor stem cell behaviors
in vivo . World J Gastroenterol 2007; 13(19): 2707-2716 - URL: https://www.wjgnet.com/1007-9327/full/v13/i19/2707.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i19.2707
Owing to lack of knowledge on the intrinsic mechanisms regulating stem cell behaviors (i.e. self-renewal, maintenance, proliferation, differentiation, apoptosis and migration/homing), early development, tissue formation and organogenesis, and tissue homeostatic maintenance in humans, currently human beings still face the great difficulties in applying stem cells in regenerative medicine and treatments for human diseases. Heretofore, some progresses have been made in understanding these fundamental mechanisms in vitro and in vivo[1], but at present, much of this kind of knowledge was emerged from the in vitro and in vivo murine models. Moreover, biomedical research in humans is largely restricted to in vitro models that lack the components and complexity of a living organism, whereas the complexity of a biologic network can only be accurately and realistically reproduced by an in vivo system.
Since these findings derived from mice and in vitro human model cannot always be extrapolated to precisely reflect the true situations in human, a preclinically and clinically relevant in vivo humanized animal model, which can and should more realistically imitate as closely as possible the in vivo situations in human, is urgently required for greatly facilitating scientists to elucidate these aforementioned mechanisms within in vivo a xenogeneic setting.
To address these issues, there are active sustaining efforts to pursue developing human/animal xenograft models (including mice, sheep, goats, monkeys and pigs) to in vivo study the related fundmental mechanisms[2-8].
Currently, by taking advantage of the window of opportunity to perform human stem cell (hSC) transplantation during the preimmune stage of development, fetal sheep[5,7] and fetal goat[8] are developed to be a unique and clinically relevant xenograft animal models for assessing the differentiative potential of hSCs in vivo, but from a scientific perspective, large animals are not a suitable model for mechanistic research.
From a scientific perspective, small animal models, such as mice and rats, are the ideal models. In vivo substitute models have been successfully developed in which human cells are transplanted by tail vein injection into the sublethally irradiated immunodeficient mice[9]. The human/mouse xenograft model derived from the immunodeficient mice has proven valuable in studying the biology of human hematopoietic stem cells (hHSCs) in vivo[9]. But the following significant drawbacks greatly limit the utilities of this model for furthering our knowledge of other human adult stem cell (hASC) biology in vivo other than human HSC biology. On the one hand, the multi-organ and multi-lineage engraftment of the transplanted hHSCs was detected in this model[10], but by nature of the transplanted model design, the engraftment levels in other organs other than hematopoietic system were very low, thus preventing from in vivo investigating mechanisms controlling the fates of hASCs residing in other tissues/organs using this model. Moreover, the mouse recipient does not have a normal functioning immune system, in other words, after birth, there is lack of completely normal physiological environment in this model, but the body’s immune system is involved in controlling stem cell fates[7].
Against this background, we took advantage of the proliferative and permissive environment of the developing pre-immune fetus to develop a human/rat xenograft animal model through in utero transplanting human low-density mononuclear cells (hMNCs) from human umbilical cord blood (hUCB) into fetal rats at the appropriate pre-immune stage. This humanized model possess of a completely functional immune system of rats and potential human immune system reconstructed by transplanted human donor-derived cells (data reported in our another upcoming paper).
Pregnant Sprague-Dawley (SD) rats, weighing 250 to 300 g, were supplied by Center of Experimental Animals, Sun Yat-sen University, Guangzhou, China. Animal care and experimentation were performed according to the Study and Ethical Guidelines for Animal Care, handling and termination established by the Subcommittee of Sun Yat-sen University. The present work was approved by the Institutional Ethics Review Committee.
The samples of hUCB were obtained from normal full-term deliveries with informed consent according to guidelines established by the Institutional Ethics Review Committee. hMNCs were separated by using a Ficoll/Hypaque density-gradient centrifugation (P = 1.077 g/mL; Amersham Pharmacia Biotech) according to the manufacturer’s instructions. After being washed with phosphate-buffered saline (PBS), hMNCs were suspended in Iscove’s modified Dulbecco media (IMDM; Gibco Laboratories, Grand Island, NY) for future use.
Human-rat hybrid animals were generated as described by Fleischman and Mintz[11], with minor modifications. Briefly, micropipettes were prepared from capillary tubing with a vertical pipette puller, and then broken by hand with jewelers’ forceps under a dissecting microscope to produce a tip 20-30 gm in diameter with a sharp-pointed bevel. A small cotton plug was inserted in the blunt end, and the sterilized pipette was held in fine plastic tubing attached to a wider mouth tube. The pregnant female rat on d 9-11 of gestation was anesthetized with nembutal, and subsequently, the fetuses were exposed through successive dorsolateral incisions. The tip of the micropipette containing the fresh cell suspension was inserted through the uterine wall into each embryo. Each injection included 5 × 106 fresh hMNCs. Rats were allowed to continue gestation, and following birth, peripheral blood (PB), bone marrow (BM) and other tissue samples from MNC-transplanted rats were obtained at various intervals and analyzed for human cell engraftment using human-specific methodologies. All time points given refer to the time length after birth.
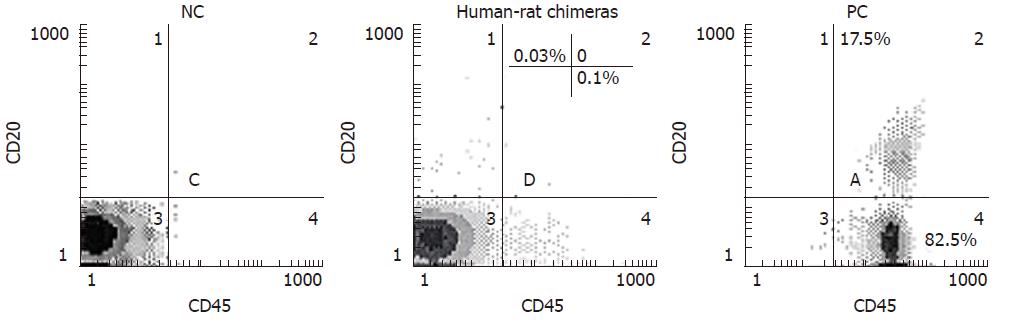
At the indicated time points, 100 μL of PB was collected from the incised tail tip of rat, then collected blood cells were incubated for 20 min at 4°C with both 20 μL of anti-human CD45 PE-Cy5 (PE-Cy5: phycoerythrin-Cy5) and 20 μL of anti-human CD3-FITC (FITC: fluorescein isothiocyanate) or CD20-PE antibodies (Pharmingen), and finally processed for flow cytometric analysis to determine human donor contribution in PB of each rat. At the end of each experiment, MNC-transplanted rats were killed by CO2 inhalation and BM cells were harvested by tibial and femural aspiration with PBS using a syringe. BM cell suspensions were filtered through a cell strainer, and then processed for cytometric analysis to determine human cell engraftment in BM of each rat after labeling with the respective antibodies to various cluster designations directly conjugated with PE, PE-Cy5 or FITC, according to the manufacturer’s recommendation. Furthermore, anti-human CD45, and CD20 monoclonal antibodies chosen here specifically react with human markers CD45, and CD20, thus human CD45+, and CD20+ cells can be easily distinguished from rat CD45, and CD20 hematopoietic cells, respectively. PB from normal rat and healthy human volunteers was used as negative control (NC) and positive control (PC), respectively.
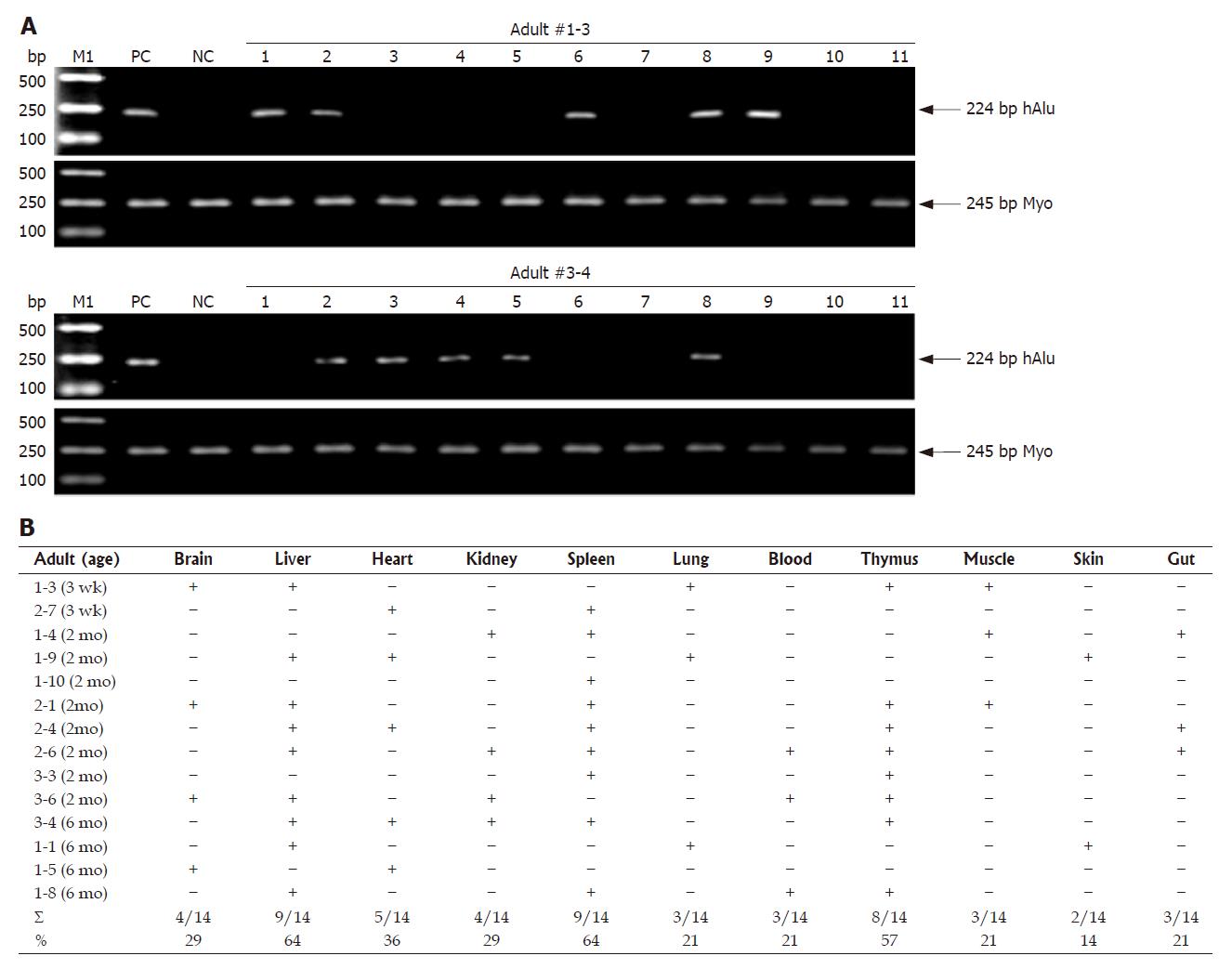
Human donor contribution in multiple tissues prior to immunohistochemical assessment was determined by human gene-specific PCR on genomic DNA prepared with standard protocols[12] from the various tissues of MNC-transplanted rats that were positive for human CD45 cells in PB detected by flow cytometry. At the indicated time points, the following tissues were isolated: brain, gut, heart, kidney, liver, lung, muscle, skin, spleen, stomach and thymus. Half of each tissue was used for genomic DNA isolation, the remaining half was embedded in paraffin. For analysis of human donor engraftment, PCR was performed with primers (Table 1) specific for human Alu repetitive sequence (hAlu) to detect human cells by standard techniques[12] and following the manufacturer’s instructions, while primers (Table 1) specific for myogenin (Myo) gene of human and rat were used for PCR internal control. PC for each PCR used the genomic DNA from human PB of healthy volunteers, while genomic DNA from normal rats was amplified as a reaction control (i.e. NC).
| Gene | Primer name | Primer sequence | Annealing temperature | PCR product size (bp) |
| hAlu | hAlu-FP: | 5'-CTGGGCGACAGAACGAGATTCTAT-3' | 56°C | 224 |
| hAlu-RP: | 5'-CTCACTACTTGGTGACAGGTTCA-3' | |||
| Myo | Myo-FP: | 5’-TTACGTCCATCGTGGACAGC-3’ | 56°C | 245 |
| Myo-RP: | 5’-TGGGCTGGGTGTTAGTCTTA-3’ |
Tissue samples harvested from rats at the indicated time points after birth were immediately excised, and then were fixed in a tube with 100 mL/L formalin. Paraffin sections (4 μm thickness) were prepared on poly-L-lysine–coated slides from formalin-fixed, paraffin-embedded tissues. To facilitate antigen detection, slides were heated in 10 mmoL/L Na-citrate buffer (pH 6.0) at 95°C for 20 min, and then cooled at room temperature. Envision system was used for the immunohistochemical analysis. Human cells in the aforementioned tissues were detected with antibodies for β2-microglobulin (Neomarkers), cytokeratin 18 (CK18) (DAKO) and CD45 (DAKO), respectively. The complex was visualized with DAB and counterstained with hematoxylin. Moreover, anti-human β2-microglobulin, CK18 and CD45 antibodies used as the primary antibody which specifically react with human β2-microglobulin, CK18 and CD45, respectively.
Essential for better understanding hSC regulation in vivo is an in vivo model which permits human donor cells to participate in the generation of a wide variety of tissue-specific human cells within recipients, and further tissue formation and organogenesis and tissue homeostatic maintenance of recipients under normal physiological conditions.
These reasons greatly encourage us to develop a more powerful small animal model (humanized) for pursuing hSC basic and pre-clinical researches before proceeding with clinical trials. The following characteristics prompt us to employ the developing fetus to produce human-rat chimeras. During the early development of the immune system, exposure to foreign antigens leads to sustained tolerance, making it possible to support the long-term engraftment and survival of transplanted human donor cells in the xenogeneic recipient settings[7].
In the developing rat fetus, some significant merits, including the existence of the naturally occurring migration patterns of stem cells, the availability of extending homing and engraftment sites and the presence of tissue- and organ-specific signals from niche, greatly facilitate the widespread distribution of human donor cells throughout the recipient body, and subsequently promote them to home and engraft into the various tissues and organs (as many as possible), in which in turn human donor cells are actively influenced by the signals from niches they reside to undergo reprogramming, proliferation and directed differentiation within the specific tissues and organs of recipient. By making use of this window of opportunity and performing stem cell transplantation at the appropriate pre-immune stage of fetal life, significant levels of multi-organ, multi-tissue, multi-lineage engraftment are very possible, as confirmed by other investigators[7,8]. More importantly, in contrast to human/mouse xenograft model from the immunodeficient mice for the study of stem cell regulation[9], such human-rat chimeras possess the normal physiological conditions and potential human immune system reconstituted by donor-derived human differentiated cells[13].
Ideally, such a human-rat chimera model permits relatively robust formation of a wide variety of donor-derived tissue-specific hASCs and their derivatives, and is also expected to be capable of maintaining the normal self-renewal, proliferation, differentiation and homing/migration of donor-derived human adult stem/progenitor cells in vivo under normal physiological conditions.
As the ability of human donor hematopoietic cells to engraft and differentiate into all blood cell lineages in the human/sheep xenograft model has been fully demonstrated[7], MNC-transplanted rats were firstly analyzed for the presence of human hemopoietic cells in PB by flow cytometry to test the expression of human marker CD45.
At 3 wk after birth, human-rat chimeras were primarily identified by measuring the expression of human leukocyte common antigen CD45 (found on all nucleated cells of the hemopoietic system) using flow cytometry, followed by confirming the presence of human CD45-positive cells engrafted in PB of recipients (Figure 1 and Table 2). In three independent experiments, 29 of the total of 36 recipient rats injected with MNCs were live-born (Table 2), while 19 out of the 29 live-born recipients (65.5%) became chimeric (Table 2). Additionally, all of 29 live-born recipient rats were normal, with no signs of malformations.
| ExperimentNo. | AnimalNo. | Phenotype | Source | Cell dose | Chimeric/totalanimals |
| 1 | 661 | MNCs | hUCB | 5 × 106 | 8/12 |
| 2 | 662 | MNCs | hUCB | 5 × 106 | 5/8 |
| 3 | 670 | MNCs | hUCB | 5 × 106 | 6/9 |
Live-born recipients were examined at 3 wk, 2 mo and 6 mo of age for signs of human cell engraftment in PB or BM. Table 3 shows human cell engraftment within recipients. Engraftment of human CD45+ cells ranged from 0.05% to 0.3% of the total cells analyzed up to 6 mo after birth (Figure 1 and Table 3), suggesting the presence of human mature lymphocytes in recipient PM.
| Marker | PB | BM | |||
| 3 wk | 2 mo | 6 mo | 2 mo | 6 mo | |
| CD45 | 0.2 ± 0.06 | 0.15 ± 0.04 | 0.09 ± 0.01 | 1.4 ± 0.36 | 0.1 ± 0.01 |
| (n = 19) | (n = 17) | (n = 4) | (n = 8) | (n = 4) |
Following flow cytometric analysis, we further assessed the tissue distribution of donor-derived human cells using PCR for hAlu, which constitutes 10% of human genome, on DNA isolated from multiple adult tissues of MNC-injected rats (n = 19) with human CD45-positive cell engraftment in PB (Figure 2 and Table 2). At 3 wk, 2 mo and 6 mo of age, 14 adult animals were analyzed by PCR of hAlu. Two representative PCR analyses of two recipients are shown in Figure 2A. As shown in Figure 2B, although the pattern of human cell distribution in individual rats differed, PCR analysis on 11 different adult tissues revealed that 14 animals had demonstrable donor-derived human cell engraftment in hematopoietic and non-hematopoietic tissues at the time of tissue collection. Donor contribution was most frequently detected by PCR in the spleen (9/14), liver (9/14) and thymus (8/14), and moderate-frequently detectable in the heart (5/14), kidney (4/14) and brain (4/14), compared to PB (3/14), muscle (3/14), gut (3/14), lung (3/14) and skin (2/14). Thus, human donor haematopoietic cells did not show preferred seeding of haematopoietic tissues. Moreover, human donor cells were frequently detected in many adult tissues up to 6 mo later by PCR.
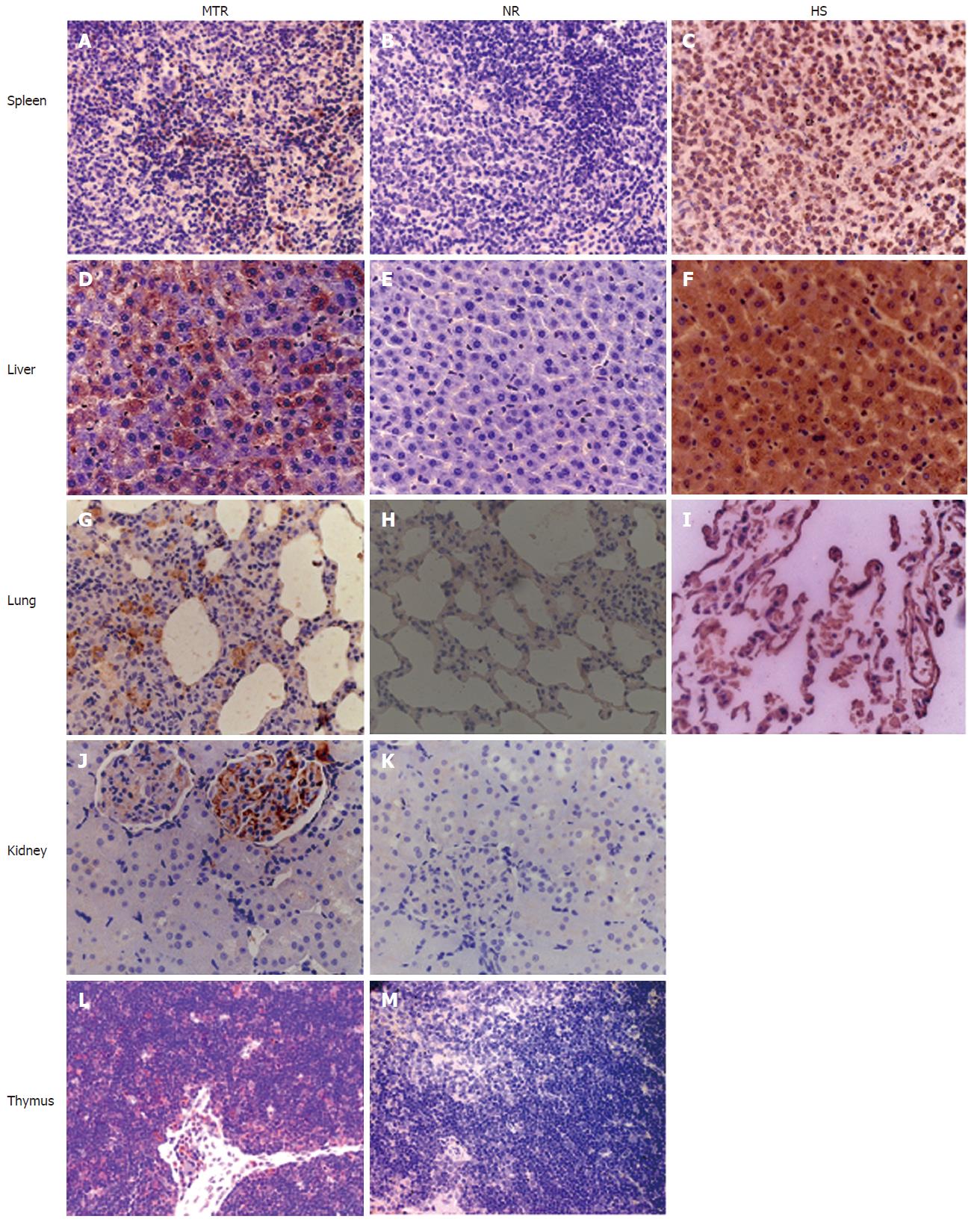
As a component of MHC class I molecules, human β2-microglobulin is present on almost all cells of the body (red blood cells are a notable exception). Thus, the presence of engrafted human cells in some PCR-positive tissues (i.e. kidney, lung, liver, spleen and thymus) was further verified by IHC using an antibody specific for human β2-microglobulin (Figure 3), indicating that the long-term engrafted human cells detected in these aforementioned tissues by PCR were not due to contamination by PB or other circulating cells. NCs consisting of age-matched tissues from normal rats were uniformly negative for β2-microglobulin, suggesting the human specificity of staining, while the normal adult human lung, liver and spleen were uniformly positive for β2-microglobulin (Figure 3). Furthermore, many human marker-positive cells in the kidney, lung, liver, spleen and thymus were detected by IHC as late as 6 mo after birth (data not shown).
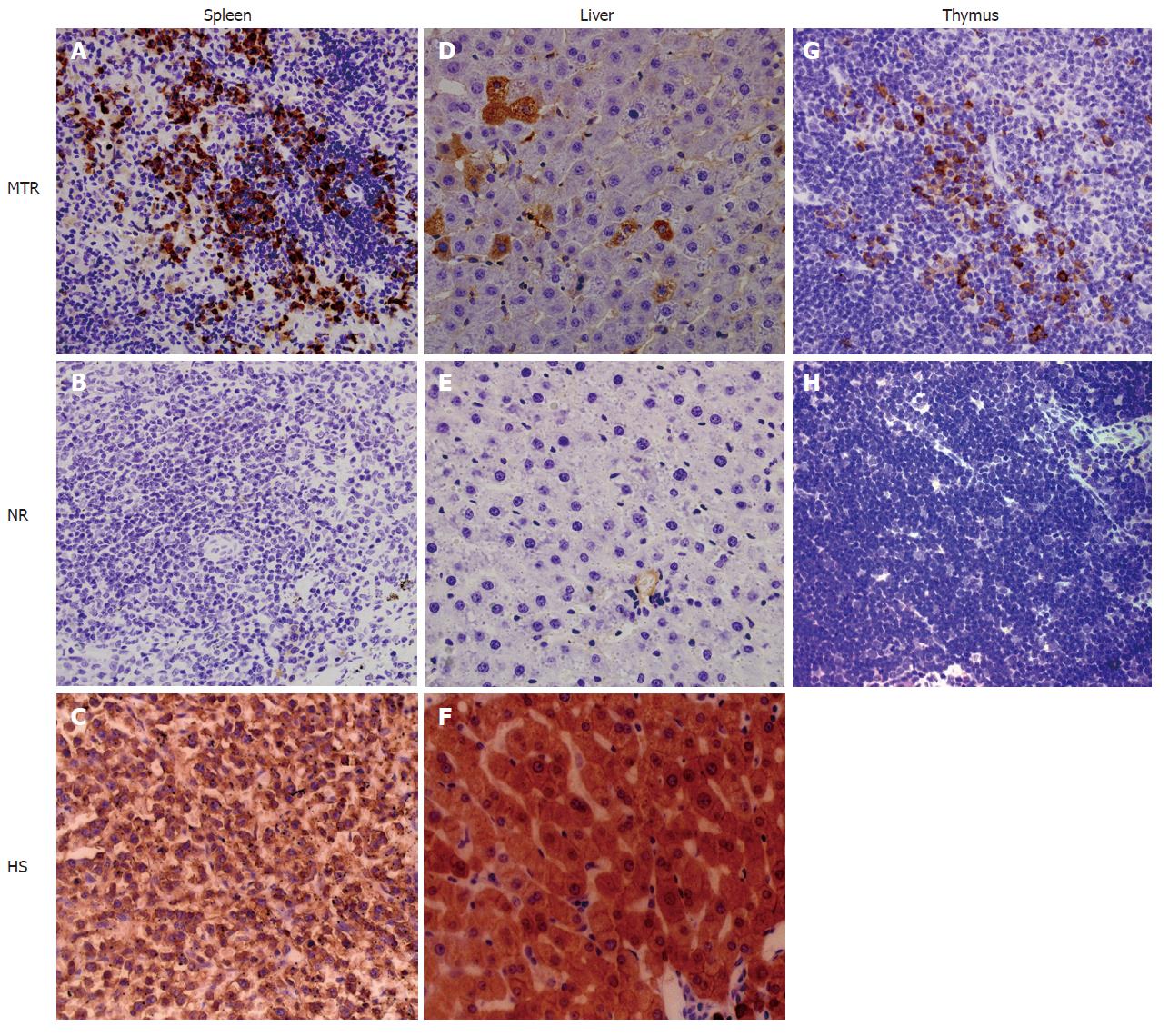
As mentioned above, the engraftment and long-term survival of donor-derived human cells in multiple adult solid tissues, such as the liver and spleen, were detected by human gene-specific PCR and IHC (Figures 2 and 3). The obvious next question was what the engrafted human donor cells had actually become. Here, human-specific differentiation markers CK18 and CD45 were further employed to examine the cellular nature of the MNC-derived human cell populations engrafted in the chimeric liver, spleen and thymus of recipients by IHC, respectively (Figure 4).
We assessed differentiation of human donor cells in the chimeric liver by IHC using a human-specific antibody against CK18 (CK18: hepatocyte marker). Cells expressing human CK18 could be found in the recipient liver as well as in human liver, but not in normal control rats (Figure 4), suggesting human liver-specific differentiation of donor-derived human cells within the xenogeneic conditions.
The human spleen serves as a major reservoir for blood and is the largest lymphatic organ that produces lymphocytes, such as B cells and T cells; human spleen cell populations contain cells expressing the leukocyte common antigen CD45 (Figure 4), a highly glycosylated cell surface protein found on all nucleated cells of the hemopoietic system. Immunohistochemical analysis revealed that in the chimeric adult spleen of recipients, many donor-derived human cell populations expressed CD45 marker (Figure 4), indicating the human spleen-specific differentiation of donor-derived human cells.
We assessed differentiation of hMNCs in the rat thymus using IHC with a human-specific antibody against CD45. At 3 wk, 2 mo and 6 mo after in utero transplantation, multiple human cells detected in the recipient thymus strongly expressed CD45 (Figure 4), while the precursor of thymic dendritic cells is thought to be the hematopoietic stem cell.
In summary, transplanted UCB-derived human cells underwent site-specific differentiation into CK18+ human cells in the recipient liver and CD45+ human cells in the recipient spleen, suggesting that after in utero transplantation of hMNCs, the transplanted human stem/progenitor cells successfully engraft into the liver and spleen of the recipients, subsequently selectively long-term survive in these organs, and then are actively influenced by niche signals to participate in organogenesis (for example liver and spleen) of recipients in the xenogeneic competitive settings.
As a source of hSCs, hUCB containing a high rate of circulating stem/progenitor cells (including hematopoietic stem/progenitor cells and mesenchymal stem/progenitor cells) is an attractive alternative to BM and PB[14]. Human stem/progenitor cells from hUCB transplanted into recipients (such as mice and sheep) can be induced in vivo to differentiate into almost all of cells of three germ layers, including blood components, heart, kidney, lymphoid organs, bone and muscle (from the mesoderm layer of the embryo), epithelial cells in the liver, lung and gut (endoderm layer), and epithelial cells in skin and neural cells (ectoderm layer)[7,8,10,13,15]. Therefore, in the present investigation, hUCB was used as a transplantable source of stem/progenitor cells to make human-animal chimeras.
Unlike previous transplantation systems which often transplanted stem cells/progenitor cells by intravenous (iv) injection into irradiated immunedeficient mice, in utero transplantation was employed to introduce MNCs into the fetal rats at the pre-immune stage. We put forward the hypothesis that the transplanted MNCs undergo adaptive processes for survival in recipients triggered by local host niche. Despite their UCB origin, these stem cells apparently adapt to rat microenvironments through mechanisms that might be more efficient for engraftment, survival, proliferation and differentiation in other tissues, such as liver, spleen and thymus, rather than blood, muscle, gut, lung and skin (Figure 2). Even within the same organ in different animals, engrafted cells were unequally distributed. It would be very interesting to demonstrate whether these observations reflects the distribution pattern of stem/progenitor cells, the existence of microenvironment favorable for human cell engraftment, or other unknown mechanisms.
In theory, foreign cells transplanted to embryonic environment (e.g. blastocyst and in utero ) may be provided with all the possible lineage options available during development[16]. But as shown in Figure 2, higher levels of human cell engraftment occurred in solid organs examined, compared with blood, which was consistent with the goat results reported by Zeng et al[8]; in addition, the chimerism was also nonuniformly distributed over all solid organs analyzed, as mentioned in mice[5,17,18]. The different patterns of human cell distribution over all tissues analyzed in individual animal may be largely owing to the xenogeneic setting diversity of recipients, cell sources, the discrepancy of cellular components of transplanted cells from various donors, the transplantation routes (such as blastocyst injection and in utero transplantation), non-consistency (such as site and time of injection) of manipulation each time and other unknown reasons. For example, among mesenchymal stem cells (MSCs), CD34+ cells and non-MSCs/CD34- cells from human BM, MSCs are the most potent component in hepatic differentiation[19]; in vitro long-term culture may alter the natures and phenotypes of stem/progenitor cells, and further influence their engrafting capability in xenogeneic settings[20].
When hUCB-derived CD34+ cells[18], human acute myeloid leukemia cells[17] and mouse FDCPmix cells (murine progenitor cells of haematopoietic origin; Petrovic et al[21]) were microinjected into murine blastocysts, respectively, they preferentially engrafted in PB and yolk sac of embryos, and less frequently detected in other tissues of embryos or adults.
While mouse and human adult stem cells were in utero transplanted into the fetus at a couple of days of gestation, adult engraftment of allogeneic and xenogeneic cells were frequently detectable in hematopoietic and non-hematopoietic organs, including PB, BM, spleen, liver, kidney, thymus, muscle, lung, gut, stomach, skin, brain and heart of the recipient sheep[5,7], mice[6,22], monkeys[3], pigs[2] and goats[8,23], and rats in this study, although the pattern of allogeneic and xenogeneic transplanted cell distribution over all tissues examined in individual recipient differed.
It is well known that multipotent adult stem/progenitor cells can in vitro and in vivo give rise to many cell types under the right conditions, while totipotent embryonic stem cells (ES cells) microinjected into blastocysts can differentiate into all tissue types in chimeras under the appropriate conditions (such as blastocyst stage)[16]. In animals and human, ES cells appear at the early developmental stage, followed by the disappearance of ES cells and the appearance of adult stem/progenitor cells. Theoretically, foreign cells, such as ES cells and adult stem/progenitor cells, introduced to embryonic environment may be provided with all the possible lineage options available during development[16], but the aforementioned findings seem to suggest that blastocyst stage is pretty suitable for the engraftment, survival, proliferation and differentiation of ES cells, but not adult stem/progenitor cells in allogeneic and xenogeneic recipient microenvironment because the engraftment, survival and proliferation of adult stem/progenitor cells in blastocyst might be greatly influenced by competition of endogenous ES cells in inner cell mass, whereas fetuses are capable of supporting the engraftment, survival, proliferation and differentiation of adult stem/progenitor cells in allogeneic and xenogeneic settings which may provide certain developmental cues that do not exist in embryonic stage, as supported by findings[24].
Currently, broader clinical applications of stem cells urgently need a humanized surrogate animal model that can more precisely and realistically mimic the in vivo environment in human more closely to greatly facilitate investigators to examine the related mechanisms in vivo. As described above, human/rat xenogeneic chimeras appear to be just in vivo small animal model.
At present, strategies and approaches for conditional loss-of-gene-function and conditional gain-of-gene-function in stem cell of human, rats and mice have been well established. When used in combination with molecular tools that can modify genetic activity in vivo in a time- and lineage-specific manner in the cell population under scrutiny, this humanized chimera will offer practically unlimited options for a precise, in-depth and large-scale analysis of gene function, and further precisely and realistically define the biological processes and pathological processes. The embryonic and adult chimeras have proven to be very useful for elucidating gene function in mice. As aforementioned, the potential applications of the humanized small animal model is not limited to follow the fates of transplanted-human donor cells engrafted in the multiple tissues of recipients (Figures 3 and 4) and be fully dissecting mechanisms controlling human stem cell fates in vivo[25], but also to the analysis of human tissue formation and organogenesis, postnatal maturation, bodily function, tissue homeostatic maintenance, and to be human disease model (e.g. cancer, hepatitis and HIV) under non-injured conditions. Human/rat xenogeneic chimeras also provide a unique in vivo system for investigating immune tolerance as well as completely evaluating the efficacy and safety of stem cell-related products and stem cell therapy strategies and programs under normal physiological conditions.
Remaining constraints of human/rat xenogeneic chimeras include (1) un-consistent engraftment of human cells within the same organs of different individuals, and (2) un-adequate quantity of human cells engrafted in PB, BM, kidney, lung, liver and spleen of human-rat chimerics. The countermeasures to eliminate the above constraints include (1) definite cell components of human stem/progenitor cells used for transplantation, (2) standard manipulation of transplantation, and (3) the need for genetic modifications to further humanize the host strain[9]. For example, the proper development and function of the transplanted cells might need the transgenic expression of human-specific cytokines, and the transgenic expression of human-specific adhesion molecules might greatly facilitate proper trafficking of human cells throughout the recipient body and subsequently to home and engraft into the various tissues and organs of recipients as many as possible, and so on. Despite these limitations, humanized rats offer great promise as in vivo small models for the pre-clinical testing of drugs and human cell-based therapeutics before their advancement to the clinic.
In conclusion, we take advantage of the highly proliferative and permissive environment of the developing pre-immune fetus to successfully create a human/rat xenograft small animal model. Humanized animals can provide insights into in vivo human biology that would otherwise not be possible owing to ethical, logistical and/or technical limitations and constraints.
We express our deepest gratitude to Dr. GA Lin (Southern Medical University, Guangzhou, China) and Professor TC Lu (Shihezi University, Xinjiang, China) for their unstinting advice and technical guidance. We are also indebted to the expert technical assistance of GG Qiu, WG Huang, FY Chen, FF Guo, GZ Yang and WW Zheng at Center of Experimental Animals, Sun Yat-sen University, and HA Li and XH Wang at Shihezi University, Xinjiang, China. We thank XH Yang, Y Li and W Zhang (Zhongshan School of Medicine, Sun Yat-sen University) for kindly providing reagents.
| 1. | Lanza R, Gearhart J, Hogan B, Melton D, Pedersen R, Thomson J, Thomas ED, West M. Essentials of stem cell biology. London: Academic Press Inc 2006; . |
| 2. | Fujiki Y, Fukawa K, Kameyama K, Kudo O, Onodera M, Nakamura Y, Yagami K, Shiina Y, Hamada H, Shibuya A. Successful multilineage engraftment of human cord blood cells in pigs after in utero transplantation. Transplantation. 2003;75:916-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Harrison MR, Slotnick RN, Crombleholme TM, Golbus MS, Tarantal AF, Zanjani ED. In-utero transplantation of fetal liver haemopoietic stem cells in monkeys. Lancet. 1989;2:1425-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 96] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia. 2002;16:1992-2003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 340] [Article Influence: 14.2] [Reference Citation Analysis (1)] |
| 5. | Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, Marshak DR, Flake AW. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6:1282-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 850] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 6. | Pixley JS, Tavassoli M, Zanjani ED, Shaft DM, Futamachi KJ, Sauter T, Tavassoli A, MacKintosh FR. Transplantation in utero of fetal human hematopoietic stem cells into mice results in hematopoietic chimerism. Pathobiology. 1994;62:238-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Porada GA, Porada C, Zanjani ED. The fetal sheep: a unique model system for assessing the full differentiative potential of human stem cells. Yonsei Med J. 2004;45 Suppl:7-14. [PubMed] |
| 8. | Zeng F, Chen MJ, Baldwin DA, Gong ZJ, Yan JB, Qian H, Wang J, Jiang X, Ren ZR, Sun D. Multiorgan engraftment and differentiation of human cord blood CD34+ Lin- cells in goats assessed by gene expression profiling. Proc Natl Acad Sci USA. 2006;103:7801-7806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 968] [Cited by in RCA: 1032] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 10. | Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2032] [Cited by in RCA: 1897] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 11. | Fleischman RA, Mintz B. Prevention of genetic anemias in mice by microinjection of normal hematopoietic stem cells into the fetal placenta. Proc Natl Acad Sci USA. 1979;76:5736-5740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 119] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Sambrook JE, Fritsch F, Maniatis T. Molecular Cloning: A Laboratory Manual. 3rd ed. New York: Cold Spring Harbor Laboratory Press 2001; 801-800. |
| 13. | Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, Manz MG. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 768] [Cited by in RCA: 795] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 14. | Kakinuma S, Tanaka Y, Chinzei R, Watanabe M, Shimizu-Saito K, Hara Y, Teramoto K, Arii S, Sato C, Takase K. Human umbilical cord blood as a source of transplantable hepatic progenitor cells. Stem Cells. 2003;21:217-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 145] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Rogers I, Casper RF. Umbilical cord blood stem cells. Best Pract Res Clin Obstet Gynaecol. 2004;18:893-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Tam PP, Rossant J. Mouse embryonic chimeras: tools for studying mammalian development. Development. 2003;130:6155-6163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 148] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Dürr M, Harder F, Merkel A, Bug G, Henschler R, Müller AM. Chimaerism and erythroid marker expression after microinjection of human acute myeloid leukaemia cells into murine blastocysts. Oncogene. 2003;22:9185-9191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Harder F, Henschler R, Junghahn I, Lamers MC, Müller AM. Human hematopoiesis in murine embryos after injecting human cord blood-derived hematopoietic stem cells into murine blastocysts. Blood. 2002;99:719-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Sato Y, Araki H, Kato J, Nakamura K, Kawano Y, Kobune M, Sato T, Miyanishi K, Takayama T, Takahashi M. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood. 2005;106:756-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 446] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 20. | Shimizu Y, Ogawa M, Kobayashi M, Almeida-Porada G, Zanjani ED. Engraftment of cultured human hematopoietic cells in sheep. Blood. 1998;91:3688-3692. [PubMed] |
| 21. | Petrovic S, Cross M, Müller AM. Differentiation potential of FDCPmix cells following injection into blastocysts. Cells Tissues Organs. 2004;178:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Wang ML, Yan JB, Xiao YP, Huang SZ. Construction of an allogenic chimeric mouse model for the study of the behaviors of donor stem cells in vivo. Chin Med J (Engl). 2005;118:1444-1450. [PubMed] |
| 23. | Zeng F, Chen M, Katsumata M, Huang W, Gong Z, Hu W, Qian H, Xiao Y, Ren Z, Huang S. Identification and characterization of engrafted human cells in human/goat xenogeneic transplantation chimerism. DNA Cell Biol. 2005;24:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Koestenbauer S, Vanderzwalmen P, Hammer A, Schoonjans L, Danloy S, Zech H, Dohr G, Zech NH. Apoptosis affects integration frequency: adult stem cells injected in blastocysts show high caspase-3 activity. Cell Biol Int. 2007;31:489-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Sun Y, Xiao D, Zhang RS, Cui GH, Wang XH, Chen XG. Formation of human hepatocyte-like cells with different cellular phenotypes by human umbilical cord blood-derived cells in the human-rat chimeras. Biochem Biophys Res Commun. 2007;357:1160-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
S- Editor Wang J L- Editor Kumar M E- Editor Che YB