Published online Apr 28, 2007. doi: 10.3748/wjg.v13.i16.2344
Revised: March 3, 2006
Accepted: March 8, 2007
Published online: April 28, 2007
AIM: To delineate the mechanisms of renal vasocon-striction in hepatorenal syndrome (HRS), we investigated the expression of typeIinositol 1, 4, 5-triphosphate receptors (IP3RI) of kidney in mice with fulminant hepatic failure (FHF).
METHODS: FHF was induced by lipopolysaccharide (LPS) in D-galactosamine (GalN) sensitized BALB/c mice. There were 20 mice in normal saline (NS)-treated group, 20 mice in LPS-treated group, 20 mice in GalN-treated group, and 60 mice in GalN/LPS-treated group (FHF group). Liver and kidney tissues were obtained at 2, 6, and 9 h after administration. The liver and kidney specimens were stained with hematoxylin-eosin for studying morphological changes under light microscope. The expression of IP3RIin kidney tissue was tested by immunohistochemistry, Western blot and reverse transcription (RT)-PCR.
RESULTS: Kidney tissues were morphologically normal at all time points in all groups. IP3RIproteins were found localized in the plasma region of glomerular mesangial cells (GMC) and vascular smooth muscle cells (VSMC) in kidney by immunohistochemical staining. In kidney of mice with FHF at 6 h and 9 h IP3RIstaining was up-regulated. Results from Western blot demonstrated consistent and significant increment of IP3RIexpression in mice with FHF at 6 h and 9 h (t = 3.16, P < 0.05; t = 5.43, P < 0.01). Furthermore, we evaluated IP3RImRNA expression by RT-PCR and observed marked up-regulation of IP3RImRNA in FHF samples at 2 h, 6 h and 9 h compared to controls (t = 2.97, P < 0.05; t = 4.42, P < 0.01; t = 3.81, P < 0.01).
CONCLUSION: The expression of IP3RIprotein increased in GMC and renal VSMC of mice with FHF, possibly caused by up-regulation of IP3RImRNA.
- Citation: Wen Y, Cui W, Liu P. Type I inositol 1, 4, 5-triphosphate receptors increase in kidney of mice with fulminant hepatic failure. World J Gastroenterol 2007; 13(16): 2344-2348
- URL: https://www.wjgnet.com/1007-9327/full/v13/i16/2344.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i16.2344
Patients with fulminant hepatic failure (FHF) are at high risk for the serious complication of hepatorenal syndrome (HRS), which is an important cause of death[1]. HRS is a functional renal failure secondary to the liver failure itself[2,3]. Renal failure will recover when there is recovery of liver function, and in the absence of a spontaneous hepatic recovery, liver transplantation will reverse the HRS. Until now, its precise pathogenesis is not clear. It is considered generally that the decrement of renal plasma flow (RPF) caused by renal vasocontraction is a key factor[4]. Multiple mechanisms are involved in renal vasoconstriction. Cell contraction is closely related to changes of intracellular calcium ([Ca2+] i) signaling. The inositol 1, 4, 5-trisphosphate receptors (IP3Rs) is the primary cytosolic target for the initiation of [Ca2+] i. Many cell types, often nonexcitable, including glomerular mesangial cells (GMC) and renal vascular smooth muscle cells (VSMC), depend on this pathway to couple external signals to intracellular Ca2+ release[5]. IP3Rs not only triggers the release of calcium from intracellular stores but also opens plasma membrane calcium channels[6,7]. This is thought to be a crucial step in allowing the cell to contract to some agonists. Many factors can affect the level of IP3Rs expression[8,9]. Is there a relationship between the changes in IP3Rs expression level and HRS progression in FHF? In order to explore the potential mechanisms, we conducted a series of studies on IP3RIexpression in the kidney of mice with FHF.
BALB/c mice were provided by Laboratory Animal Center in China Medical University. D-galactosamine (GalN) and lipopolysaccharide (LPS) were obtained from Sigma. Polyclonal antibody of IP3RIwas purchased from US biological. BCIP/NBT liquid substrste system was purchased from Sigma. RT-PCR kit was purchased from TskaRa.
One hundred and twenty six-week-old male BALB/c mice were divided into 4 groups: 20 mice in NS-treated group, 20 mice in LPS-treated group, 20 mice in GalN-treated group, and 60 mice in GalN/LPS-treated group (20 mice at 2, 6, 9 h, respectively). Mice with FHF were given an intraperitoneal injection of D-galactosamine (GalN, 800 mg/kg body weight), followed by lipopolysaccharide (LPS, 10 μg/kg body weight)[10]. In control groups, mice were given an intraperitoneal injection of GalN (800 mg/kg body weight), LPS (10 μg/kg body weight), NS (similar volume to GalN and LPS), respectively. Liver and kidney tissues were fixed for histopathologic analysis and immunohistochemistry under light microscopy. Frozen specimens were stored for quantitative analysis of IP3RIby Western blot and RT-PCR .
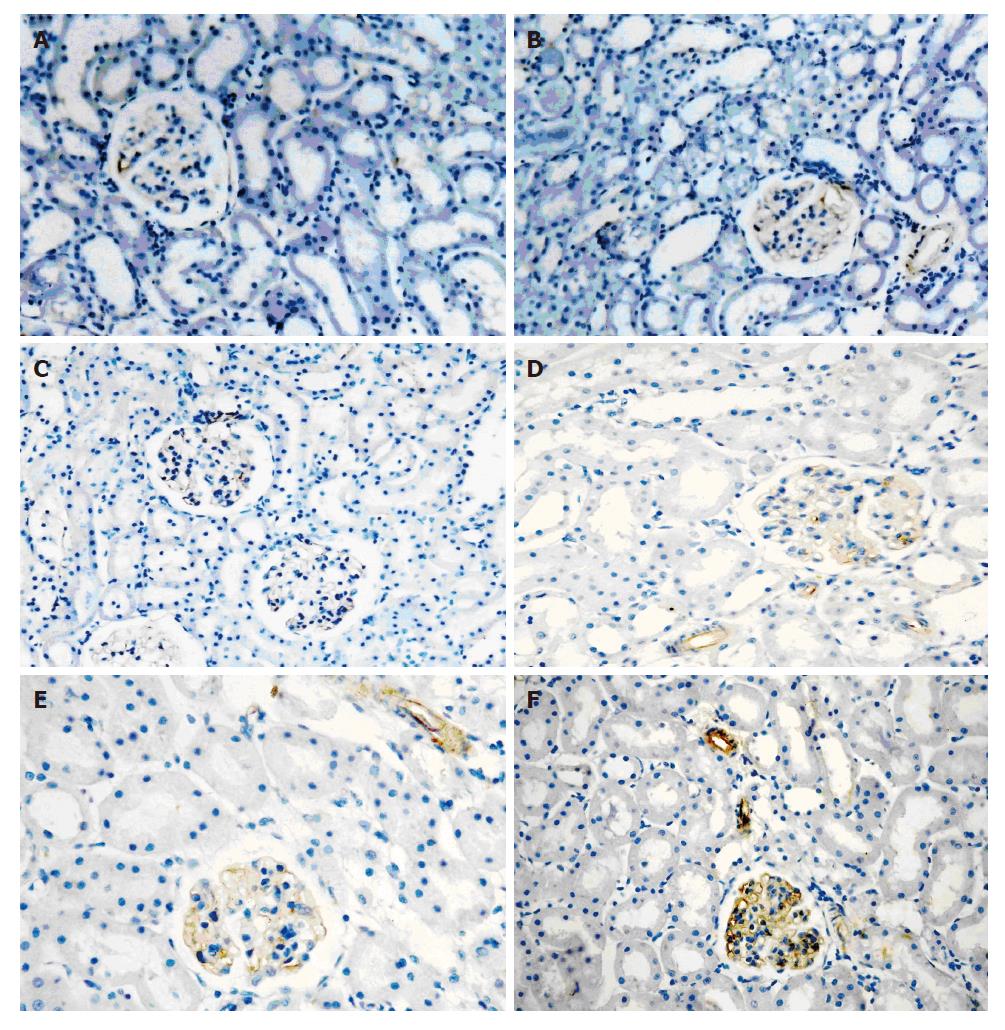
Sections were indirectly immunolabeled with an ABC kit according to the manufacturer's instructions. Sections were deparaffined, blocked with normal goat serum for 30 min, and incubated with primary antibody (rabbit anti-mouse IP3RIdiluted in phosphate-buffered saline (PBS) 1:100) for 12 h in a humidified chamber at 4°C. These sections were then rinsed thrice for 10 min in PBS, and the secondary antibody (goat anti-rabbit IgG diluted 1:100 in PBS) was applied for 2 h at room temperature. Sections were rinsed in PBS and then in distilled water. Fresh peroxidase reaction mixture containing equal amounts of 0.02% hydrogen peroxide in H2O and 0.1% diaminobenzidine in PBS were prepared. We chose four fields of vision at random, and analyzed the optical density using the “multi-system color/RGB monitor” computer image processing system.
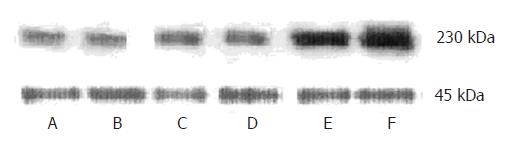
Total protein of kidney tissues were extracted in lysis buffer (50 mmol/L Tris-HCl (pH 7.2), 150 mmol/L NaCl, 1% Triton X-100, 1 mmol/L EDTA, 1 mmol/L PMSF, and 5 mg/mL each of aprotinin and leupeptin). Protein concentrations were determined by BCA protein assay and equal amounts of protein were run on a 6% SDS-PAGE gel, and transferred to PVDF membrane. The PVDF membrane was then blocked with 5% nonfat dry milk for 2 h. After being blocked, PVDF membrane was immunoblotted with the primary antibody (IP3RIantibody) for 2 h. The membrane was washed three times with TBS to remove unbound primary antibody. Then the membrane was incubated with horseradish peroxidase (HRP)-conjugated goat-anti-rabbit IgG for 2 h, washed three times with TTBS to remove unbound secondary antibody, and incubated with BCIP/NBT liquid substrate reagent for 5 min, the reaction was stopped by H2O. β-actin was used for internal control, and 230KD bands of IP3R I and 45KD bands of β-actin were quantitated using a densitometer (model GS-700, Bio-Rad Laboratories). Band volumes were calculated with the ratio of IP3RIto β-actin bands.
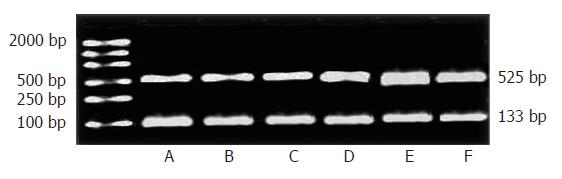
Total RNA was extracted using TRIzol following manufacturers' protocol. RNA concentrations were determined by UV analysis and diluted to 100 ng/μL with DEPC water. RNA was incubated at 30°C for 10 min followed by 42°C for 30 min and 99°C for 5 min for reverse transcription (RT). cDNA underwent 35 cycles of PCR (94°C for 30 s, 55°C for 30 s, and 72°C for 1 min). The sense and antisense primers used were 5'-GGTTTCATCTGCAAGCTAATAAAA -3’ and 5'-AATGCTTTCATGGAATACTCGGTC -3’, respectively. After PCR, 5 μL sample was run on the gel and a PCR amplified product of 525 bp was observed under an ultraviolet illuminator. GAPDH was used for internal control. The sense and antisense primers of GAPDH were 5’-GACAACTTTGGCATCGTGGA-3’ and 5'-ATGCAGGGATGATGTTCTGG-3’. A PCR product of 133 bp was observed, 525 bp bands of IP3RIand 133 bp bands of GAPDH were quantitated using a densitometer. Band volumes were calculated with the ratio of IP3RIto GAPDH bands.
Software SPSS 11.0 was used in statistical analysis. Each parameter was expressed as mean ± SE, and compared using Student's t-test. P < 0.05 was considered statistically significant.
Administration of LPS (10 μg/kg body weight) and GalN (800 mg/kg body weight) can induce FHF. Nine hours after GalN/LPS administration, the mortality of mice reached 60%. Liver tissues stained with hematoxylin-eosin presented severe hemorrhage and hepatic necrosis in 2, 6 and 9 h FHF groups. Kidney tissues were morphologically normal at all time points in all groups. Therefore, we concluded that the animal models of FHF were established successfully.
IP3RIproteins were localized to plasma region of GMC and renal VSMC. IP3RIprotein expressed at low levels in 3 control groups. There was no difference between 2 h FHF group and 3 control groups. IP3RI-staining was up-regulated in 6 h, 9 h FHF groups. The maximal effect was seen at 9 h (Figure 1).
In order to quantify the changes of IP3RIprotein, we carried out Western blot analysis for IP3RIprotein expression. An antibody raised to the C terminus of the mouse IP3RIrecognized a 230-kDa polypeptide. The expression of the IP3RIprotein was similar among 3 control groups. There was no difference between 2 h FHF group and 3 control groups. The expression of the IP3RIprotein increased to 167% ± 4% by 6 h, 196% ± 5% by 9 h with respect to control values (t = 3.16, P < 0.05; t = 5.43, P < 0.01).The maximal effect was seen at 9 h, which is identical to the result by immunohistochemistry (Figure 2).
To determine if the effects of increment of the IP3RIprotein expression are caused by an increment of new protein synthesis, we evaluated possible regulation at the mRNA level of IP3RI. Quantitative analysis demonstrated an increase to 155% ± 12% of control IP3RImRNA expression as early as 2h FHF, and it remained increased at 9 h FHF (173% ± 10%) (t = 2.97, P < 0.05; t = 3.81, P < 0.01). The maximal effect was seen at 6 h, and IP3RImRNA expression was increased to 192% ± 4% of control values (t = 4.42, P < 0.01). Thus, the increment in mRNA for the IP3RIpreceded the increment in protein (Figure 3).
HRS is a common complication in patients with FHF and end stage cirrhosis. HRS develops in approximately 55% of all patients with FHF[11]. There are only functional changes and relatively few histological changes in the kidneys. The pathogenesis of HRS involves in the development of hyperdynamic circulation, lowering of renal perfusion pressure, the activation of sympathetic nervous system, and increased synthesis of a variety of vasoactive mediators, which renders the kidneys more susceptible to decrease in perfusion pressure. Patients with HRS have an obvious reduction in renal blood flow and the glomerular filtration rate (GFR). The hallmark of HRS is renal vasoconstriction. Multiple mechanisms may be involved in renal vasoconstriction[12]. The circulating concentrations of many vasoconstrictors including endothelin and angiotensin II were markedly high in HRS[13,14]. These mediators can cause renal vasoconstriction, but more importantly they can also decrease the glomerular capillary ultrafiltration coefficient (Kf), thus causing a decline of GFR over and above that caused by renal vasoconstriction alone. As it is known, contraction of renal VSMC can result in a reduction in renal blood flow, while contraction of GMC can lower the filtration fraction and glomerular Kf[15]. Both GMC and renal VSMC are sensitive to vasoconstrictor. As a result, GFR was decreased obviously in HRS. Both endothelin and angiotensin II were important renal vasoconstrictors that stimulate IP3-mediated [Ca2+] i mobilization via the IP3Rs, followed by release of stored intracellular Ca2+ and Ca2+ entry through plasma membrane channels[16]. IP3Rs are the intracellular Ca2+ release channels that play a key role in Ca2+ signal in cells[17]. Cell contraction is closely connected with changes in intracellular Ca2+ concentration. IP3Rs is localized to endoplasmic reticulum . There is also evidence suggesting the presence of functional IP3Rs on the plasma membrane[18]. IP3Rs contains four independent ligand binding sites that are cooperative with respect to calcium channel opening[19]. In mammalian cells, there are at least three isoforms of the IP3R derived from three distinct genes. IP3RIis predominant in the GMC and renal VSMC, but absent in other renal cells[20]. Therefore, our study focused on IP3RIas a candidate protein that may facilitate intracellular Ca2+ release. The level of IP3RIexpression can be affected by many factors such as some cytokines. There might be a relationship between the changes in IP3RIexpression level and renal vasoconstriction in HRS. Theoretically, changes in IP3RI expression level are linked to regulation of functional [Ca2+] i stores and [Ca2+] i concentration. Wang JY et al[21] had discovered the up-regulation of expression of IP3RIon rat glomerular and afferent arterioles in a model of liver cirrhosis by immunohistochemical method. Does IP3RIof kidney also increase in mice with FHF? In order to explore the possible pathogenesis of HRS, we examined the expression level of IP3RIof kidney in mice with FHF by immunohistochemistry, Western blot and RT-PCR.
Our study shows that IP3RIprotein was localized to the plasma region of GMC and renal VAMC, which is consistent with previous studies. IP3RIexpressed at low levels in control groups. There was no differrence between NS-treated group, LPS-treated group and GalN-treated group. Its expression level was still low in 2 h FHF, while increased obviously in 6 h and 9 h FHF. This phenomenon was testified by Western blot quantitative analysis. The results from Western blot showed that the expression of the IP3RIprotein increased to 167% ± 4% by 6 h, and 196% ± 5% by 9 h with respect to control values. The maximal effect was seen at 9 h, and IP3RIexpression was increased to 196% ± 5% of control values. In order to elucidate the reason of increased protein expression of IP3RI, we evaluated the expression level of IP3RImRNA of kidney in mice with FHF by RT-PCR. We concluded that increased protein expression of IP3R I is caused by increased synthesis of the new IP3RIprotein as an increase in mRNA levels was found in mice with FHF at 2 h, 6 h and 9 h. Up-regulation of the IP3RImRNA was demonstrated as early as 2 h FHF. The maximal effect 192% ± 4% of control values was seen at 6 h. There was mild decrement at 9 h in contrast to the level at 6 h, but it was still higher than control values. Our result that the increment in mRNA for IP3RIprecedes the increment in protein is consistent with the time sequence of increased mRNA levels prior to occurrence of increased protein levels. Our finding demonstrates that up-regulation of IP3RImRNA levels can be explained by the increase in transcription of the IP3RIgene. Our observation also indicates that factors that can not directly induce Ca2+ flux may also regulate [Ca2+] i concentration by affecting IP3RIexpression. Increased IP3RIprotein expression provided more ligand binding sites for IP3 that is more beneficial to [Ca2+] i release. Consequently, kidney became more sensitive to agonists that stimulate IP3-mediated [Ca2+] i mobilization via the IP3RI. Overexpression of IP3RImay lead to modulatory influences on renal blood flow and GFR. Interaction between IP3RIup-regulation and agonists may enhance the contracting ability of GMC and VSMC to vasoconstrictors. Overexpression of IP3RImay be an important factor in contributing to HRS progression.
| 1. | Gerbes AL, Gülberg V. [Renal impairment in liver diseases]. Praxis (Bern 1994). 2006;95:1535-1538. |
| 2. | Sural S, Sharma RK, Gupta A, Sharma AP, Gulati S. Acute renal failure associated with liver disease in India: etiology and outcome. Ren Fail. 2000;22:623-634. |
| 3. | Rivera-Huizar S, Rincón-Sánchez AR, Covarrubias-Pinedo A, Islas-Carbajal MC, Gabriel-Ortíz G, Pedraza-Chaverrí J, Alvarez-Rodríguez A, Meza-García E, Armendáriz-Borunda J. Renal dysfunction as a consequence of acute liver damage by bile duct ligation in cirrhotic rats. Exp Toxicol Pathol. 2006;58:185-195. |
| 4. | Demirtaş S, Can M, Yarpuzlu A. Hepatorenal syndrome. Clin Chem Lab Med. 2006;44:379-386. |
| 5. | Woodcock EA, Matkovich SJ. Ins(1,4,5)P3 receptors and inositol phosphates in the heart-evolutionary artefacts or active signal transducers? Pharmacol Ther. 2005;107:240-251. |
| 6. | Yao YM, Hu SJ, Huang YW, Yang CH, Sun J, Zhu ZH, Wu T. [Effects of tumor necrosis factor alpha on expression of phospholamban and intracellular calcium in cardiomyocytes]. Zhongguo Yixue Kexueyuan Xuebao. 2005;27:767-771. |
| 7. | Dellis O, Dedos SG, Tovey SC, Taufiq-Ur-Rahman SJ, Taylor CW. Ca2+ entry through plasma membrane IP3 receptors. Science. 2006;313:229-233. |
| 8. | McGowan TA, Sharma K. Regulation of inositol 1,4,5-trisphosphate receptors by transforming growth factor-beta: implications for vascular dysfunction in diabetes. Kidney Int Suppl. 2000;77:S99-S103. |
| 9. | Krizanova O, Kvetnansky R, Jurkovicova D. Effect of two distinct stressors on gene expression of the type 1 IP3 receptors. Gen Physiol Biophys. 2005;24:237-246. |
| 10. | LV SA, Song hl, Wang JY, Liu P. Blood brain barrier permeability in acute liver necrosis of mice. Shijie Huaren Xiaohua Zazhi. 2004;12:1346-1348. |
| 11. | Ytrebø LM, Sen S, Rose C, Davies NA, Nedredal GI, Fuskevaag OM, Ten Have GA, Prinzen FW, Williams R, Deutz NE. Systemic and regional hemodynamics in pigs with acute liver failure and the effect of albumin dialysis. Scand J Gastroenterol. 2006;41:1350-1360. |
| 12. | Moore K. Renal failure in acute liver failure. Eur J Gastroenterol Hepatol. 1999;11:967-975. |
| 13. | Anand R, Harry D, Holt S, Milner P, Dashwood M, Goodier D, Jarmulowicz M, Moore K. Endothelin is an important determinant of renal function in a rat model of acute liver and renal failure. Gut. 2002;50:111-117. |
| 14. | Zaza S, Bonny O, Liaudet L. [Hepatorenal syndrome in patients with liver cirrhosis]. Nephrol Ther. 2005;1:174-182. |
| 15. | Kuo HT, Shin SJ, Kuo MC, Chen HC. Effects of specific endothelin-1 receptor antagonists on proliferation and fibronectin production of glomerular mesangial cells stimulated with Angiotensin II. Kaohsiung J Med Sci. 2006;22:371-376. |
| 16. | Zhu Z, Arendshorst WJ. Angiotensin II-receptor stimulation of cytosolic calcium concentration in cultured renal resistance arterioles. Am J Physiol. 1996;271:F1239-F1247. |
| 17. | White C, McGeown JG. Inositol 1,4,5-trisphosphate receptors modulate Ca2+ sparks and Ca2+ store content in vas deferens myocytes. Am J Physiol Cell Physiol. 2003;285:C195-C204. |
| 18. | Taylor CW, Dellis O. Plasma membrane IP3 receptors. Biochem Soc Trans. 2006;34:910-912. |
| 19. | Hamada K, Miyata T, Mayanagi K, Hirota J, Mikoshiba K. Two-state conformational changes in inositol 1,4,5-trisphosphate receptor regulated by calcium. J Biol Chem. 2002;277:21115-21118. |
| 20. | Monkawa T, Hayashi M, Miyawaki A, Sugiyama T, Yamamoto-Hino M, Hasegawa M, Furuichi T, Mikoshiba K, Saruta T. Localization of inositol 1,4,5-trisphosphate receptors in the rat kidney. Kidney Int. 1998;53:296-301. |
| 21. | Wang JY, Liu HY, Liu P. [Expression of type I inositol 1,4,5-triphosphate receptor on rat glomerular and afferent arterioles in a model of liver cirrhosis]. Zhonghua Ganzangbing Zazhi. 2004;12:609-611. |
S- Editor Wang J L- Editor Ma JY E- Editor Chen GJ