Published online May 7, 2007. doi: 10.3748/wjg.v13.i17.2484
Revised: December 8, 2006
Accepted: March 1, 2007
Published online: May 7, 2007
AIM: To evaluate whether the combination of recom-binant chicken fibroblast growth factor receptor -1 (FGFR-1) protein vaccine (cFR-1) combined with low-dose gemcitabine would improve anti-tumor efficacy in a mouse CT26 colon adenocarcinoma (CT26) model.
METHODS: The CT26 model was established in BABL/c mice. Seven days after tumor cell injection, mice were randomly divided into four groups: combination therapy, cFR-1 alone, gemcitabine alone, and normal saline groups. Tumor growth, survival rate of tumor-bearing mice, and systemic toxicity were observed. The presence of anti-tumor auto-antibodies was detected by Western blot analysis and enzyme-linked immunospot assay, microvessel density (MVD) of the tumors and tumor cell proliferation were detected by Immunohistochemistry staining, and tumor cell apoptosis was detected by TdT-mediated biotinylated-dUTP nick end label staining.
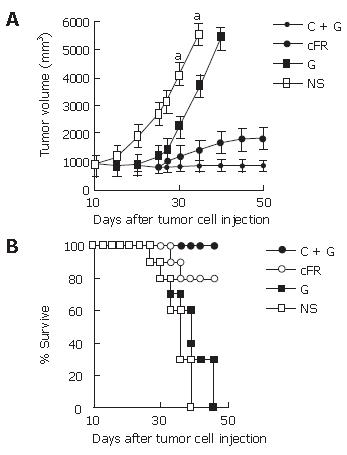
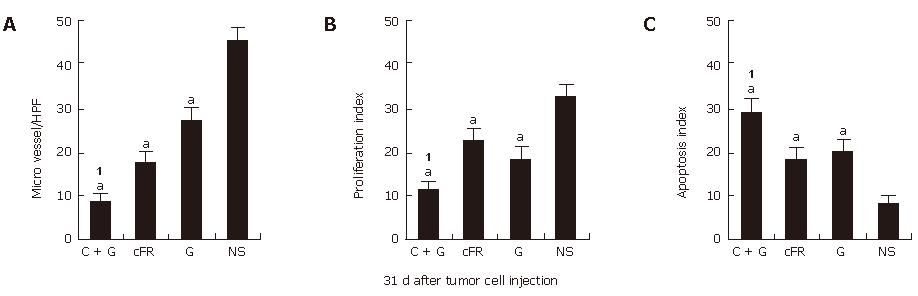
RESULTS: The combination therapy results in apparent decreases in tumor volume, microvessel density and tumor cell proliferation, and an increase in apoptosis without obvious side-effects as compared with either therapy alone or normal control groups. Also, both auto-antibodies and the antibody-producing B cells against mouse FGFR-1 were detected in mice immunized with cFR-1 vaccine alone or with combination therapy, but not in non-immunized mice. In addition, the deposition of auto-antibodies on endothelial cells from mice immunized with cFR-1 was observed by immunofluorescent stain-ing, but not on endothelial cells from control groups. Synergistic indexes of tumor volume, MVD, cell apoptosis and proliferation in the combination therapy group were 1.71 vs 1.15 vs 1.11 and 1.04, respectively, 31 d after tumor cell injection.
CONCLUSION: The combination of cFR-1-mediated anti-angiogenesis and low-dose gemcitabine synergistically enhances the anti-tumor activity without overt toxicity in mice.
- Citation: Zheng SJ, Zheng SP, Huang FY, Jiao CL, Wu RL. Synergistic anti-tumor effect of recombinant chicken fibroblast growth factor receptor-1-mediated anti-angiogenesis and low-dose gemcitabine in a mouse colon adenocarcinoma model. World J Gastroenterol 2007; 13(17): 2484-2489
- URL: https://www.wjgnet.com/1007-9327/full/v13/i17/2484.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i17.2484
Colorectal cancer is the third most common malignancy in the world and the fourth killer among all tumors in China[1,2]. The prognosis of advanced colorectal cancer remains poor, estimated 5-year survival rates less than 10%[1-3]. Thus, it is essential to seek multidisciplinary approaches for the treatment of colorectal cancer.
It is generally believed that the growth and metastases of a tumor are angiogenesis-dependent and thus that anti-angiogenic therapy, which targets genetically stable endothelial cells as a strategy for cancer therapy, is highly warranted[4-6]. At present, basic fibroblast growth factor (bFGF) has been shown to be one of the most important angiogenic growth factors for tumor angiogenesis. bFGF serves its biological function through interaction with its high-affinity receptor, fibroblast growth factor receptor-1 (FGFR-1), which is markedly expressed both in active endothelial cell and in many different forms of tumor and plays an important role in tumor angiogenesis and tumor growth[7-10]. Accumulating evidence indicates that an FGFR-1-mediated anti-angiogenesis target for tumor immunotherapy could suppress angiogenesis and further inhibit tumor growth[11-13]. Our data indicate that vaccination with the cFR-1 protein can induce auto-antibodies against FGFR-1 in mice[14].
Chemotherapy remains one of the major systemic therapies for cancer, but acquired drug resistance is one of the major hindrances to chemotherapy. Recently, evidence has confirmed that low dosages of conventional chemotherapeutic drugs can damage or kill the endothelial cells of tumor neovasculature through various direct or indirect mechanisms, and delay acquired resistance to these chemotherapeutic drugs[15-17]. Other findings have also demonstrated that anti-angiogenic therapy combined with various chemotherapy drugs could more effectively inhibit tumor growth without overt toxicity relative to either therapy alone[16-20]. Gemcitabine is a new deoxycytidine analog that inhibits DNA synthesis and has shown cytotoxicity against a wide range of cancer cell lines in vitro and applied widely in clinical anti-tumor therapy[20-22]. Moreover, studies have also confirmed that the combination of an anti-angiogenic biotherapy with a low-dose gemcitabine strategy can effectively suppress tumor angiogenesis without increased overt toxicity relative to either therapy alone[20]. Thus, in this study, we primarily evaluated the anti-tumor activities of the recombinant cFR-1 protein vaccine in combination with low-dose gemcitabine in a mouse tumor model.
The lyophilized recombinant proteins of cFR-1 and mouse FGFR-1 (mFR-1) were dissolved in NS and mixed with an equal volume of aluminum hydroxide adjuvant at 4 mg/mL for 60 min before use in vaccination[23].
The CT26 tumor model was established in BALB/c mice to evaluate whether the combination of cFR-1 vaccine and low-dose gemcitabine would improve the anti-tumor efficacy. Six to eight-week-old female mice were transplanted with 1 × 106 live tumor cells. After tumors had grown for 7 d, the mice were randomly divided into the following four groups of 10 mice each. Group 1 mice, treated with a combination of cFR-1 vaccine and low-dose gemcitabine (C + G), received cFR-1 vaccine plus low-dose gemcitabine as follows: after d 0 (7 d after tumor cell injection), cFR-1 vaccine was injected subcutaneously (s.c.) once a week for 4 wk with a dose of 10 μg per mouse. At d 7 (14 d after tumor cell injection), 20 mg/kg of gemcitabine was injected intraperitoneal (i.p.) at an interval of every 3 d for a total of 4 doses. Group 2 mice, treated with cFR-1 vaccine alone (cFR), received cFR-1 vaccine in a similar scheme as that in group 1, except that it lacked gemcitabine. Group 3 mice, treated with low-dose gemcitabine alone (G), received the same dose of gemcitabine as group 1, but they did not receive the cFR-1 vaccine. Group 4 mice, the untreated group (NS), received sterile NS s.c. as the scheme of vaccination or were treated i.p. as in group 1, respectively. Tumor growth was evaluated every 3 d and tumor volume was estimated using the formula for an ellipsoid (0.5 × length × width × height). At the end of the experiment, the tumor tissues, major organs and blood samples of the mice were collected for subsequent histologic and immunologic investigations. All studies involving mice were approved by the Institute's Animal Care and Use Committee.
Western blot analysis was performed as described pre-viously[14]. Briefly, the recombinant proteins were separated by 12 % SDS-PAGE. Gels were transblotted with the Mini Polyacrylamide Gel System (Bio-Rad, USA) onto a polyvinylidene difluoride membrane. Membrane blots were blocked at 4°C in 5% nonfat dry milk, washed and probed with mouse sera at a 1:500 dilution. Blots were then washed and incubated with goat anti-mouse IgG HRP-labeled secondary antibody and then stained with the Vectastain ABC kit (Vector, Burlingame, USA).
The ELISPOT assay for the enumeration of antibody-producing B cells (APBCs) has been described[14]. Briefly, PVDF-bottomed, 96-well filtration plates (Millipore, Bedford, USA) were coated with 30 μg/mL of recombinant FGFR-1 protein. Mononuclear cells prepared from spleen were incubated on the plates at 37°C for 4 h. IgG bound to the membrane was revealed as spots with alkaline phosphatase-conjugated anti-mouse IgG antibodies.
For microvessel density (MVD) and cell proliferation analyses, frozen sections were fixed in acetone, incubated and stained with antibodies reactive to either CD31 or proliferating cell nuclear antigen (PCNA) (BD Pharmingen, USA), respectively. The MVD was determined by counting the number of microvessels and the proliferation index was calculated as the ratio of the proliferation cell number to the total cell number per high-power field in tumor sections as described[24].
To identify the endothelial deposition of auto-antibody by immunofluorescent staining, frozen sections were fixed in acetone, washed with PBS, and incubated with FITC-conjugated antibody against mouse IgG, IgA, or IgM (Sigma, St. Louis, USA). Moreover, sections of tissue were fixed with 1% paraformaldehyde in PBS and stained for apoptosis analysis by using the TdT-mediated biotinylated-dUTP nick end labeling (TUNEL) assay according to the manufacturer's instructions (In Situ Cell Death Detection Kit; Roche, UK). These slides were imaged using a fluorescence microscope and the apoptosis index was calculated as the ratio of the apoptotic cell number to the total cell number in each high-power field.
Calculation of synergistic indexes: Mean values of tumor volume, MVD, cell apoptosis and proliferation were used for calculation of the correspondent synergistic indexes using the methods described before[24]. Briefly, the mean tumor volume, MVD, cell apoptosis index or proliferation index in each treatment group was obtained by dividing the mean value by that in the untreated control group. The expected relative ratio of the combination treatment group was obtained by timing the observed relative ratio of the xenogeneic FGFR-1 vaccine treatment group to that of the low-dose gemcitabine treatment group. Then, the corresponding synergistic index of tumor volume, MVD or proliferation (compared to the untreated control group, the resultant value was decreased) was obtained by dividing the expected relative ratio by the observed relative ratio, whereas the synergistic index of apoptosis (compared to the untreated control group, the resultant value was increased) was obtained by dividing the observed relative ratio by the expected relative ratio. The synergistic indexes of tumor volume, MVD, cell apoptosis and proliferation are further detailed in Table 1. An index of greater than 1 indicates a synergistic effect, whereas an index of less than 1 indicates a less than additive effect.
| Day2 | FGFR-1 | Gemcitabine | Combination therapy | Index4 | |
| Expected3 | Observed | ||||
| Tumor volume index | 0.48 | 0.60 | 0.29 | 0.17 | 1.71 |
| MVD index | 0.35 | 0.66 | 0.23 | 0.20 | 1.15 |
| Apoptosis index | 0.48 | 0.43 | 0.21 | 0.19 | 1.11 |
| Proliferation index | 0.49 | 0.46 | 0.22 | 0.21 | 1.04 |
Results were presented as mean ± SD. All statistical analyses were carried out using SPSS 12.0 for Windows statistical software (SPSS Inc, USA). For comparison of individual time points, ANOVA and an unpaired Student's t were used. Survival curves were constructed according to the Kaplan-Meier method. Statistical significance was determined by the log-rank test. P < 0.05 was considered statistically significant.
In the first part, cFR-1 vaccine or low-dose gemcitabine treatment resulted in the inhibition of tumor growth, to a certain extent, compared with the untreated control group. Remarkably, the combination therapy resulted in more significant anti-tumor activity (Figure 1A). The relative ratio of tumor volume in the combination group showed a synergistic relationship about 31 d after tumor cell transplantation in the tumor model (Table 1). In addition, the survival of tumor-bearing mice had similar results to that of tumor growth (Figure 1B).
The possibility that the cFR-1 vaccine alone or the com-bination treatment induces production of anti-tumor auto-immunity in the mouse model was examined by using Western blot analysis, ELISPOT assay and immunofluo-rescent staining.
In Western blot analysis, sera from these cFR-1-immunized mice recognized a protein as indicated by the positive staining of an about 40 kDa band (Figure 2A and C) that was not stained by sera from the untreated control or low-dose gemcitabine treatment groups (Figure 2B and D).
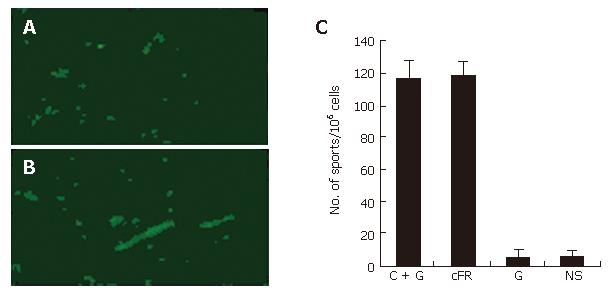
The endothelial deposition of auto-antibodies was found within tumor tissues from cFR-1-immunized mice, as detected by immunofluorescent staining (Figure 3A and 3B); however this deposition was not detected in the non-immunized control groups. In addition, detectable deposition of auto-antibodies was not found within the major organs of immunized and non-immunized mice.
The number of APBCs, which were detected by ELISPOT assay, was significantly elevated in the spleen of mice immunized with cFR-1 vaccine, both in the vaccine alone and in the combination treatment groups, as compared with those in the non-immunized groups (Figure 3C). Figure 3C shows that the number of APBCs was not different between the vaccine alone and combination treatment groups, which suggested that the low-dose gemcitabine scheme did not inhibit the immune response to cFR-1 immunization.
In this study, the combination treatment resulted in more significant synergistic suppression of tumor growth than treatment with the single agents individually. MVD was determined in tumor tissue sections stained with antibody reactive to CD31, cell apoptosis was evaluated by TUNEL, and cell proliferation was assayed by the presence of PCNA (Figure 4). About 31 d after tumor cell transplantation, the synergistic indexes of tumor volume, MVD, cell apoptosis and proliferation in the combination therapy group were 1.71 vs 1.15 vs 1.11 and 1.04, respectively (Table 1).
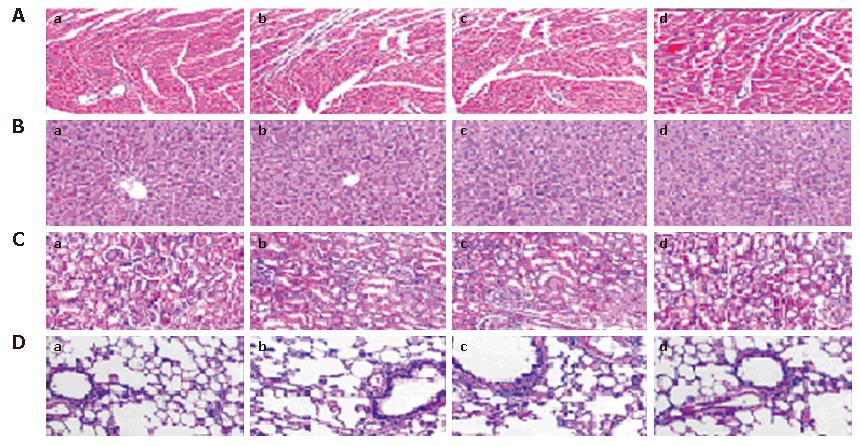
In this study, potential toxicity in gross measures was not observed in either the combination or single agent groups. The gross measures include such things as ruffling of fur behavior, body weight and life span. It should be noted that there was less feeding in mice treated with gemcitabine, which was both minor and transient, compared to those that did not receive chemotherapy. In addition, no pathologic changes in liver, lung, kidney or heart tissue sections, which were stained with hematoxylin and eosin (HE), were observed by microscopic examination (Figure 5). Furthermore, no detectable toxicity of bone marrow, kidney or liver was found by complete blood count and enzyme analyses (data not shown).
Angiogenesis is critical to the growth and metastasis of a tumor. Due to the genetic stability and accessibility to systemically delivered therapeutic agents, endothelial cells that line tumor blood vessels are attractive targets for anti-tumor therapy[5]. Since FGFR-1 is an important molecule for angiogenesis in solid tumors, as described previously, it is conceivable that breaking immune tolerance against FGFR-1-involved angiogenesis in solid tumors may be used as a useful and new approach for cancer therapy with active immunity. Some recent data have confirmed that xenogeneic homologous molecules can induce a cross-immunity reaction against self-homologous molecules that is responsible for anti-tumor activity[14,25,26]. Although anti-angiogenic therapy has proven to be effective at stopping tumor growth in many preclinical studies, it remains uncertain whether it is tumoricidal. Many studies have also concluded that this therapeutic limitation may be overcome by using a combination of angiogenic inhibitors with various chemotherapeutic drugs, such as cisplatin, gemcitabine, oxaliplatin, etc[16-20]. Thus, the strategy of combining anti-angiogenic biotherapy with chemotherapeutic drugs shows potential and promise for anti-tumor therapy.
Both acquired drug resistance and considerable systemic toxicities are major reasons for the limited advances made in cancer chemotherapy and have resulted in the failure of treatments. Gemcitabine is a new deoxycytidine analog that has been widely applied in clinical anti-tumor therapy. Many studies have also confirmed that the combination of anti-angiogenic biotherapy with low-dose gemcitabine can suppress tumor growth more effectively than conventional chemotherapy or anti-angiogenic biotherapy alone, including reversal of acquired drug resistance and minimization or elimination of systemic toxicity[17,20]. The purpose of our study was to evaluate the anti-tumor efficacy of cFR-1 protein vaccine combined with low-dose gemcitabine and the potential toxicity of the treatments in a mice colorectal cancer model.
Our present studies demonstrate that the combination strategy resulted in more effective inhibition of tumor growth, not only by induction of more effective anti-angiogenesis, but also by promotion of apoptosis and up-regulation of the suppression of cell proliferation in tumor tissues as compared with either therapy alone or with untreated groups, without obvious side-effects. The mechanism responsible for the interaction between cFR-1 vaccine and low-dose gemcitabine therapy may involve a synergistic anti-angiogenic effect and synergistic apoptosis and proliferation of tumor cells. On the one hand, the immunotherapy with cFR-1 vaccine could induce a special anti-tumor immunity reaction through induction of the production of auto-antibodies against FGFR-1, which could block bFGF/FGFR-1 signal transduction and further inhibit tumor growth by anti-angiogenesis. On the other hand, low-dose gemcitabine therapy could interfere with DNA synthesis and induce DNA breakage, thus resulting in tumor cell apoptosis[20-22]. Without acquisition of necessary oxygen and nutrients, there would be an increase in tumor cell apoptosis coupled with less proliferation and tumor angiogenesis. Moreover, the low-dose gemcitabine did not inhibit the host cross-immune response, but it potentiated anti-tumor effects as was demonstrated in the synergistic indexes of tumor volume, MVD, apoptosis and proliferation, and the presence of both antibodies and APBCs in the cFR-1-immunized mice which indicate gemcitabine has an anti-tumor effect. Therefore, the combination therapy strategy showed effective and synergistic anti-tumor activity.
In conclusion, our findings demonstrated that the combination therapy strategy of cFR-1 vaccine combined with low-dose metronomic gemcitabine effectively and synergistically suppressed tumor growth via inhibition of tumor angiogenesis without systemic toxicity in mice.
| 1. | Hawk ET, Levin B. Colorectal cancer prevention. J Clin Oncol. 2005;23:378-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 156] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 2. | Li M, Gu J. Changing patterns of colorectal cancer in China over a period of 20 years. World J Gastroenterol. 2005;11:4685-4688. [PubMed] |
| 3. | Coluccia AM, Benati D, Dekhil H, De Filippo A, Lan C, Gambacorti-Passerini C. SKI-606 decreases growth and motility of colorectal cancer cells by preventing pp60(c-Src)-dependent tyrosine phosphorylation of beta-catenin and its nuclear signaling. Cancer Res. 2006;66:2279-2286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Folkman J. Angiogenesis-dependent diseases. Semin Oncol. 2001;28:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 205] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 5. | Fan TP, Jaggar R, Bicknell R. Controlling the vasculature: angiogenesis, anti-angiogenesis and vascular targeting of gene therapy. Trends Pharmacol Sci. 1995;16:57-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 125] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | He Y, Zhou J, Wu JS, Dou KF. Inhibitory effects of EGFR antisense oligodeox ynucleotide in human colorectal cancer cell line. World J Gastroenterol. 2000;6:747-749. [PubMed] |
| 7. | Powers CJ, McLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer. 2000;7:165-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 947] [Cited by in RCA: 979] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 8. | Chaudhuri MM, Moscatelli D, Basilico C. Involvement of the conserved acidic amino acid domain of FGF receptor 1 in ligand-receptor interaction. J Cell Physiol. 1993;157:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Cross MJ, Lu L, Magnusson P, Nyqvist D, Holmqvist K, Welsh M, Claesson-Welsh L. The Shb adaptor protein binds to tyrosine 766 in the FGFR-1 and regulates the Ras/MEK/MAPK pathway via FRS2 phosphorylation in endothelial cells. Mol Biol Cell. 2002;13:2881-2893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Udayakumar TS, Klein RD, Maliner MS, Nagle RB, Bowden GT. Aberrant expression of fibroblast growth factor receptor-1 in prostate epithelial cells allows induction of promatrilysin expression by fibroblast growth factors. Int J Cancer. 2001;91:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Hori A, Sasada R, Matsutani E, Naito K, Sakura Y, Fujita T, Kozai Y. Suppression of solid tumor growth by immunoneutralizing monoclonal antibody against human basic fibroblast growth factor. Cancer Res. 1991;51:6180-6184. [PubMed] |
| 12. | Valesky M, Spang AJ, Fisher GW, Farkas DL, Becker D. Noninvasive dynamic fluorescence imaging of human melanomas reveals that targeted inhibition of bFGF or FGFR-1 in melanoma cells blocks tumor growth by apoptosis. Mol Med. 2002;8:103-112. [PubMed] |
| 13. | Wang F, McKeehan K, Yu C, McKeehan WL. Fibroblast growth factor receptor 1 phosphotyrosine 766: molecular target for prevention of progression of prostate tumors to malignancy. Cancer Res. 2002;62:1898-1903. [PubMed] |
| 14. | Zheng S, Huang F, Zheng S, Wang W, Yin H, Wu R. Vaccination with a recombinant chicken FGFR-1 bypasses immunological tolerance against self-FGFR-1 in mice. J Huazhong Univ Sci Technolog Med Sci. 2006;26:389-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Boehm T, Folkman J, Browder T, O'Reilly MS. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature. 1997;390:404-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1201] [Cited by in RCA: 1132] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 16. | Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, Bohlen P, Kerbel RS. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15-R24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 852] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 17. | Bocci G, Nicolaou KC, Kerbel RS. Protracted low-dose effects on human endothelial cell proliferation and survival in vitro reveal a selective antiangiogenic window for various chemotherapeutic drugs. Cancer Res. 2002;62:6938-6943. [PubMed] |
| 18. | Browder T, Butterfield CE, Kräling BM, Shi B, Marshall B, O'Reilly MS, Folkman J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878-1886. [PubMed] |
| 19. | Sweeney CJ, Miller KD, Sissons SE, Nozaki S, Heilman DK, Shen J, Sledge GW. The antiangiogenic property of docetaxel is synergistic with a recombinant humanized monoclonal antibody against vascular endothelial growth factor or 2-methoxyestradiol but antagonized by endothelial growth factors. Cancer Res. 2001;61:3369-3372. [PubMed] |
| 20. | Bruns CJ, Shrader M, Harbison MT, Portera C, Solorzano CC, Jauch KW, Hicklin DJ, Radinsky R, Ellis LM. Effect of the vascular endothelial growth factor receptor-2 antibody DC101 plus gemcitabine on growth, metastasis and angiogenesis of human pancreatic cancer growing orthotopically in nude mice. Int J Cancer. 2002;102:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 122] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Hertel LW, Boder GB, Kroin JS, Rinzel SM, Poore GA, Todd GC, Grindey GB. Evaluation of the antitumor activity of gemcitabine (2',2'-difluoro-2'-deoxycytidine). Cancer Res. 1990;50:4417-4422. [PubMed] |
| 22. | Jiang PH, Motoo Y, Sawabu N, Minamoto T. Effect of gemcitabine on the expression of apoptosis-related genes in human pancreatic cancer cells. World J Gastroenterol. 2006;12:1597-1602. [PubMed] |
| 23. | Zheng SJ, Huang FY, Zheng SP, Wang W, Wu RL. Construction and identification of recombinant plasmid encoding extracellular domain of FGFR-1 in chicken. Huazhong Kejidaxue Xuebao Yixueban. 2006;35:433-435. |
| 24. | Wild R, Ramakrishnan S, Sedgewick J, Griffioen AW. Quantitative assessment of angiogenesis and tumor vessel architecture by computer-assisted digital image analysis: effects of VEGF-toxin conjugate on tumor microvessel density. Microvasc Res. 2000;59:368-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 105] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Gregor PD, Wolchok JD, Turaga V, Latouche JB, Sadelain M, Bacich D, Heston WD, Houghton AN, Scher HI. Induction of autoantibodies to syngeneic prostate-specific membrane antigen by xenogeneic vaccination. Int J Cancer. 2005;116:415-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Srinivasan R, Wolchok JD. Tumor antigens for cancer immunotherapy: therapeutic potential of xenogeneic DNA vaccines. J Transl Med. 2004;2:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
Co-first-authors: Shao-Jiang Zheng and Shao-Ping Zheng
S- Editor Wang J L- Editor Xia HHX E- Editor Chen GJ