Published online May 14, 2007. doi: 10.3748/wjg.v13.i18.2615
Revised: January 15, 2007
Accepted: January 31, 2007
Published online: May 14, 2007
AIM: To study the correlations of Pancreas duodenal homeobox-1 with pancreatic cancer characteristics, including pathological grading, TNM grading, tumor metastasis and tumor cell proliferation.
METHODS: Reverse transcriptase-polymerase chain reaction (RT-PCR) was used to detect PDX-1 mRNA expression in pancreatic cancer tissue and normal pancreatic tissue. The expression of PDX-1 protein was measured by Western blot and immunohistochemistry. Immunohistochemistry was also used to detect proliferative cell nuclear antigen (PCNA). Correlations of PDX-1 with pancreatic cancer characteristics, including pathological grading, TNM grading, tumor metastasis and tumor cell proliferation, were analyzed by using χ2 test.
RESULTS: Immunohistochemistry showed that 41.1% of pancreatic cancers were positive for PDX-1 expression, but normal pancreatic tissue except islets showed no staining for PDX-1. In consistent with the result of imunohistochemistry, Western blot showed that 37.5% of pancreatic cancers were positive for PDX-1. RT-PCR showed that PDX-1 expression was significantly higher in pancreatic cancer tissues than normal pancreatic tissues (2-3.56 ± 0.35vs 2-8.76 ± 0.14, P < 0.01). Lymph node metastasis (P < 0.01), TNM grading (P < 0.05), pathological grading (P < 0.05) and tumor cell proliferation (P < 0.01) were significantly correlated with PDX-1 expression levels.
CONCLUSION: PDX-1 is re-expressed in pancreatic cancer, and PDX-1-positive pancreatic cancer cells show more malignant potential compared to PDX-1-negative cells. Therefore, PDX-1-positive cells may be tumor stem cells and PDX-1 may act as alternate surface marker of pancreatic cancer stem cells.
- Citation: Liu T, Gou SM, Wang CY, Wu HS, Xiong JX, Zhou F. Pancreas duodenal homeobox-1 expression and significance in pancreatic cancer. World J Gastroenterol 2007; 13(18): 2615-2618
- URL: https://www.wjgnet.com/1007-9327/full/v13/i18/2615.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i18.2615
According to the hypothesis of cancer stem cells, only a rare, phenotypically distinct subset of cells has the capacity of forming new tumors and that this subgroup could be considered cancer stem cells, the other cells in cancers are filial generation of these cells[1]. Recently, it was reported that cancer stem cells existed in some solid malignancies, including breast, brain, prostate and lung cancers[2-6]. Although the cellular origin of cancer stem cells has not been definitively determined, evidence suggest that they may be derived from mutation of somatic stem cells caused by interaction of microenvironment, carcinogen and hereditary factors. Therefore, somatic stem cells and cancer stem cells may express the same surface markers[1].
It is reported that 90% of pancreatic cancers originate from pancreatic ducts, and PanIN (pancreatic intraepithelial neoplasia), which is a precancerous lesion, also originates from pancreatic ducts[7,8]. In addition, signal molecules that regulate proliferation and differentiation of embryonic stem cell are activated in human pancreatic cancers and mice model of pancreatic cancer. Considering that there are pancreatic stem cells located in pancreatic ducts, it is possible that pancreatic cancer originates from these stem cells[9].
Pancreas duodenal homeobox-1 is a key transcription factor in embryonic pancreas development, it is critical for ensuring proper embryonic development of the endocrine pancreas and normal islet function and act as a marker of pancreatic stem cells[10]. PDX-1 is found in the mouse primitive gut as early as the stage of 8.5 d, it directs the pancreatic stem cells differentiation by promoting expression of molecules which are associated with endocrine cells and exocrine cells development. At the stage of 13.5 d, the expression of PDX-1 increased to promote expression of molecules which are associated with endocrine cells differentiation, which plays a key role in beta cells maturation and differentiation[11]. In mature pancreas, PDX-1 is mainly expressed in beta cells other than ductal cells[12], but it was found to be re-expressed in ductal cells in some conditions, such as partial pancreatectomy and pancreatitis, thereby suggesting that activation of stem cells is located in the pancreatic duct[13-16].
We deduce that pancreatic cancer may originate from the mutation of pancreatic stem cells located in the pancreatic ducts and PDX-1 may be activated extraordinarily in this process. To investigate whether PDX-1 is re-expressed in pancreatic cancer and its role in pancreatic cancer development, expression of PDX-1 was detected in pancreatic cancer tissues at protein and mRNA levels. At the same time, expression of PCNA was determined, and also correlation of PDX-1 expression with tumor characteristics was explored.
Fifty-six pancreatic cancer patients (29 men and 27 women, age ranged from 47 to 69 years) who underwent pancreaticoduodenectomy at Union Hospital, Wuhan, China between 2000 and 2004 were enrolled in this study. Lymph node metastasis was found in 36 of 56 (64.3%) patients. Of 56 samples, 17 were well-differentiated, 21 were moderately differentiated and 18 were poorly differentiated. According to the criteria of the TNM classification (UICC), 19 patients had stage I and II, 37 patients had stage III and IV cancer. Six normal pancreas tissue samples were used as control.
Goat anti-PDX-1 monoclonal antibody was purchased from Santa Cruz, USA. Rabbit anti-PCNA monoclonal antibody was purchased from Boster, China. S-P immunohistochemistry kit was from Zymed, USA, and DAB coloration kit was from Zhongshan Biotec, China.
Samples were fixed in 4% phosphate-buffered paraformaldehyde overnight at 4°C, dehydrated in ethanol, embedded in paraffin, cut into 3-μm thick sections, and stained with H&E. S-P immunohistochemistry was used to detect the expression of PDX-1 and PCNA. Cells stained with brown yellow in cytoplasm were defined as positive. Ten optical fields at 400 × magnification were randomly selected in each slide, and 50 cells were counted in every field. Sections with less than 15% positive cells were defined as negative and that with ≥ 15% positive cells were defined as positive.
Total RNA was extracted separately from tissues with TRIZOL reagent following the manufacturer's instruc-tions. For reverse transcription, 4 μL of total RNA and 0.5 μL of Oligo (dt) were added to 6.5 μL of distilled water, annealed for 5 min at 70°C and immediately cooled on ice. Then 4.0 μmol/L 5 × first strand buffer, 2.0 μL of 10 mmol/L dNTP, 0.5 μL of RNasin and 0.5 μL of RTase were added to get a total reaction volume of 20 μL. The reaction was allowed to proceed at 37°C for 60 min, then at 95°C for 5 min to inactivate the enzyme. In real-time PCR, each assay was repeated three times. The total PCR volume consisted 1 μL of cDNA, 1 μL of SYBRGreen PCR I, 5 μL of 10 × buffer, 1.6 μL of primers (PDX-1: 5'-CTTGGGTATGGATCTGTGG-3' and 5'-CGGACTCATCGTACTCCTGCTT-3'; beta-actin: 5'-CCATCATGAAGTGTGACGTGG-3' and 5'-GTCCGCCTAGAAGCATTTGCG-3'), 7 μL of MgCl2, 0.5 μL of Taq DNA Polymerase, 1 μL of dNTP and 33 μL of distilled water. After denaturation of the enzyme for 2 min at 94°C, the PCR assays were carried out for 45 amplification cycles, each cycle consisted of denaturation at 94°C for 30 s, annealing at 57°C for 30 s and extension at 72°C for 30 s. Fluorometric PCR was performed with the FTC-2000 System. Relative gene expression was determined by calculating the ratio of each transporter protein gene and β-actin.
Total protein was obtained from tissues as previously described[14]. Briefly, tissue was lysed in 50 mmol/L Tris (pH 7.5), 0.3 mol/L NaCl, 5 mL/L Triton X-100, 1 g/L sodium azide, with a cocktail of antiprotease (chymostatin, leupeptin, aprotinin, pepstatin (CLAP), 100 μmol/L each; Sigma Aldrich). Then protein samples (50 μg) were separated on 100 g/L SDS-PAGE and transferred from gel onto nitrocellulose membrane and blocked in a blocking solution (50 g/L dry milk and 3 mL/L Tween 20 in PBS) for 1 h. Membranes were then incubated with goat anti-rat PDX-1 antibody (1:10000 dilution) and horseradish peroxidase-labeled secondary antibody (1:5000 dilution) (Amersham, Japan). Finally, the protein was visualized on enhanced chemiluminescence film (Hyperfilm, Amersham, Japan) by applying Amershem's Enhanced Chemiluminescence Western blotting Detection System. Band intensity was quantitated with Gel Analysis software (GreyStone-Iconix). Samples which expressed higher PDX-1 than normal pancreatic tissues were defined as positive, otherwise negative.
Chi-square test was used to analyze the data. P < 0.05 was considered statistically significant.
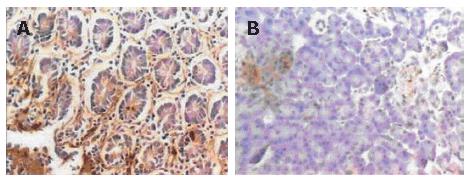
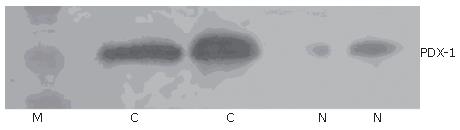
Immunohistochemical staining showed that 41.1% (23/56) samples were positive for PDX-1. PDX-1 was mainly expressed in cytoplasm, with strong positivity located mainly at the leading edge of infiltration (Figure 1A). PDX-1 was also weekly expressed in the beta cells of pancreatic islet in normal pancreatic tissues, but not found in the pancreatic duct (Figure 1B). Western blot showed that 37.5% (21/56) samples were positive for PDX-1, which was in consonance with the result of imunohistochemistry (Figure 2).
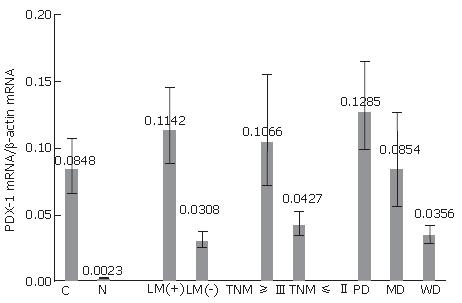
The 2-ΔΔCT method was used to analyze mRNA expression. Relative to beta-actin, the PDX-1 mRNA in pancreatic cancers was 0.0848 (range: 0.0665-0.1080) whereas that of normal pancreatic tissues was 0.0023 (range: 0.0021-0.0025) (Figure 3).
We found a significant relationship between PDX-1 expression and lymph node metastasis (P < 0.01) (Table 1): 55.6% (20/36) tumor tissues with lymph node metastasis were PDX-1-positive, whereas only 15% (3/20) tumor tissues without lymph node metastasis were PDX-1-positive. PDX-1 expression was also markedly related to TNM staging (P < 0.05) and pathology staging (P < 0.05).
PDX-1 mRNA was over-expressed in the pancreatic cancer tissues compared to normal pancreatic tissues (Figure 3). Interestingly, PDX-1 mRNA expression in cancer tissues with lymph node metastasis, late TNM staging and lower pathology staging was much higher than their counterpart.
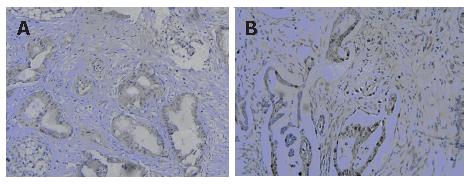
To study the correlation of PDX-1 expression with cell proliferation, we detected the expression of PCNA in pancreatic cancer by imunohistochemistry. The results showed that 53.6% (30/56) pancreatic cancers were positive for PCNA; 78.26% (18/23) PDX-1-positive samples were positive for PCNA, whereas only 36.36% (12/33) PDX-1-negative samples were positive for PCNA. These results indicated that PCNA expression was obviously related to PDX-1 expression in pancreatic cancer tissues (Figure 4).
In mammals, pancreas is well known to inherit a strong regeneration potential. The remnant pancreas can re-generate and recover the function of insulted pancreas after pancreatitis or partial pancreatectomy[14-16]. Previous studies suggested that this process was correlated with proliferation and differentiation of pancreatic stem cells, i.e. on stimulation of impaired factors, quiescent stem cells, which are regarded as located in the pancreatic duct, were activated to proliferate and differentiate into impaired cells. In a previous study, we observed that mature duct cells were main progenitor source for pancreatic growth in rat pancreatectomy model and PDX-1 was re-activated in the ductal cells, thereby suggesting that re-activation of PDX-1 is a marker of pancreatic stem cells.
There is overwhelming evidence that cancer stem cells, which are considered to originate from somatic stem cells, should be responsible for tumors malignancy[17]. Miyamoto et al[9] found that signal molecules that regulate proliferation and differentiation of embryonic stem cell were activated in human pancreatic cancers. Thus, we deduced that PDX-1 may be re-activated in the process of transformation from pancreatic stem cells to pancreatic cancer stem cells.
To investigate whether PDX-1 was re-expressed in pancreatic cancer and its role in pancreatic cancer development, we detected PDX-1 protein and mRNA expression in 56 pancreatic cancer tissues. To validate the used methods, we also analyzed normal human pancreatic tissues. Results of immunohistochemistry indicated that PDX-1 was only weekly expressed in beta cells of the pancreatic islet in normal pancreatic tissues, which is in agreement with previous studies in mice and human pancreas[11,12], but PDX-1 was re-activated in pancreatic cancers. About 41.1% samples showed positive immunostaining for PDX-1 in the cytoplasm and nuclei of cells, especially in the cells located at the leading edge of infiltration, thereby implying that PDX-1 plays a role in pancreatic cancer infiltration. Results of Western blot were in consonance with that of immunohistochemistry. In addition, we analyzed the correlation of PDX-1 expression with tumor characteristics. We found that lymph node metastasis, TNM grading and pathological grading were all significantly correlated with PDX-1 expression.
To study the correlation of PDX-1 expression with cell proliferation, we detected the expression of PCNA in pancreatic cancer by immunohistochemistry. The positive rate of PCNA in PDX-1-positive samples was as high as 78.2%, whereas that in PDX-1-negative samples was only 36.4%, suggesting that PDX-1-positive cells had higher potential of proliferation. Many studies have demonstrated high frequency of mutations of oncogene such as K-ras and tumor suppressor genes such as p16, p53 in pancreatic cancer tissues and cell lines[8,18,19]. Based on our study, we deduced that the alteration of PDX-1 expression might represent one of those genetic changes.
In summary, PDX-1 is re-expressed in pancreatic cancers, and its expression level has a significant correlation with pathological grading, TNM grading, tumor metastasis and tumor cell proliferation. Since PDX-1 is considered a marker of pancreatic stem cells, we tentatively put forward that cells that express PDX-1 in pancreatic cancers may be cancer stem cells. How does the PDX-1 molecule affect the malignancy of a particular cell is awaited to be studied in our future work.
The authors are grateful to the technical assistance from Yuan Tian and Jin-Hui Zhang, the Research Laboratory of General Surgery, Union Hospital, Wuhan.
| 1. | Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3:895-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 1115] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 2. | Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4851] [Cited by in RCA: 4929] [Article Influence: 170.0] [Reference Citation Analysis (1)] |
| 3. | Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983-3988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7830] [Cited by in RCA: 7800] [Article Influence: 339.1] [Reference Citation Analysis (0)] |
| 4. | Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821-5828. [PubMed] |
| 5. | Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946-10951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1998] [Cited by in RCA: 2045] [Article Influence: 102.3] [Reference Citation Analysis (0)] |
| 6. | Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1571] [Cited by in RCA: 1621] [Article Influence: 77.2] [Reference Citation Analysis (0)] |
| 7. | Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, Sharma A, O'Neil JJ. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci USA. 2000;97:7999-8004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 751] [Cited by in RCA: 738] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 8. | Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J, Redston MS, DePinho RA. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112-3126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 772] [Cited by in RCA: 829] [Article Influence: 36.0] [Reference Citation Analysis (9)] |
| 9. | Miyamoto Y, Maitra A, Ghosh B, Zechner U, Argani P, Iacobuzio-Donahue CA, Sriuranpong V, Iso T, Meszoely IM, Wolfe MS. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell. 2003;3:565-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 505] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 10. | Edlund H. Pancreatic organogenesis--developmental mechanisms and implications for therapy. Nat Rev Genet. 2002;3:524-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 352] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 11. | Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1357] [Cited by in RCA: 1335] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 12. | Gerrish K, Gannon M, Shih D, Henderson E, Stoffel M, Wright CV, Stein R. Pancreatic beta cell-specific transcription of the pdx-1 gene. The role of conserved upstream control regions and their hepatic nuclear factor 3beta sites. J Biol Chem. 2000;275:3485-3492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 127] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Risbud MV, Bhonde RR. Models of pancreatic regeneration in diabetes. Diabetes Res Clin Pract. 2002;58:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Sharma A, Zangen DH, Reitz P, Taneja M, Lissauer ME, Miller CP, Weir GC, Habener JF, Bonner-Weir S. The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes. 1999;48:507-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 241] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 15. | Hosotani R, Ida J, Kogire M, Fujimoto K, Doi R, Imamura M. Expression of pancreatic duodenal hoemobox-1 in pancreatic islet neogenesis after surgical wrapping in rats. Surgery. 2004;135:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Marshak S, Benshushan E, Shoshkes M, Havin L, Cerasi E, Melloul D. Functional conservation of regulatory elements in the pdx-1 gene: PDX-1 and hepatocyte nuclear factor 3beta transcription factors mediate beta-cell-specific expression. Mol Cell Biol. 2000;20:7583-7590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 95] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6844] [Cited by in RCA: 6981] [Article Influence: 279.2] [Reference Citation Analysis (0)] |
| 18. | Jeong J, Park YN, Park JS, Yoon DS, Chi HS, Kim BR. Clinical significance of p16 protein expression loss and aberrant p53 protein expression in pancreatic cancer. Yonsei Med J. 2005;46:519-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Khorana AA, Hu YC, Ryan CK, Komorowski RA, Hostetter G, Ahrendt SA. Vascular endothelial growth factor and DPC4 predict adjuvant therapy outcomes in resected pancreatic cancer. J Gastrointest Surg. 2005;9:903-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
S- Editor Liu Y L- Editor Kumar M E- Editor Wang HF