Published online Apr 28, 2007. doi: 10.3748/wjg.v13.i16.2369
Revised: February 1, 2007
Accepted: February 8, 2007
Published online: April 28, 2007
AIM: To investigate whether hypoxia inducible factor-1α (HIF-1α) is linked to the protective effects of ischemic preconditioning (IP) on sinusoidal endothelial cells against ischemia/reperfusion injury.
METHODS: Sinusoidal endothelial cell lines ECV-304 were cultured and divided into four groups: control group, cells were cultured in complete DMEM medium; cold anoxia/warm reoxygenation (A/R) group, cells were preserved in a 4°C UW solution in a mixture of 95% N2 and 5% CO2 for 24 h; anoxia-preconditioning (APC) group, cells were treated with 4 cycles of short anoxia and reoxygenation before prolonged anoxia-preconditioning treatment; and anoxia-preconditioning and hypoxia inducible factor-1α (HIF-1α) inhibitor (I-HIF-1) group, cells were pretreated with 5 μm of HIF-1α inhibitor NS398 in DMEM medium before subjected to the same treatment as group APC. After the anoxia treatment, each group was reoxygenated in a mixture of 95% air and 5% CO2 incubator for 6 h. Cytoprotections were evaluated by cell viabilities from Trypan blue, lactate dehydrogenase (LDH) release rates, and intracellular cell adhesion molecule-1 (ICAM-1) expressions. Expressions of HIF-1α mRNA and HIF-1α protein from each group were determined by the RT-PCR method and Western blotting, respectively.
RESULTS: Ischemia preconditioning increased cell viability, and reduced LDH release and ICAM-1 expressions. Ischemia preconditioning also upregulated the HIF-1α mRNA level and HIF-1α protein expression. However, all of these changes were reversed by HIF-1α inhibitor NS398.
CONCLUSION: Ischemia preconditioning effectively inhibited cold hypoxia/warm reoxygenation injury to endothelial cells, and the authors showed for the first time HIF-1α is causally linked to the protective effects of ischemic preconditioning on endothelial cells.
- Citation: Shi LB, Huang JH, Han BS. Hypoxia inducible factor-1α mediates protective effects of ischemic preconditioning on ECV-304 endothelial cells. World J Gastroenterol 2007; 13(16): 2369-2373
- URL: https://www.wjgnet.com/1007-9327/full/v13/i16/2369.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i16.2369
Cold ischemia/warm reperfusion injury has been well demonstrated that this process results in inevitable pathophysiology in clinical organ transplantation, and contributes to diverse serious post-transplantation complications such as chronic dysfunction or primary non-function of transplantations, and increase the rate of acute organ repellence[1,2]. The injury comes from cold ischemia during organ preservation, also from subsequent reperfusion[2]. At the cellular level, both hepatocytes and sinusoidal endothelial cells were damaged. However, recent studies indicated that sinusoidal endothelial cells are more vulnerable and damaged prior to hepatocyte during ischemia/reperfusion[3-5]. This endothelial dysfunction, at least in coronary endothelial cells, is not a transient phenomenon, perhaps persisting several months after reperfusion[6,7]. Therefore, reducing the damage to endothelial cells caused by ischemia/reperfusion is pivotal to maintain the normal function of grafts.
Ischemic preconditioning (IP) is a novel and potent protective approach that effectively prevents ischemia and reperfusion injury, and is already applied in liver transplantation in the animal model[8,9]. However, the underlying mechanisms remain unclear. Hypoxia inducible factor-1 (HIF-1) is a transcriptional factor. It responds to low oxygen conditions and acts as a master regulator of gene expression. Genes regulated by HIF-1 provide protection to cells through augmenting tissue oxygen supply or increasing tolerance to severe oxygen deprivation[10]. It was shown that HIF-1 played an important role in protecting brain against ischemia injury[11]. Thus we hypothesize that HIF-1 is involved in the potent protective effects of IP on sinusoidal endothelial cells. In the present study, we used ECV304 sinusoidal endothelial cells as a model to imitate cold ischemia/reperfusion process of clinical organ transplantation, and we indicated that, for the first time, HIF-1 is causally linked to the protective effects of IP on sinusoidal endothelial cells, suggesting a potential clinical application in the future.
ECV 304 endothelial cells obtained from Shanghai Institute for Biological Sciences were maintained in complete DMEM medium containing 10% fetal bovine serum in a humidified atmosphere of 5% CO2 at 37°C. The cultured medium was replaced by fresh medium every three days. After further synchronization in complete DMEM medium for 12 h, cells were divided into four groups: control group, cells were further cultured in complete DMEM medium in a mixture of 95% N2 and 5% CO2 incubator for 24 h at 37°C; A/R group, cells were preserved in a 4°C UW solution (Du Pont Pharma, Canada) in a mixture of 95% N2 and 5% CO2 for 24 h; APC group, cells were followed by 4 cycles of 15 min of anoxia and 15 min of reoxygenation treatment before subjecting to the prolonged anoxia-preconditioning treatment as A/R group; and AP + HIF-1α inhibitor group (I-HIF), cells were cultured in DMEM medium with 0.1 mmol/L HIF-1α inhibitor NS398 (Jingmei Ltd., China) before subjecting to anoxia-preconditioning treatment as the AP group. Before cold anoxia storage, expressions of HIF-1 mRNA and HIF-1α protein from each group were determined by RT-PCR and western blotting, respectively. After anoxia treatment each group was reoxygenated in a mixture of 95% air and 5% CO2 incubator for 6 h. Cell viability was evaluated by Trypan blue dye absorbance rate and lactic dehydrogenase (LDH) release rate, respectively. Intracellular cell adhesion molecule (ICAM-1) expressions were detected by flow cytometer.
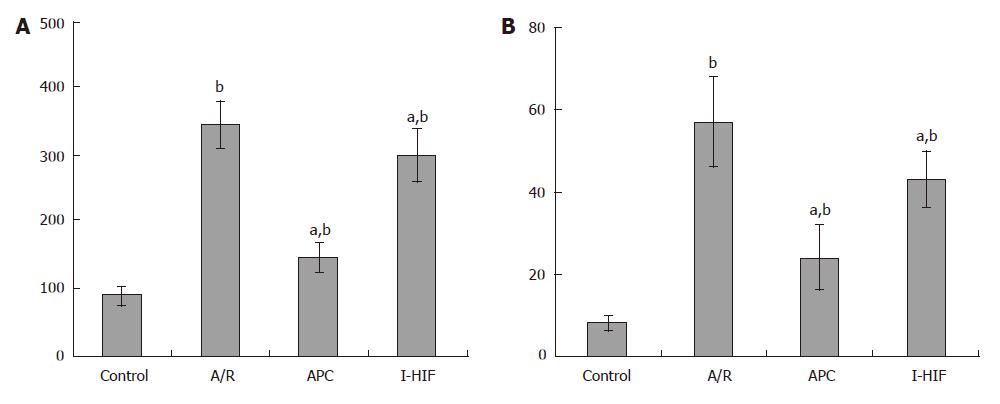
Determination of Trypan blue dye absorbing rate (TBDAR): cells from each group were trypsinized using an 0.25% Trypsin solution, then were adjusted to 106 cells/mL single cell suspension. After adding nine drops of the suspension into a cuvette, 1 drop of 0.4% Trypan blue dye solution was added into each group. Samples from each group were mixed thoroughly before transferring immediately to the edge of the hemocytometer chamber. The number of stained cell and total cell number was counted within 3 min. The cells viability percentage was obtained by the following mathematical equation: TBDAR = (SC/TC) × 100, where SC is the stained cell number, and TC is the total cell number.
Determination of lactate dehydrogenase (LDH) level: upper serum from each group was taken, and determined by Automatic Biochemistry Analyzer CL-2000 (Shimatsuna, Japan).
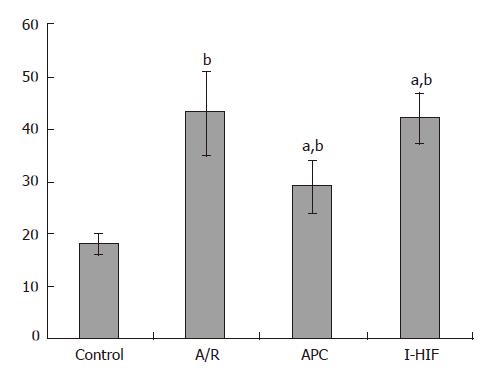
Aliquots of cell suspensions of 1.8 mL at a density of 2.2 × 105 cells/dish in complete medium were placed in a 3.5 mm dish. Each group was trypsinized using an 0.25% Trypsin solution, followed by washing twice and collecting with phosphate buffered saline (PBS). After disposing the upper serum after centrifuging at 2000 r/min for 10 min, samples from each group were exposed to 5 μL FITC-conjugated murine anti-human ICAM-1 obtained from Santa Cruz, USA. (Cat. No. sc-107) for 30 min at room temperature evading sunlights. The samples were washed twice with PBS to be free from staining solutions. After centrifuging and discarding the upper serum, the final samples were fixed by 1 mL of 0.5% paraformaldehyde. Lumps of the cells were removed using a 40 μm nylon mesh. Green (FITC) fluorescence was collected through a 530 nm band filter using a Coulter Epics Elite Flow Cytometer (Miami, FL), and at least 10 000 cells were measured each time.
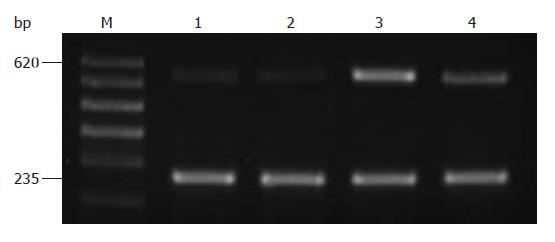
Total RNA was extracted by one step rapid use of Trizol reagent (Invitrogen, USA. Cat. No. 15596-026), 1 μg of total RNA was used for reverse transcription in an RT-PCR volume of 50 μL using RT-PCR superscriptTM III kits from Invitrogen, USA. (Cat. No. 12574-018). PCR increased HIF-1α illumination with GAPDH as the internal reference. For HIF-1α, upstream primer 5'-CCT GCA CTC AAT CAA GAA GTT GC-3' and downstream primer 5'-TTC CTG CTC TGT TTG GTG AGG CT-3' were used, and the PCR product was 620 bp. PCR conditions were as follows: predenaturation for 5 min at 94°C; 27 cycles for 1 min at 94°C, 1 min at 64°C, and 1 min at 72°C; and finally 10 min at 72°C. Upstream GAPDH primer 5'-TGG GGA AGG TGA AGG TCG GA-3' and downstream GAPDH primer 5'-GGG ATC TCG CTG CTC GAA GA-3' were employed, and the PCR product was 235 bp. After 2% agarose gel electrophoresis, PCR products were observed and photographed under ultraviolet. Results were analyzed by scoring optical density scanning using the Gel Doc2000 digital imaging system, and the ratio between HIF-1α and GAPDH was calculated.
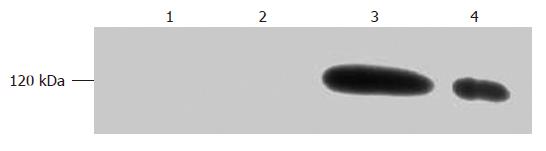
Cells were washed twice with PBS, and lysed in RIPA buffer on ice for 20 min. After centrifuging at 12 000 ×g for 20 min, upper serum was collected and the protein level was analyzed by the Lowry method. The reaction solution was analyzed on a discontinuous 7.5% SDS/polyacrylamide gel eletrophoresis (SDS-PAGE) system by 30 μg/hole. After transferring proteins to polyvinylidene difluoride (PVDF) membrane by electroblotting (220 V for 2 h), the PVDF membrane was blocked at 37°C for 1 h in Tris-buffered saline (TBS) plus 0.3% bovine serum albumin (TBS-BSA). Then the membrane was incubated with 1:1000 dilution of murine anti-human HIF-1α antibody obtained from Santa Cruz, USA (Cat. No. sc-13515) at 4°C overnight. After washing with TBS, 1:2000 dilution of alkaline phosphatase labeled goat anti-mouse IgG obtained from Santa Cruz, USA (Cat. No. sc-2302) was added and further incubated at 37°C for 0.5 h. Detection was performed by alkaline phosphatase system coloration.
All results were expressed as mean value ± standard deviation. Group measurements were assessed by one-way Anova examination. Ratio calculations were carried out by χ2. And P < 0.05 was considered statistically significant.
There was high upregulation of TBDAR and LDH level in the A/R treatment group, when compared to the control, the P value is less than 0.01, suggesting a significant damage to ECV 304 endothelial cells by A/R treatment. In APC group, TBDAR and LDH levels were reduced (P < 0.05) when compared to the A/R group. But in the I-HIF group, by using HIF-1α inhibitor, both TBDAR and LDH levels were reversed, the P value is less than 0.05 when compared to the A/R group, indicating a reduced damage to cells. All three groups, A/R, APC and I-HIF showed higher TRDAR and LDH levels (P < 0.01), suggesting AP had no complete protective effects on endothelial cells (Figure 1).
Flurescence results demonstrated ICAM-1 expression was at a low level of 14% ± 2.3% in control, and up-regulated up to 53% ± 7.6% (P < 0.01). After intervention by anoxia preconditioning, the rate decreased again (P < 0.05) compared to the A/R group. In the I-HIF group, ICAM-1 expression rose to 39% ± 7.1% (P < 0.05) compared to APC, and no significance was found when compared to A/R group, suggesting a reverse effect of HIF-1α inhibitor NS398 (Figure 2).
The length of PCR products was 620 bp for HIF-1α mRNA and 235 bp for GAPDH as an internal reference. In each column, two bands were seen. The results showed that after the anoxia-preconditioning treatment, the level of 620 bp HIF-1α mRNA was highly increased (P < 0.05), whereas the cell group pretreated with HIF-1α inhibitor NS398 which highly inhibited 620 bp HIF-1α mRNA (Figure 3).
Normal endothelial cells have certain HIF-1α mRNA, but the protein expression is either undetectable or extremely rare. After the anoxia-preconditioning treatment, the level of 120 kDa protein expression was highly increased (P < 0.05), while the cell group was pretreated with HIF-1α inhibitor NS398 which greatly inhibited the 120 kDa protein expression (Figure 4).
Many studies have indicated that during cold ischemia/reperfusion, endothelial cells are susceptible to cell swelling, detachment from their matrix, necrosis, and apoptosis[12,13]. Furthermore, sinusoidal endothelial damage was highly associated with microcirculatory failure following cold ischemia/reperfusion[14]. Adhesion of granulocyte endothelial cells, manifested as intracellular cell adhesion molecule expression such as ICAM-1, play a key role in this process[15]. In addition, activated endothelial cells act as antigen presenting cells, which increase the acute organ repellence rate[1]. In our study, we confirmed that endothelial cells were severely damaged after 24 h of cold hypoxia storage and 6h of warm reoxygenization demonstrated by the significant increase of the Trypan blue dye absorbing rate, release of LDH, and upregulation of ICAM-1 expression. These results showed a severe endothelial cell damage caused by ischemia/reperfusion injury, which were consistent with previous studies.
Ischemia preconditioning, defined as a short period of ischemia and reperfusion prior to a prolonged ischemic insult, is a novel and potent method of avoiding ischemia/reperfusion injury. It was first discovered in research of cardial IP treatment on dogs[16], and already proved in rodents and larger mammals such as pigs[4,17]. Our results demonstrated an IP reduced Trypan blue dye absorbing rate, LDH release level, and inhibited ICAM-1 expression after 24 h of cold hypoxia storage and 6h of warm reoxygenization. These data strongly showed that anoxia preconditioning treatment can protect ECV304 endothelial cells. To further investigate the underlying mechanism, we examined the expression of HIF-1 α mRNA and protein expression with or without HIF-1 α inhibitor NS398.
HIF-1 is a nuclear transcriptional factor related to tolerance on ischemia anoxia. It is composed of α and β subunits. Although HIF-1 DNA and mRNA and β proteins exist in normal cells, HIF-1α proteins are not detectable. When chronic anoxia occurs, HIF-1α proteins are highly increased and then combine with HIF-1 β protein forming active heterogeneous HIF-1 dimer[18]. The dimers regulate the transcription of the proliferation gene expression of many important cells. These proteins are either capable of improving oxygen supplies for ischemic tissues or to decrease cell cytometabolism in order to alleviate oxygen shortage to reach homeostasis, therefore they increase the tolerance and adaptability to ischemia[10]. It was reported several key genes involved in maintenance of endothelial cells and responsible for endothelium-dependent vasodilation, were regulated by HIF-1 inculding inducible NO synthase( iNOS), vascular endothelial growth factor(VEGF), heme oxygenase-1[19]. Our results demonstrated that the protective effects of IP were accompanied by increased HIF-1 mRNA and protein expression. Thus increased HIF-1 expression level may mediate the protective effects of IP by improving microcirculatory function. Furthermore, using HIF-1α inhibitor NS398, we found all protective effects caused by IP were reversed. From this result, we can draw a conclusion that HIF-1α is causally linked to the protective effects of ischemic preconditioning on ECV-304 endothelial cells.
Recently several drugs or preservation solutions were shown to precondition ischemia injury, termed as “chemical preconditioning” [20,21]. But to date, there is no agent in research that targets HIF-1α. Thus our study not only provides a new mechanism by which IP exerts its protective effects against ischemia/reperfusion injury, but also provides a new drug target. New drugs based on this target, used either alone or in combination with the above-mentioned agents will further promote the effects of chemical preconditioning in the future.
Cold ischemia/warm reperfusion injury frequently results in inevitable pathophysiology in clinical organ transplantation, and diverse serious post-transplantation complications. Ischemic preconditioning (IP) is a novel and potent protective approach that effectively prevents ischemia and reperfusion injury. But the underlying mechanisms remain unclear.
Recent studies indicated that sinusoidal endothelial cells are more vulnerable and damaged prior to hepatocyte during ischemia/ reperfusion. So damage to endothelial cells was pivotal in the process. Hypoxia inducible factor -1α (HIF-1α) is a nucleus transcriptional factor and regulates transcriptions of many genes related to tolerance on ischemia anoxia. The authors investigated whether IP has protective effects on endothelial cells and the possible connection between the effects and HIF-1α.
This is the first report to show that there is strong correlation between HIF-1α upregulation and the protective effects of ischemia preconditioning on ECV-304 endothelial cells.
HIF-1α is expected as a new drug target and provides a potential use in clinical organ transplantation.
This is an interesting and well-performed study, pointing to the potential clinical application of HIF-1α.
| 1. | Bilzer M, Gerbes AL. Preservation injury of the liver: mechanisms and novel therapeutic strategies. J Hepatol. 2000;32:508-515. |
| 2. | Ohkohchi N. Mechanisms of preservation and ischemic/reperfusion injury in liver transplantation. Transplant Proc. 2002;34:2670-2673. |
| 3. | Matsumoto K, Honda K, Kobayashi N. Protective effect of heat preconditioning of rat liver graft resulting in improved transplant survival. Transplantation. 2001;71:862-868. |
| 4. | Tsukamoto S, Ohkohchi N, Endoh T, Seya K, Satomi S, Mori S. Procurement of liver grafts by an artificial heart-lung machine using leukocyte-depleted washed red blood cells in non-heart-beating donors. Transplant Proc. 1996;28:197-200. |
| 5. | Gao WS, Takei Y, Marzi I, Lindert KA, Caldwell-Kenkel JC, Currin RT, Tanaka Y, Lemasters JJ, Thurman RG. Carolina rinse solution--a new strategy to increase survival time after orthotopic liver transplantation in the rat. Transplantation. 1991;52:417-424. |
| 6. | Kaeffer N, Richard V, François A, Lallemand F, Henry JP, Thuillez C. Preconditioning prevents chronic reperfusion-induced coronary endothelial dysfunction in rats. Am J Physiol. 1996;271:H842-H849. |
| 7. | Laude K, Beauchamp P, Thuillez C, Richard V. Endothelial protective effects of preconditioning. Cardiovasc Res. 2002;55:466-473. |
| 8. | Adam R, Arnault I, Bao YM, Salvucci M, Sebagh M, Bismuth H. Effect of ischemic preconditioning on hepatic tolerance to cold ischemia in the rat. Transpl Int. 1998;11 Suppl 1:S168-S170. |
| 9. | Schulz R, Walz MK, Behrends M, Neumann T, Gerken G, Heusch G. Minimal protection of the liver by ischemic preconditioning in pigs. Am J Physiol Heart Circ Physiol. 2001;280:H198-H207. |
| 10. | Genc S, Akhisaroglu M, Kuralay F, Genc K. Erythropoietin restores glutathione peroxidase activity in 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine-induced neurotoxicity in C57BL mice and stimulates murine astroglial glutathione peroxidase production in vitro. Neurosci Lett. 2002;321:73-76. |
| 11. | Sharp FR, Ran R, Lu A, Tang Y, Strauss KI, Glass T, Ardizzone T, Bernaudin M. Hypoxic preconditioning protects against ischemic brain injury. NeuroRx. 2004;1:26-35. |
| 12. | Gao W, Bentley RC, Madden JF, Clavien PA. Apoptosis of sinusoidal endothelial cells is a critical mechanism of preservation injury in rat liver transplantation. Hepatology. 1998;27:1652-1660. |
| 13. | Huet PM, Nagaoka MR, Desbiens G, Tarrab E, Brault A, Bralet MP, Bilodeau M. Sinusoidal endothelial cell and hepatocyte death following cold ischemia-warm reperfusion of the rat liver. Hepatology. 2004;39:1110-1119. |
| 14. | Arii S, Imamura M. Physiological role of sinusoidal endothelial cells and Kupffer cells and their implication in the pathogenesis of liver injury. J Hepatobiliary Pancreat Surg. 2000;7:40-48. |
| 15. | Sakamoto S, Okanoue T, Itoh Y, Nakagawa Y, Nakamura H, Morita A, Daimon Y, Sakamoto K, Yoshida N, Yoshikawa T. Involvement of Kupffer cells in the interaction between neutrophils and sinusoidal endothelial cells in rats. Shock. 2002;18:152-157. |
| 16. | Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124-1136. |
| 17. | Arai M, Thurman RG, Lemasters JJ. Ischemic preconditioning of rat livers against cold storage-reperfusion injury: role of nonparenchymal cells and the phenomenon of heterologous preconditioning. Liver Transpl. 2001;7:292-299. |
| 18. | Schumacker PT. Hypoxia-inducible factor-1 (HIF-1). Crit Care Med. 2005;33:S423-S425. |
| 19. | Wenger RH. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 2002;16:1151-1162. |
| 20. | Riepe MW, Ludolph AC. Chemical preconditioning: a cytoprotective strategy. Mol Cell Biochem. 1997;174:249-254. |
| 21. | Janssen H, Janssen PH, Broelsch CE. UW is superior to Celsior and HTK in the protection of human liver endothelial cells against preservation injury. Liver Transpl. 2004;10:1514-1523. |
S- Editor Liu Y L- Editor Ma JY E- Editor Ma WH