Published online Mar 28, 2007. doi: 10.3748/wjg.v13.i12.1851
Revised: January 13, 2006
Accepted: March 18, 2007
Published online: March 28, 2007
AIM: To study the inhibitory effect of mononuclear bone marrow cell (BMC) transplantation on carbon tetrachloride (CCl4) -induced liver fibrosis in rats.
METHODS: Rat liver fibrosis models were induced by CCl4 and alcohol administration. After 8 wk, twenty rats were randomly allocated into treatment group (n = 10) and control group (n = 10). BMC were infused into the rats in treatment group via the portal vein, while heparinized saline was infused in control group. CCl4 was hypodermically injected into the rats twice a week for 4 wk. At the end of wk 12, all rats were humanely sacrificed. Liver samples were taken and stained with HE or Masson trichrome. The general conditions, liver fibrosis (hydroxyproline and collagen fibre) and liver pathological grades in rats were evaluated.
RESULTS: The general conditions of the rats in treatment group improved markedly, but not in control group. Hydroxyproline was 504.6 ± 128.8 μg/g in treatment group, and 596.0 ± 341.8 μg/g in control group. The percentage of collagen fibre was 3.75% ± 0.98% in treatment group and 5.02% ± 0.44% in control group. There was a significant difference between the two groups (P < 0.05). Liver pathological grade decreased from grade IV to grade III partially in treatment group (P < 0.05) with no obvious improvement in control group (P > 0.05). There was a significant difference between treatment group and control group (P < 0.05).
CONCLUSION: Transplantation of BMC can improve liver fibrosis due to chronic liver injury in rats.
- Citation: Cao BQ, Lin JZ, Zhong YS, Huang SB, Lin N, Tang ZF, Chen R, Xiang P, Xu RY. Contribution of mononuclear bone marrow cells to carbon tetrachloride-induced liver fibrosis in rats. World J Gastroenterol 2007; 13(12): 1851-1856
- URL: https://www.wjgnet.com/1007-9327/full/v13/i12/1851.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i12.1851
Liver fibrosis is caused by the excessive accumulation of extracellular matrix (ECM) proteins including collagen in most types of chronic liver disease[1]. Accumulation of ECM proteins distorts the hepatic architecture by forming fibrous scars. The subsequent development of nodules of regenerating hepatocytes defines cirrhosis. Cirrhosis leads to hepatocellular dysfunction and increases intrahepatic resistance to blood flow, resulting in hepatic insufficiency and portal hypertension[2], and often requires liver transplantation. However, donor livers are limited[3,4]. Cell transplantation has become the better choice of treatment for liver regeneration.
As a general rule, replication of existing hepatocytes is the quickest and most efficient way to generate hepatocytes for liver regeneration and repair. Nevertheless, there is evidence that the replicative activity of hepatocytes diminishes in advanced cirrhosis of humans and in chronic liver injury of mice, reaching a state of “replicative senescence”, perhaps as a consequence of telomere shortening[5-7]. Recent reports have shown that bone marrow cells (BMC) are able to differentiate into non-hematopoietic stem cells[8-12]. The ability of BMC to differentiate into hepatocytes and intestinal cells has been shown by Y-chromosome detection in autopsy analysis of human female recipients of BMC from male donors[13,14].
Bone marrow is a complex tissue containing hematopoietic stem cells, mesenchymal stem cells, and other kinds of cell factors. Mesenchymal stem cells and cell factors are precursors of nonhematopoietic tissues. The precursors of nonhematopoietic tissues can serve as a feeding layer that supports nonhematopoietic stem cell growth, self-renewing without differentiation, and become one phenotype, indicating that BMC with remarkable plasticity have become an attractive cell source in regenerative medicine.
However, contribution of BMC to the generation of hepatocytes in liver regeneration remains uncertain. In the present study, we employed a rat model of fibrosis induced by CCl4 to evaluate the effect of BMC on fibrosis. Our results suggest that BMC treatment can improve fibrosis in rat models.
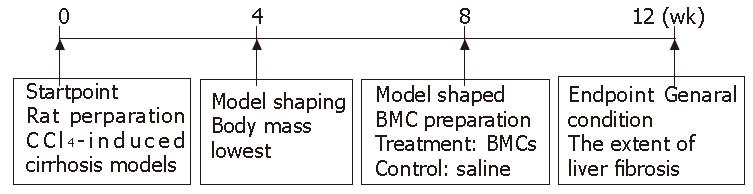
Wistar rats weighing 200-300 g, purchased from Experimental Animal Center of Nanfang Medical University (Guangzhou, China), were housed in cages (5 in each cage) and subjected to 12-d/12-night cycle with free access to basic food. Rat hepatic cirrhosis model was induced by CCl4 (Figure 1). On d 0, 3 mL/kg CCl4 dissolved in paraffin oil (Guangzhou Chemical Factory, China) was injected subcutaneously into the rats (Guangzhou Chemical Factory, China) twice a week for 8 wk, with free access to alcohol gavage and high-fat diet. From the fourth week, a rat was killed weekly and liver fibrosis was determined by histopathology. At the end of wk 8, twenty rats were randomly divided into treatment group (n = 10) and control group (n = 10). BMC were infused into the rats in treatment group via the portal vein, while heparinized saline solution was infused in control group. CCl4 was hypodermically injected into the rats twice a week for 4 wk. Then the trial ended, all rats were sacrificed to assess the extent of liver fibrosis at the end of experiment.
For BMC isolation, Fisher 344 rats (6-wk old) were killed by cervical dislocation and the limbs were removed. BMC were flushed with Dulbecco modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) from medullary cavities of tibias and femurs using a 25-G needle. According to the blood volume to be separated, 50 mL centrifuge tubes were filled with either 20 mL lymphocyte separation medium (D= 1077 g/L, at 20°C), then 25 mL heparinized bone marrow was diluted with an equal volume of PBS and poured over the lymphocyte separation solution. The separation process was performed by centrifugation at 1200 ×g for 20 min. Mononuclear BMC concentrated in the interphase (white layer) between plasma and separation solution. They were subsequently extracted with a sterile Pasteur pipette and washed twice with heparinized saline before final resuspension in heparinized saline. The concentration of mononuclear cells was 6 × 109/L. After cirrhosis models induced by CCl4 were confirmed, rats were placed in a dryer and anaesthetized with ether. Portal vein was exposed with abdominal midline and right transverse incision. Mononuclear BMC (0.5 mL, 3 × 106) and 0.5 mL heparinized saline were infused into liver respectively via portal vein in treatment group and control group.
This experiment was approved by the Committee of Animal Experiment Ethics at the Sun Yat-Sen University School of Medicine and was carried out under the guidelines for animal experiments at Sun Yat-Sen University School of Medicine.
Hyp was determined by the Kivirikko’s method with modifications. Briefly, liver specimens were weighed, and 0.5 g freeze-dried sample was hydrolyzed in 6 mol/L HCl at 110°C in an autoclave at a pressure force of 1.2 kg/cm2 for 24 h, re-dissolved in water, and centrifuged to remove any impurities. Samples were incubated for 10 min in 0.05 mol/L chloramine-T (Fisher, Fair Lawn, NJ, USA) at room temperature, followed by 15-min incubation in Ehrlich's-perchloric acid solution at 65°C. Sample absorbency was measured at 560 nm and resulting values were compared to a standard hyp curve. Each sample was assayed in duplicate. The hyp was expressed as micrograms per gram of wet liver.
Liver tissues were fixed in formalin and embedded in paraffin according to standard procedures (glass slide was cleaned with 950 ml/L ethanol, treated with APES solution and air dried). The liver tissue from the right lobes of liver was cut into 5-μm thick sections using microtome and applied to slides, deparafinized in xylenes and changed three times, each for 5 min. Hydrate sections were gradually passed through graded alcohol, washed twice in 100% ethanol, then twice in 95% ethanol for 10 min each, and finally washed in deodorized water for 1 min. Hematoxylin and eosin (HE) and masson trichrome (MT) staining was performed according to the standard procedure.
The liver fibrosis area with picro-sirius red staining was quantified under Zeiss Axiotron microscope equipped with a KY-F30B 3-CCD camera (JVC, Japan). Briefly, the red area was considered as the fibrotic area and was assessed by computer-aided image analysis with Meta-Morph software (Universal Imaging Corporation, Downingtown, PA) at a magnification of × 400. The semiquantitative data were expressed as percentage of positive staining. The average of scores taking from 5 randomly selected fields (4 corners and 1 center) per sample was used as the percent area of fibrosis.
Fibro-proliferation in liver sections was graded as 0: normal liver; 1: few collagen fibrils extending from the central vein and portal tract; 2: obvious extension of collagen fibrils without encompassing the whole lobule; 3: collagen fibrils extending and encompassing the whole lobule; 4: collagen fibrils extending and separating lobule into pseudo-lobule mainly in square form; 5: formation of pseudo-lobule mainly in circle form. Two pathologists who had no knowledge about their sources and each other's assessment examined the stained slide independently.
Results are presented as mean ± SD. Differences between groups were analyzed by one-way ANOVA using SPSS 13.3. The grade of liver histopathology was examined using the χ2 test.
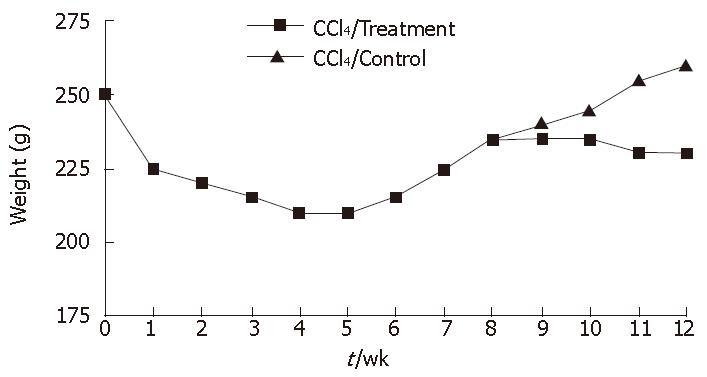
In rat fibrosis model induced by CCl4, the mean loss body mass was 40.67 g ± 10.88 g in the first 4 wk. The body mass increased by 25.93 g ± 8.67 g in the later 4 wk. The body mass of rats in treatment group was 26.57 g ± 8.58 g from wk 8 to the end of experiments, while body mass stopped increasing in rats of the control group (Figure 2).
In CCl4-induced model, rats became lethargic, slower in reaction and diarrhea occurred in 40% of the rats. The fur of rats became gray, ulceration occurred in the injection area. Up to wk 8, cirrhosis model was pathologically confirmed with a mortality rate of 20% (6/30). After infusion of mononuclear bone marrow cells or heparinized saline, morphological and behavioral changes were observed in rats of the treatment group showing improvement in reaction, no diarrhea, fur scar healing and no ulceration. The rats in control group showed slow reaction, diarrhea, incurable fur ulceration, even new ulceration. Their eyes were pale and urine was yellow, and some animals had labored respiration. The mortality rate was 10% (1/10) in two groups. Some rats had a large amount of ascites at the time when they were killed, which might be a reason for their weight gain.
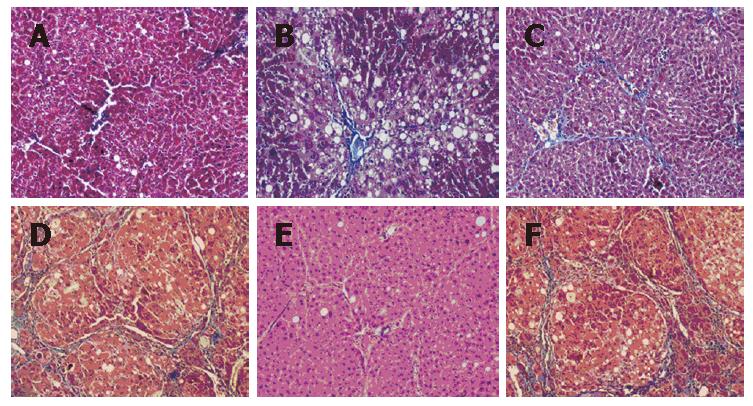
Two weeks after CCl4 injection, histological examina-tion revealed fat denaturalization, centrilobular congestion and hemorrhagic necrosis, but no liver fibrosis (Figure 3A). Four weeks later, extensive fatty changes, centrilobular necrosis and intense neutrophilic infiltration were observed (Figure 3B). By 6 wk, liver fibrosis surrounding the central veins could be seen, with collagen fiber deposition, severe centrilobular necrosis and bridging necrosis, bile duct proliferation (Figure 3C). Massive fibrosis, extensive bridging, pseudolobules formed in the liver after exposure to CCl4 for 8 wk (Figure 3D).
Surprisingly, 4 wk after BMC transplantation (12 wk after CCl4 injection), the BMC-transplanted liver showed that the architecture of liver became better, with inflammation reduced, pseudolobules resolved, collagen accumulation and fatty degeneration alleviated, necrosis of hepatocytes replaced by the regenerated hepatocyres, and the thickened septal fibrosis became thinner or disappeared (Figure 3E). The BMC-transplanted liver showed improvement in liver fibrosis compared with the liver treated with CCl4 alone (Figure 3F), although CCl4 was injected throughout the experimental period.
BMC transplantation significantly reduced hydroxyproline in wet liver to 504.6 ± 128.8 μg/g liver in the treatment group compared to 596.0 ± 3418.0 μg/g liver in control group. Hyp was significantly reduced in treatment group compared with control group (P < 0.05, Table 1). Quantitative image analysis of liver fibrosis indicated that the percent area of liver fibrosis 4 wk after BMC transplantation was 3.75% ± 0.98 % in treatment group and 5.02% ± 0.44% in control group. Quantification of MT staining demonstrated that collagen was prominently decreased in rats of the treatment group compared to control group (P < 0.05, Table 1).
| Group | Hydroxyproline(μg/g) | Collagen fibre(%) |
| Treatment | 504.6 ± 128.8a | 3.75 ± 0.98a |
| Control | 596.0 ± 341.8 | 5.02 ± 0.44 |
Moreover, the degree of fibrosis was decreased in treatment group compared to control group. Liver pathological grades were improved from grade IV to grade III in treatment group (P < 0.05) compared to control group (P > 0.05). There was a significant difference between treatment and control groups (P < 0.05, Table 2).
| Group | 0 | 1 | 2 | 3 | 4 | 5 | χ2 | P |
| Treatment | ||||||||
| Pre-treatment | 0 | 0 | 0 | 0 | 0 | 10 | 10.55 | 0.005 |
| Post-treatment | 0 | 0 | 0 | 0 | 4 | 6 | ||
| Control | ||||||||
| Pre-treatment | 0 | 0 | 0 | 0 | 0 | 10 | - | 1.0 |
| Post-treatment | 0 | 0 | 0 | 0 | 0 | 10 | ||
In this study, experimental fibrosis model was induced by CCl4 and alcohol administration. The general conditions of rats improved after infusion of BM-derived mononuclear cells, suggesting that mononuclear bone marrow cells can improve liver fibrosis. CCl4-induced liver fibrosis is a classical experimental fibrosis model. In this experiment, cirrhosis model was effectively induced by CCl4 combined with alcohol gavage. CCl4 could directly damage hepatocytes, thus leading to fatty degeneration and necrosis of hepatocytes. Alcohol is an enzyme inducer which damages mitochondria, increases oxygen consumption and enhances the toxicity of CCl4 on hepatocytes, thus promoting formation of cirrhosis. During formation of cirrhosis, the body weight of rats dramatically reduced within the first 4 wk, then slowly increased within 4-8 wk. From wk 8, the body weight of rats slightly increased in treatment group with no obvious change in control group (Figure 1), showing that rats become adapted to toxicity. It was reported that this kind of model could undergo spontaneous resolution of fibrosis after CCl4 challenging was stopped[15]. So it is necessary to establish a cirrhosis model to unceasingly inject CCl4. We did not find recovery of liver fibrosis in this experiment.
BMC administration could repair injured liver by reducing inflammation, collagen deposition and remodeling[16,17]. In the current study, transplanted mononuclear bone marrow cells could degrade collagen fibers and improve liver fibrosis.
The classical paradigm that intrahepatic cell populations are replenished from resident oval cells has been revised to include extrahepatic stem cells[18]. Circulating bone marrow–derived cells contribute to the regeneration of a wide variety of organs. There is evidence that bone marrow-derived stem cells are able to enter parenchymal organs and participate in regenerative and structural modeling of transplanted tissues[8,10,11,19]. However, the mechanism by which extrahepatic stem cells contribute to the repair process (including regeneration) following injury remains controversial. The type of injury seems to play a major role in determining whether stem cells differentiate into hepatocytes (the process of stem cell “plasticity”), fuse with hepatocytes (the process of cell “fusion”), or change to form other cell lineages (the process of “trans-differentiation”)[20,21]. Our previous study showed that BM-derived stem cells can inhibit the proliferation and activation of hepatic stellate cells by secreting some cytokines which might protect the liver against fibrosis[22].
In the current study, bone marrow-derived cells were infused without bone marrow ablation. It was reported that reconstitution of haematopoietic stem cells following ablation is necessary for their engraftment in the liver[23]. Additionally, significant engraftment of bone marrow-derived cells during liver injury is thought to require in vivo selection. However, Yamamoto et al[24,25] support the notion that bone marrow ablation is not required for stem cell engraftment in the liver. Mononuclear bone marrow cells include mesenchymal stem cells (MSC), hematopoietic stem cells (HSC), and many kinds of cell factors. These cells and cell factors may reduce fibrosis by interations between them.
Currently stem cells are mostly infused through peripheral vein in animal experiments of intrahepatic transplantation. Though this method is simple, the number of stem cells eventually reaching the liver is extremely limited, because of powerful macrophage phagocytosis in the lung. It severely impairs the efficacy of stem cell therapy. This problem may be solved by direct infusion of stem cells into the liver. In this experiment, the rats were anesthetized with ether and BMC were directly injected into the portal vein, which significantly improved liver fibrosis. However, Liechty et al[26] reported that BMC bear immune tolerance to allogenes even in different races. It may be related with the fact that BMC can inhibit the proliferation of T cells and induce T cell inaction[27-29]. Immune tolerance offers hope for BMC to be used in clinical practice.
The therapeutic potential of bone marrow-derived stem cells has led to increasing research interest in liver regeneration, especially in models of liver injury. Available data suggest that bone marrow-derived stem cells play a limited role in normal liver. The results of the current study demonstrate that stem cell administration can improve fibrosis and plays a significant role in liver injury and fibrogenesis.
The authors thank Professor Chun-Kui Shao for his help in histopathology and the staff of Center for Stem Cell Biology and Tissue Engineering, Sun Yat-Sen University.
Liver fibrosis is due to the excessive accumulation of extracellular matrix (ECM) proteins including collagen that occurs in most types of chronic liver disease. Accumulation of ECM proteins distorts the hepatic architecture by forming fibrous scars, and subsequent development of nodules of regenerating hepatocytes defines cirrhosis. Cirrhosis leads to hepatocellular dysfunction and increases intrahepatic resistance to blood flow, which results in hepatic insufficiency and portal hypertension, and often requires liver transplantation. However, donor livers are limited. Cell transplantation has been used for liver regeneration.
Recent reports showed that bone marrow cells (BMC) are able to differentiate into non-hematopoietic cell lineages. The ability of BMC to differentiate into hepatocytes and intestinal cells has been shown by Y-chromosome detection in autopsy analysis of human female recipients of BMC from male donors. So, BMC have an attractive cell source for regenerative medicine.
It has recently been recognized that circulating bone marrow-derived cells contribute to the regeneration of a wide variety of organs. There is evidence that marrow-derived stem cells are able to enter parenchymal organs and participate in regenerative and structural modeling of transplanted tissues.
Bone marrow is a complex tissue containing hematopoietic stem cells, mesenchymal stem cells, and other kinds of cell factors. Mesenchymal stem cells and cell factors are precursors of nonhematopoietic tissues, indicating that BMC have become an attractive cell source in regenerative medicine. However, the contribution of BMC to the generation of hepatocytes in liver regeneration remains uncertain. In the present study, we employed a model of fibrosis induced by CCl4 to evaluate the effect of BMC on fibrosis. Our results suggest that BMC treatment could improve fibrosis in rat models.
Bone marrow is a complex tissue containing hematopoietic stem cells, mesenchymal stem cells, and other many kinds of cell factors. It has been reported that infusion of hematopoietic stem cells or mesenchymal stem cells can improve liver fibrosis.
In this study, rats were anesthetized with ether, and portal vein was exposed by abdominal midline and right transverse incision. BMC were directly injected into portal vein. The results showed liver fibrosis was significantly improved.
Finally, our experiment used different genetic background rats between the donor and recipient. However, the results showed that BMC could bear immune tolerance in allogenes even between different races,suggesting that BMC can inhibit the proliferation of T cells and inactivate T cells. Immune tolerance offers hope for use of BMC in clinical practice.
The current study demonstrated that stem cell administration could improve fibrosis and BMC play a significant role in liver injury and fibrogenesis. Stem cell therapy will become the choice of treatment for liver disease in near future.
Stem cell therapy: Stem cells have become an attractive cell source in regenerative medicine. Infusion of stem cells can repair or replace damaged cells.
In this manuscript, the authors described the antifibrotic effect of mononuclear BMC infusion on carbon tetrachloride-induced liver fibrosis in rats. Intraportal infusion of mononuclear BMC improved the general conditions of rats and decreased hydroxyproline and the areas of collagen fiber in liver tissue. The results of this study are of certain importance.
| 1. | Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38 Suppl 1:S38-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1199] [Cited by in RCA: 1314] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 2. | Ginès P, Cárdenas A, Arroyo V, Rodés J. Management of cirrhosis and ascites. N Engl J Med. 2004;350:1646-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 483] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 3. | Iredale JP. Cirrhosis: new research provides a basis for rational and targeted treatments. BMJ. 2003;327:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Lee DS, Gil WH, Lee HH, Lee KW, Lee SK, Kim SJ, Choi SH, Heo JS, Hyon WS, Kim GS. Factors affecting graft survival after living donor liver transplantation. Transplant Proc. 2004;36:2255-2256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Falkowski O, An HJ, Ianus IA, Chiriboga L, Yee H, West AB, Theise ND. Regeneration of hepatocyte 'buds' in cirrhosis from intrabiliary stem cells. J Hepatol. 2003;39:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 150] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Rudolph KL, Chang S, Millard M, Schreiber-Agus N, DePinho RA. Inhibition of experimental liver cirrhosis in mice by telomerase gene delivery. Science. 2000;287:1253-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 307] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 7. | Wiemann SU, Satyanarayana A, Tsahuridu M, Tillmann HL, Zender L, Klempnauer J, Flemming P, Franco S, Blasco MA, Manns MP. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB J. 2002;16:935-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 388] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 8. | Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1795] [Cited by in RCA: 1670] [Article Influence: 61.9] [Reference Citation Analysis (3)] |
| 9. | Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, Henegariu O, Krause DS. Liver from bone marrow in humans. Hepatology. 2000;32:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 900] [Cited by in RCA: 854] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 10. | Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 779] [Cited by in RCA: 750] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 11. | Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2032] [Cited by in RCA: 1897] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 12. | Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1764] [Cited by in RCA: 1638] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 13. | Körbling M, Katz RL, Khanna A, Ruifrok AC, Rondon G, Albitar M, Champlin RE, Estrov Z. Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N Engl J Med. 2002;346:738-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 545] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 14. | Okamoto R, Yajima T, Yamazaki M, Kanai T, Mukai M, Okamoto S, Ikeda Y, Hibi T, Inazawa J, Watanabe M. Damaged epithelia regenerated by bone marrow-derived cells in the human gastrointestinal tract. Nat Med. 2002;8:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 287] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 15. | Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJ. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 840] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 16. | Fang B, Shi M, Liao L, Yang S, Liu Y, Zhao RC. Systemic infusion of FLK1(+) mesenchymal stem cells ameliorate carbon tetrachloride-induced liver fibrosis in mice. Transplantation. 2004;78:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 204] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 17. | Zhao DC, Lei JX, Chen R, Yu WH, Zhang XM, Li SN, Xiang P. Bone marrow-derived mesenchymal stem cells protect against experimental liver fibrosis in rats. World J Gastroenterol. 2005;11:3431-3440. [PubMed] |
| 18. | Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 518] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 19. | Direkze NC, Forbes SJ, Brittan M, Hunt T, Jeffery R, Preston SL, Poulsom R, Hodivala-Dilke K, Alison MR, Wright NA. Multiple organ engraftment by bone-marrow-derived myofibroblasts and fibroblasts in bone-marrow-transplanted mice. Stem Cells. 2003;21:514-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 184] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 20. | Shackel NA, Rockey DC. Stem cells and liver disease: promise laced with confusion and intrigue. Gastroenterology. 2004;127:346-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Hüttmann A, Li CL, Dührsen U. Bone marrow-derived stem cells and "plasticity". Ann Hematol. 2003;82:599-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Zhao DC, Chen R, Yu WH, Lei JX, Peng YW, Liu Y, Zhang XM, Li SN, Xiang P. Inhibition of hepatic stellate cell proliferation and activation by bone marrow mesenchymal stem cells in vitro. Zhongguo Bingli Shengli Zazhi. 2005;21:1139-1142. |
| 23. | McTaggart RA, Feng S. An uncomfortable silence em leader while we all search for a better reporter gene in adult stem cell biology. Hepatology. 2004;39:1143-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Yamamoto N, Terai S, Ohata S, Watanabe T, Omori K, Shinoda K, Miyamoto K, Katada T, Sakaida I, Nishina H. A subpopulation of bone marrow cells depleted by a novel antibody, anti-Liv8, is useful for cell therapy to repair damaged liver. Biochem Biophys Res Commun. 2004;313:1110-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Nagai H, Terada K, Watanabe G, Ueno Y, Aiba N, Shibuya T, Kawagoe M, Kameda T, Sato M, Senoo H. Differentiation of liver epithelial (stem-like) cells into hepatocytes induced by coculture with hepatic stellate cells. Biochem Biophys Res Commun. 2002;293:1420-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, Marshak DR, Flake AW. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6:1282-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 851] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 27. | Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838-3843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2455] [Cited by in RCA: 2378] [Article Influence: 99.1] [Reference Citation Analysis (0)] |
| 28. | Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821-2827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 841] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 29. | Rasmusson I, Ringdén O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005;305:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 374] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
S- Editor Wang J L- Editor Wang XL E- Editor Chin GJ