Published online Mar 28, 2007. doi: 10.3748/wjg.v13.i12.1870
Revised: January 6, 2007
Accepted: March 6, 2007
Published online: March 28, 2007
AIM: To investigate the correlation between hepatitis B virus surface antigen (HBsAg), hepatitis C virus (HCV) expression in hepatocellular carcinoma (HCC), the HAI score of the noncancerous region of the liver and the serum Alpha fetoprotein (AFP) level.
METHODS: The patterns of HBsAg and HCV in 100 cases of HCC and their surrounding liver tissues were studied on paraffin-embedded sections with immuno-histochemistry, the histological status was determined by one pathologist and one surgeon simultaneously using the hepatitis activity index (HAI) score, and AFP was detected by radioimmunity. The study included 100 consecutive patients who underwent curative resection for HCC. Based on HBsAg and HCV expression, the patients were classified into 4 groups: patients positive for HBsAg (HBsAg group), patients positive for HCV (HCV group), patients negative for both HCV and HBsAg (NBNC group) and patients positive for both HBsAg and HCV (BC group).
RESULTS: The BC group had significantly higher HAI scores than the other three groups. (BC > HCV > HBsAg > NBNC). HBV and HCV virus infection was positively correlated with HAI (rs = 0.39, P = 0.0001). The positive rate of AFP (85.7%) and the value of AFP (541.2 ng/mL) in the group with HBV and HCV co-infection were the highest among the four groups. The positive rate (53.3%) of AFP and the value of AFP ( 53.3 ng/mL) in the group with none-infection of HBV and HCV were the lowest. HBV and HCV virus infection was positively correlated with AFP(rs = 0.38, P = 0.0001).
CONCLUSION: The AFP increase in patients with liver cancer was positively correlated with the infection of HBV and HCV. The serum AFP elevation by the infection of HBV and HCV is one of mechanisms which lead to hepatocarcinogenesis, and the antivirus intervening treatment of hepatitis is significant for the prognosis of liver cancer. From our Spearman’s rank correlation analysis, we can conclude that the severity of virally induced inflammation is correlated with HBsAg and HCV expression in HCC tissues and noncancerous tissues. Prior co-infection of HBV in HCV patients may be an adverse risk factor for intrahepatic inflammation.
- Citation: Xuan SY, Xin YN, Chen H, Shi GJ, Guan HS, Li Y. Significance of hepatitis B virus surface antigen, hepatitis C virus expression in hepatocellular carcinoma and pericarcinomatous tissues. World J Gastroenterol 2007; 13(12): 1870-1874
- URL: https://www.wjgnet.com/1007-9327/full/v13/i12/1870.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i12.1870
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide[1,2]. Although H pylori can be detectable in the HCC tissues[3], HBV has been reported to be the prime cause for the development of HCC with regard to the major microorganism etiologies of HCC. The incidence of HCC is clearly linked to the rate of chronic HBV infection. A large percentage of HCC patients show serological or histological markers of past contact with hepatitis B virus (HBV)[4]. Likewise, hepatitis B virus (HCV) infection predisposes to HCC[5].
Most patients with HCC in China have hepatitis virus-induced chronic liver disease (HBV or HCV). The infection rate of HBV has reached 10% in China, and HCV has reached 3.2%. This causes loss of hepatic reserve, and subsequently limits the extent of resection for HCC patients. Poor hepatic reserve can also be a major obstacle to multidisciplinary treatments for post-hepatectomy recurrences. HBsAg and HCV expression can really reflect HBV and HCV infection in HCC. In order to discuss the relationship between the patients’ viral histological status and the degree of virally induced inflammation, we studied the clinical data of HCC and examined HBsAg and HCV expression in HCC tissues and noncancerous tissues.
All patients participating in this study gave informed consent before surgery. A total of 100 patients (66 males and 34 females, mean age 53.6 ± 24.1 years, range 22-78 years) who underwent hepatectomy for HCC from January 2000 to September 2005 were included in this study. The patients, who had undergone hepatectomy and hepatic arterial chemoembolization (TACE) or who had alcoholic liver cirrhosis, were excluded. HCC tissues were obtained from all patients. HCC tissues and surrounding liver tissues were examined for HBsAg and HCV. We also investigated the HBsAg and HCV expression in normal liver tissues without HCC cells or HBV and HCV, which was surgically obtained from the pathological specimens of patients undergoing surgical resection for other diseases or at autopsy.
The pathologic diagnosis and classification of variables were based on the criteria recommended in the General Rules for Clinical and Pathological Study of Primary Liver Cancer (Liver Cancer Study Group of China 2001). Clinicopathological parameters analyzed included sex, age, liver pathology (HAI score) (Table 1) and Alpha fetoprotein (AFP) (Table 2). Eighty-seven patients were positive for HBsAg (HBsAg group), 27 were positive for HCV (HCV group), 6 were negative for both HCV and HBsAg (NBNC group) and 21 were positive for both HBsAg and HCV (BC group).
| Groups | n | AFP negative | AFP positive | Positive rates of AFP(%) | Mean of AFP(ng/mL) | Average sum of ranks |
| NBNC | 6 | 4 | 2 | 33.3 | 53.3 | 14.3 |
| HBsAg | 66 | 22 | 44 | 66.7 | 352.6 | 48.7 |
| HCV | 7 | 3 | 4 | 57.1 | 348.2 | 50.1 |
| BC | 21 | 3 | 18 | 85.7 | 541.2 | 66.7 |
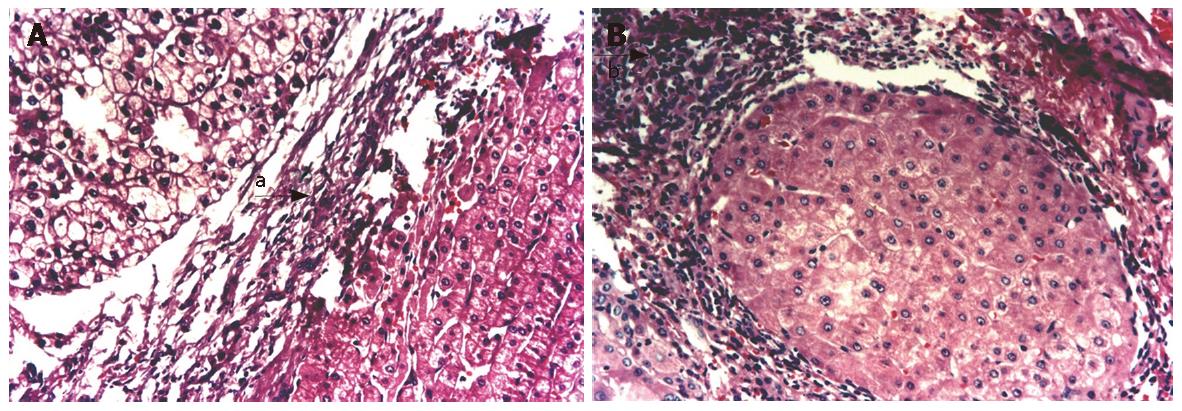
Based on the microscopic findings of the non-cancerous region of the surgical specimen by hematoxylin and eosin staining, the histological status was determined by one pathologist and one surgeon simultaneously using the hepatitis activity index (HAI) score. The inflammatory activity of coexisting hepatitis was graded according to the histological activity index (HAI score) of Knodell et al[6] as follows (Table 3). The scores for three features (degree of hepatocellular periportal necrosis and bridging, degree of introlobular degeneration and focal hepatocellular necrosis, and degree of portal inflammation) were calculated for each individual. The HAI score in each group was expressed as the average of the scores in three visual fields. The degree of histopathological derangement was comparable in the HBsAg and HCV groups; however, the HAI score in the BC group was significantly higher than that in the other three groups (Table 1).
| Periportal Bridging Necrosis | Intralobular degeneration and focal necrosis | Portal inflammation | Fibrosis |
| 0: None | 0: None | 0: None | 0: None |
| 1: Mild | 1: Mild | 1: Mild | 1: Fibrous |
| (< 1/3 lobules) | (< 1/3 lobules) | portal expansion | |
| 3: Moderate | 3: Moderate | 3: Moderate | 3: Bridging |
| (< 50% circumference) | (< 2/3 lobules) | (< 2/3 lobules) | fibrosis |
| 4: Marked | 4: Marked | 4: Marked ,dense | 4: Cirrhosis |
| (> 2/3 lobules) | (> 2/3 lobules) | ||
| 5: Moderate | |||
| + bridging | |||
| 6: Marked | |||
| + bridging | |||
| 10: Multiple | |||
| lobular necrosis |
All primary antibodies (polyclonal antibodies) to HBsAg, HCV and secondary antibodies were purchased from Zhongshan Biotechnology Limited Company (Beijing, China).
To obtain more accurate dada, we selected the tissue blocks containing HCC and surrounding liver tissues. Each specimen was cut into 4-μm thick sections. Tissue wax sections were unfolded on glass sheet. Immunohistochemistry (strepolin-biotin-peroxidase method) was used to detect the expression of HBsAg and HCV. Briefly, paraffin-embedded tissue sections were dewaxed, treated with 3% H2O2 at 37°C, washed with PBS, incubated with HBsAg and HCV antibodies separately, washed with PBS for 15 min, incubated again with strepolin-biotin-peroxidase at 37°C. Finally, the sections were washed with PBS for 15 min, visualized with DAB reagent and counterstained with hematoxylin. Negative and positive controls were used simultaneously to ensure specificity and reliability of the staining process. The negative controls were incubated with PBS instead of primary antibody and a positive section supplied by the manufacturer of the staining kit was taken as positive control. Sections were observed under microscope after being mounted.
Immunostaining of HBsAg and HCV showed brown-yellowish granules either in the cytoplasma or the nucleus with a positive expression. Results estimation: the specimens with no positive cells or positive cells < 10% were negative (-); with light brownish yellow cells or positive cells > 10% were positive (+).
All statistical analyses were performed using the Microsoft SPSS 12.0 software. A P value less than 0.05 was considered to indicate significance. Quantitative data were expressed as mean ± SD. Chi-square test was used for comparison between groups. Rank-sum test and Spearman’s rank correlation were used in the four groups.
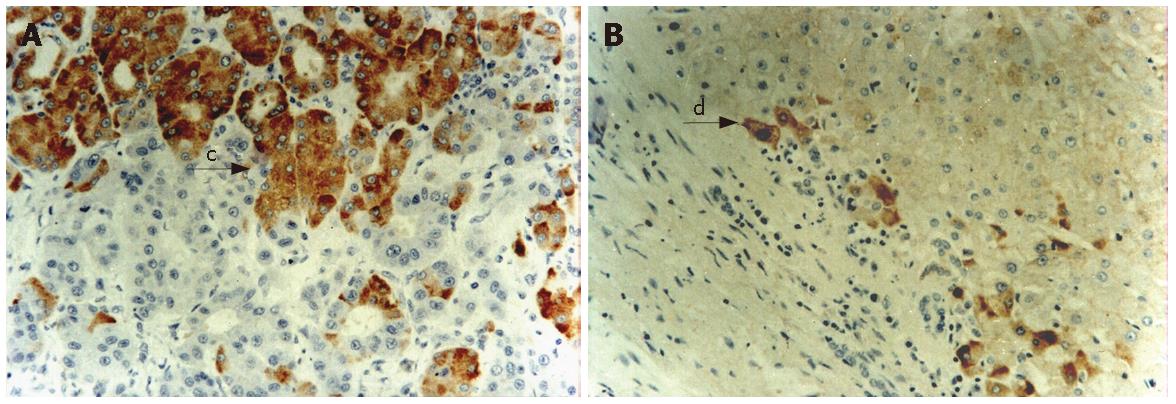
Expressions of HBsAg and HCV in HCC and noncancerous tissues (Figures 1 and 2). Different expressions of HBsAg and HCV were observed in HCC and noncancerous tissues. We presumed HBsAg positive when HBsAg was detected either in HCC or noncancerous tissues. Of the 100 biopsies, HBsAg was detected in the hepatocytes in 87 (87%), HCV 28 (28%), and HBsAg and HCV 21 (21%), respectively.
Three patterns of HBsAg staining-cytoplasmic, submembranous and membranous, have been described in patients with chronic HBV infection. In our study, all patients with high replication (HBeAg-positive) had discrete cytoplasmic HBsAg expression. A mixed pattern of staining was seen in the other two groups. There was no difference in membranous HBsAg expression and histological activity in these groups. There was a significant difference in the positive expressions of HBsAg between HCC and noncancerous tissues. (23% vs 79%, χ2 = 62.75, P < 0.001).
HCV Ag could be seen in the nuclei and/or cytoplasms of cancer cells and/or pericancerous hepatocytes of HCC. In the 28 positive cases of HCC, HCV Ag was more often seen in nucleus than in cytoplasm (20 cases in nuclei, only 8 in cytoplasms). The HCV Ag positive cells were scattered singly or gathered in small groups in some parts. The detecting rate of HCV Ag in cancer tissues was 15% (15/100), whereas in the pericancerous tissues it was as high as 23% (23/100). There was no significant difference in the positive expressions of HCV between HCC and noncancerous tissues (15% vs 23%, χ2 = 2.08, P = 0.15).
The score of HAI in the group with co-infection of HBV and HCV is the highest among the four groups (12.62 ± 3.88) while that in the group without infection of HBV and HCV is the lowest (6.67 ± 2.58). HBV and HCV infection was positively correlated with HAI (rs = 0.39, P = 0.0001) (Table 1).
HCC is one of the most common malignant tumors, representing more than 5% of all cancers. The estimated annual number of cases exceeds 500 000[7], with a mean annual incidence of around 3%-4%. In terms of relative frequencies, HCC ranks as the fifth most common cancer in the world (the fifth among men and eighth among women) and the second among cancers of the digestive tract after stomach cancer[8]. Chronic hepatitis B and C, mostly in the cirrhotic stage, are responsible for the majority of the hepatocellular carcinomas worldwide. In cases with persistent HBV infection, HBV is one of the most important risk factors for HCC. It has been noted that the probability of acquiring HCC increases with severity of liver disease. The annual risk of HCC is 0.5% for asymptomatic HBsAg carriers and 0.8% for patients with chronic hepatitis B[9]. Patients with HBV-cirrhosis have a 1000 times higher risk of developing HCC compared to a HBsAg negative control group. The incidence of HCC in compensated cirrhosis due to HBV from Asia was 2.7%. In Japan, the mean interval between the time of initial infection with HBV and the occurrence of HCC is 50 years. As most people are infected at birth, HBV related liver cirrhosis usually develops in patients in their 40’s and HCC in their 50’s[10].
Likely, HCV is associated with HCC, and HCV infection enhances the development of liver diseases. HCV affects the initiative period of HCC and induces the malignant phenotypic alteration of hepatocytes[11].
Co-infection of HBV and HCV seems to result in more severe liver diseases than either infection alone[12]. The risk of developing HCC in subjects with both infections has been investigated in a meta-analysis of 32 epidemiological studies between 1993 and 1997[13]. The odds ratio for development of HCC in HBsAg positive and anti-HCV/HCV RNA negative subjects was 20.4; in HBsAg negative and anti-HCV/HCV RNA positive subjects 23.6; and subjects positive for both markers 135. These data suggest a more than additive but less than multiplicative effect of HBV and HCV co-infection on the relative risk for HCC. The viruses may act through common as well as different pathways in the carcinogenic process.
AFP is a fetal specific glycoprotein produced primarily by the fetal liver. Normally, its serum concentration falls rapidly after birth and its synthesis in adult life is repressed. However, greater than 70% of HCC patients have a high serum concentration of AFP because of the tumor excretion. Forty years after its discovery, serum AFP remains a most useful tumor marker in screening HCC patients. The serum concentration of 20 ng/mL is the most commonly used cut-off value to differentiate HCC patients from healthy adults in clinical researches. Alpha-fetoprotein expression is a potential prognostic marker in hepatocellular carcinoma. In our study, there was direct correlation between HBsAg, HCV expression in HCC tissues, noncancerous tissues and serum AFP. The level of AFP in BC group is the highest among the four groups. We conclude that HBV/HCV infection is related to the increased level of AFP. That is to say, the increased level of serum AFP in HBV/HCV infection is associated with HCC. AFP, as a hepatoma-promoting factor, has become a new target of anti-hepatoma agents. Wang XW et al[14] suggested that it is a new approach for the treatment of tumors to inhibit or block oncogene expression. The effect of AFP on the growth of tumor has been confirmed. The functional mechanism of AFP on the growth of tumors may be attributed, at least in part, to receptor mediated cAMP pathway and/or calcium signaling resulting in overexpression of certain genes. The increase of AFP in the patients with liver cancer was positively correlated with the infection of HBV and HCV. The AFP increase by the infection of HBV and HCV viruses was one of mechanisms which lead to hepatocarcinogenesis, and the antivirus intervening treatment of hepatitis is significant for the prognosis of liver cancer. We therefore, can prevent HCC through decreasing the level of AFP by anti-virus therapy.
HAI score describes the histological status of accompanying chronic hepatitis and was established by pathologists. A reprospective trial has been performed that the patients with moderate hepatitis (HAI score of 6–9) had the highest rate of intrahepatic metastatic recurrence among the various HAI groups[15]. In our study, the BC group had significantly higher HAI scores than the other three groups. (BC > HCV > HBsAg > NBNC). From our Spearman’s rank correlation analysis, we can conclude that the severity of virally induced inflammation was well correlated with HBsAg and HCV expression in HCC tissues and noncancerous tissues. Prior co-infection of HBV in HCV patients may be an adverse risk factor for intrahepatic inflammation. As for the tissues of liver cancer with virus infection background, the HAI is obviously higher than that without virus infection. HBV and HCV virus infection was correlated with HAI significantly, perennial viremia will aggravate pathological changes of liver tissue.
Based on our experiments, therefore, HCC might be prevented by early antiviral therapy. In patients with chronic hepatitis B or C, antiviral therapy in a noncirrhotic stage is protective for HCC development in responders, probably by prevention of cirrhosis development.
| 1. | Kiyosawa K, Umemura T, Ichijo T, Matsumoto A, Yoshizawa K, Gad A, Tanaka E. Hepatocellular carcinoma: recent trends in Japan. Gastroenterology. 2004;127:S17-S26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 231] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 2. | El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27-S34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 729] [Article Influence: 33.1] [Reference Citation Analysis (1)] |
| 3. | Xuan SY, Li N, Qiang X, Zhou RR, Shi YX, Jiang WJ. Helicobacter infection in hepatocellular carcinoma tissue. World J Gastroenterol. 2006;12:2335-2340. [PubMed] |
| 4. | Blakely TA, Bates MN, Baker MG, Tobias M. Hepatitis B carriage explains the excess rate of hepatocellular carcinoma for Maori, Pacific Island and Asian people compared to Europeans in New Zealand. Int J Epidemiol. 1999;28:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Caselmann WH, Alt M. Hepatitis C virus infection as a major risk factor for hepatocellular carcinoma. J Hepatol. 1996;24:61-66. [PubMed] |
| 6. | Desmet VJ. Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis Hepatology 1981; 1: 431-435. J Hepatol. 2003;38:382-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2558] [Cited by in RCA: 2519] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 7. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3252] [Cited by in RCA: 3258] [Article Influence: 130.3] [Reference Citation Analysis (1)] |
| 8. | Bosch FX, Ribes J, Borràs J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999;19:271-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 672] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 9. | Liaw YF, Tai DI, Chu CM, Lin DY, Sheen IS, Chen TJ, Pao CC. Early detection of hepatocellular carcinoma in patients with chronic type B hepatitis. A prospective study. Gastroenterology. 1986;90:263-267. [PubMed] |
| 10. | Kiyosawa K, Sodeyama T, Tanaka E, Gibo Y, Yoshizawa K, Nakano Y, Furuta S, Akahane Y, Nishioka K, Purcell RH. Interrelationship of blood transfusion, non-A, non-B hepatitis and hepatocellular carcinoma: analysis by detection of antibody to hepatitis C virus. Hepatology. 1990;12:671-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 927] [Cited by in RCA: 884] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 11. | Zhang LF, Peng WW, Yao JL, Tang YH. Immunohistochemical detection of HCV infection in patients with hepatocellular carcinoma and other liver diseases. World J Gastroenterol. 1998;4:64-65. [PubMed] |
| 12. | Sato S, Fujiyama S, Tanaka M, Yamasaki K, Kuramoto I, Kawano S, Sato T, Mizuno K, Nonaka S. Coinfection of hepatitis C virus in patients with chronic hepatitis B infection. J Hepatol. 1994;21:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 104] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Donato F, Boffetta P, Puoti M. A meta-analysis of epidemiological studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma. Int J Cancer. 1998;75:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 14. | Wang XW, Xie H. Alpha-fetoprotein enhances the proliferation of human hepatoma cells in vitro. Life Sci. 1999;64:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Ueno S, Tanabe G, Yoshida A, Yoshidome S, Takao S, Aikou T. Postoperative prediction of and strategy for metastatic recurrent hepatocellular carcinoma according to histologic activity of hepatitis. Cancer. 1999;86:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
S- Editor Liu Y L- Editor Ma JY E- Editor Zhou T