Published online Apr 21, 2007. doi: 10.3748/wjg.v13.i15.2247
Revised: February 20, 2006
Accepted: February 14, 2007
Published online: April 21, 2007
We report a case of multiple duodenal, pancreatic, and gastric carcinoids. A 67-year old woman was admitted to our hospital for treatment of a duodenal carcinoid. Laboratory tests revealed that the patient was associated with macrocytic anemia and hypergastrinemia, and type A gastritis was shown by gastrofiberscopy. During surgery, another tumor was incidentally found in the head of the pancreas. The tumors in the duodenum and pancreas were completely excised by pancreatoduodenectomy and immunohistologically diagnosed as gastrin-and serotonin-producing carcinoids, respectively. Pathological examination revealed that in addition to the grossly found carcinoids, there were subclinical carcinoids, one of which was an endocrine cell micronest, located in the stomach and duodenum. The tumors in the duodenum, pancreas, and stomach showed different characteristics from one another morphologically and immunochemically. Although no definitive evidence has been obtained, some sort of genetic anomaly may have been involved in this case, and hypergastrinemia due to duodenal gastrinoma may induce multiple gastric carcinoids.
- Citation: Bamba T, Kosugi SI, Kanda T, Tsubono T, Sakai Y, Musha N, Ishihara N, Hatakeyama K. Multiple carcinoids in the duodenum, pancreas and stomach accompanied with type A gastritis: A case report. World J Gastroenterol 2007; 13(15): 2247-2249
- URL: https://www.wjgnet.com/1007-9327/full/v13/i15/2247.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i15.2247
Gastrointestinal carcinoids are relatively rare tumors and usually occur singly. According to the organ distribution of carcinoid tumors as determined by an analysis of 11842 reported cases[1], the frequencies of occurrence of gastric, duodenal, and pancreatic carcinoids are 8.3%, 11.6%, and 1.1%, respectively. Therefore, the likelihood that multiple primary carcinoids would occur simultaneously in these organs is quite low. Recently, researchers have accumulated clinical evidence of gastric carcinoid associated with type A gastritis, which frequently leads to multiple tumors[2,3]. We report here a case of multiple primary carcinoids in the duodenum, pancreas, and stomach that was associated with type A gastritis. We also discuss the possible mechanisms underlying the occurrence of multiple carcinoids in our case with reference to a review of the literature.
On June 5, 2001, a 67-year old woman with no subjective symptoms was admitted to Saiseikai Niigata Daini Hospital for a detailed examination and treatment of a duodenal tumor. A submucosal tumor in the duodenum of the patient had been followed up by a family doctor since 1997. The patient had a 30-year history of bronchial asthma treatment but no particular family history of anemia, hypercalcemia, pituitary tumor, and malignant diseases. Hematological examination on admission showed macrocytic anemia (RBC: 230 × 104/mm3; Hb: 8.8 mg/dL; MCV: 113.0 μm3) with a low vitamin B12 level (below 100 pg/mL). The blood calcium level was normal (9.0 mg/dL). The fasting serum gastrin level was extremely high (1630 pg/mL). Serum serotonin and urine 5-hydroxyindole acetic acid concentrations were within the normal range (63 μg/dL and 5.70 ng/mL, respectively). Gastrointestinal endoscopy showed a sessile tumor with a diameter of nearly 18 mm and covered by intact mucosa at the upper curve of the duodenal bulb, as well as extensive mucosal atrophy in the fundus of the stomach corresponding to type A gastritis. The tumor in the duodenum was diagnosed as a carcinoid by histological examination of biopsy specimens. Computed tomography scans showed no metastasis and no other tumor in the abdomen.
On July 2, 2001, the patient underwent surgery to excise the duodenal carcinoid. During the operation, however, another tumor about 1 cm in size was incidentally found in the head of the pancreas. Pathological examination using frozen sections revealed that the tumor was an endocrine neoplasm. Both tumors were completely excised by pancreatoduodenectomy. The serum gastrin level returned to normal postoperatively. Presently, the patient has survived for 47 mo since the surgery with no sign of recurrence.
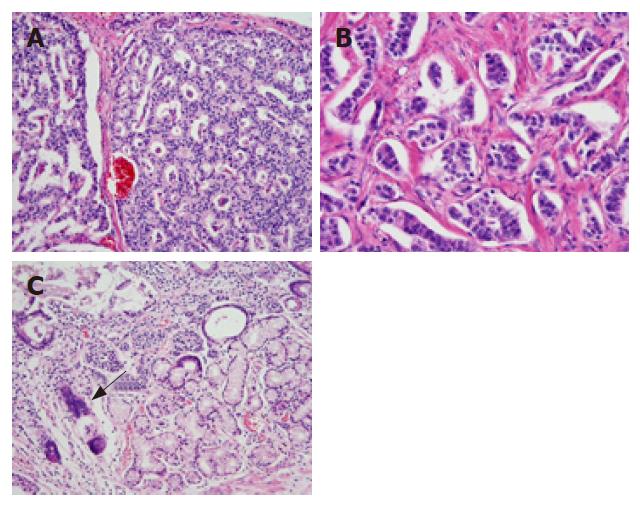
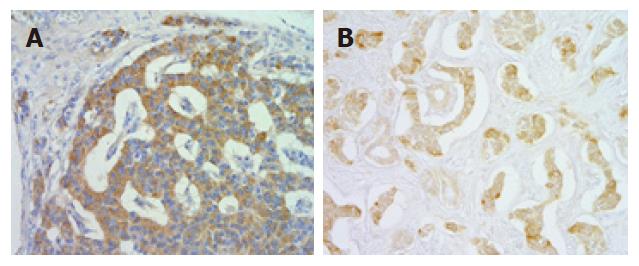
Pathological examination of an excised specimen revealed that the endocrine neoplasm in the pancreatic head was a carcinoid. Furthermore, a detailed examination using serial sections revealed that there were four subclinical carcinoid tumors that were smaller than 2 mm in size in the antrum and duodenum. Thus, 6 carcinoid tumors, one of which was endocrine cell micronest (ECM)[4], were present in the excised specimen (2 in the duodenum, 1 in the pancreas, and 3 in the stomach). Histologically, the grossly found tumor in the duodenum showed an anastomotic ribbon-like appearance, and the interstitum was composed of enriched vasculature (Figure 1A). The carcinoid in the pancreas showed a palisade-like arrangement and relatively dense connective tissues in the intersitium (Figure 1B). On the other hand, the tumor nests in the gastric carcinoids exhibited a vesicular appearance (Figure 1C). That is, the carcinoids in the three organs differed from one another histomorphologically. Furthermore, we characterized the tumors in the duodenum, pancreas and stomach by immunohistochemical analysis using anti-human gastrin polyclonal antibody (DAKO, Japan) and anti-human serotonin polyclonal antibody (DAKO, Japan). The tumor cells in the duodenal carcinoid were strongly positive (Figure 2A) for gastrin and very weakly positive for serotonin. Conversely, the carcinoid in the pancreas was weakly positive for gastrin and strongly positive for serotonin (Figure 2B). On the other hand, a carcinoid tumor in the stomach was unequivocally negative for the two hormones. Thus, the carcinoid tumors in the duodenum, pancreas, and stomach also showed different endocrinological features from one another. The pathological and immunochemical features of the six tumors are summarized in Table 1.
| Tumor | Site | Size (mm) | Gastrin | Serotonin |
| Carcinoid | Duodenum | 14 × 13 | +++ | + (slightly) |
| Carcinoid | Pancreas | 15 × 13 | + (slightly) | +++ |
| Carcinoid | Stomach | 1.2 | - | - |
| Carcinoid | Stomach | 1.8 | NA | NA |
| Carcinoid | Duodenum | 0.5 | NA | NA |
| ECM | Stomach | ≤ 0.1 | NA | NA |
We reported a case of multiple carcinoids that showed different histomorphological and immunohistological characteristics. Rindi et al[5] have classified gastric carcinoids into the following three types: (1) those that arise in a background of type A gastritis, (2) those associated with Zollinger-Ellison syndrome, usually in combination with multiple endocrine neoplasia type 1 (MEN1), and (3) those that occur sporadically. Based on an analysis of 1094 gastric carcinoid cases that were reported in the literature, the incidence of multiple tumors is 16.7% in patients not associated with type A gastritis[6]. On the other hand, in patients associated with type A gastritis, the incidence is 64.0%, which is significantly higher than that in patients not associated with type A gastritis. The presumed mechanism underlying the occurrence of multiple gastric carcinoids in type A gastritis is as follows: a low or no acid state caused by mucosal atrophy in the fundic gland region induces hyperplasia of gastrin-producing cells in the gastric antrum through feedback, which causes a rise of blood gastrin levels. Then, the unusually and constantly high levels of gastrin stimulate the proliferation of enterochromaffin-like (ECL) cells in the stomach. It is thought that hyperplasia and the subsequent tumorigenic transformation of ECL cells are the cause of multiple carcinoids[7,8].
Our case was clinically diagnosed as type A gastritis with pernicious anemia, and the blood gastrin level was high (1630 pg/mL). Histological examination of the excised specimen, however, showed no hyperplasia of gastrin producing cells in the gastric antrum. Concerning the hypergastrinemia in the present case, the relevance of a gastrin-producing tumor in the duodenum should be taken into consideration. Some of carcinoids are capable of functioning and producing gastrointestinal hormones. A duodenal carcinoid tumor in the present case was unequivocally positive for gastrin in an immunohistochemical analysis. Moreover, the hypergastrinemia rapidly remitted after excision. These findings suggest the possibility that hypergastrinemia in the present case was caused largely by a gastrin-producing tumor in the duodenum, that is duodenal gastrinoma. Interestingly, atrophy in the fundic gland region due to type A gastritis seemed to prevent the typical symptoms of Zollinger-Ellison syndrome/gastrinoma, such as refractory peptic ulcer disease, severe diarrhea and gastric acid hypersecretion. In the present case, all four carcinoid tumors in the stomach were small and included ECM. It is likely that ECL cells in the stomach were constitutively stimulated and consequently transformed into neoplasms.
On the other hand, the relevance of type A gastritis and/or hypergastrinemia to the development of pancreatic or duodenal carcinoids remains unclear. Thus, we should take the genetic background of multiple carcinoids into consideration. A MEN-1 type genetic anomaly is known to cause carcinoid tumors as lesions secondary to (1) pituitary gland tumors, (2) parathyroid gland tumors, and (3) pancreatic endocrine neoplasms[9]. The causative gene (MEN-1) has been identified, and the penetration is said to reach 99% by 50 years of age in autosomal dominant inheritance. Although no genetic examination was conducted in this case, it is unlikely that a MEN-1 type genetic anomaly was related to the multiple carcinoids because our case had neither family history nor parathyroid gland lesion, that has a high occurrence rate (90%-100%) and generally occurs in youth. However, Imamura et al[10] have recently reported that endocrine neoplasm occurs frequently in the duodenum and pancreas in gastrinoma patients with MEN-1 type genetic anomaly, and that gastrinomas are immunochemically positive for gastrin in the duodenum and negative for gastrin in the pancreas. This endocrinological feature of gastrointestinal tumors in MEN-1 is in agreement with our case. Because carcinoid is a relatively rare tumor, the chance of multiple primary carcinoids occurring simultaneously in three organs is quite small. Therefore, the possibility that an unknown genetic anomaly similar to the MEN-1 type anomaly may be involved in the occurrence of multiple carcinoids is undeniable.
The mechanism underlying the occurrence of multiple carcinoids in the present case was discussed from various perspectives. The specific mechanism of multiple carcinoids would be established by the accumulation of additional similar cases in the near future.
The authors are grateful to Jun Soga, MD, (Professor Emeritus of Niigata University) for his scientific advice and critical review.
| 1. | Soga J. Carcinoids and their variant endocrinomas. An analysis of 11842 reported cases. J Exp Clin Cancer Res. 2003;22:517-530. [PubMed] |
| 2. | Kinouchi M, Shiiba K, Ishii S, Mizoi T, Oyama J, Matsuno S, Sasaki I. A case of multiple gastric carcinoid associated with hypergastrinemia. Jpn J Gastroenterol. 2004;37:1616-1621. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Satake S, Ishado Y, Nakai R, Mukaeyama Y, Maeda S. Two cases of gastric carcinoid tumor associated with type A gastritis treated by distal gastrectomy. Jpn J Gastroenterol. 2003;36:1173-1177. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Itsuno M, Watanabe H, Iwafuchi M, Ito S, Yanaihara N, Sato K, Kikuchi M, Akiyama N. Multiple carcinoids and endocrine cell micronests in type A gastritis. Their morphology, histogenesis, and natural history. Cancer. 1989;63:881-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Rindi G, Luinetti O, Cornaggia M, Capella C, Solcia E. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology. 1993;104:994-1006. [PubMed] |
| 6. | Soga J. Gastric carcinoids: a statistical evaluation of 1,094 cases collected from the literature. Surg Today. 1997;27:892-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Hodges JR, Isaacson P, Wright R. Diffuse enterochromaffin-like (ECL) cell hyperplasia and multiple gastric carcinoids: a complication of pernicious anaemia. Gut. 1981;22:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Solcia E, Rindi G, Silini E, Villani L. Enterochromaffin-like (ECL) cells and their growths: relationships to gastrin, reduced acid secretion and gastritis. Baillieres Clin Gastroenterol. 1993;7:149-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Glascock MJ, Carty SE. Multiple endocrine neoplasia type 1: fresh perspective on clinical features and penetrance. Surg Oncol. 2002;11:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Imamura M, Komoto I, Doi R. Recent trend of diagnosis and treatment for Zollinger-Ellison syndrome. Gan To Kagaku Ryoho. 2005;32:147-151. [PubMed] |
S- Editor Liu Y L- Editor Wang XL E- Editor Wang HF