Published online Mar 7, 2006. doi: 10.3748/wjg.v12.i9.1367
Revised: May 1, 2005
Accepted: October 10, 2005
Published online: March 7, 2006
AIM: To study the blood-brain barrier integrity in prehepatic portal hypertensive rats induced by partial portal vein ligation, at 14 and 40 d after ligation when portal pressure is spontaneously normalized.
METHODS: Adult male Wistar rats were divided into four groups: Group I: Sham14d , sham operated; Group II: PH14d , portal vein stenosis; (both groups were used 14 days after surgery); Group III: Sham40d, Sham operated and Group IV: PH40d Portal vein stenosis (Groups II and IV used 40 d after surgery). Plasma ammonia, plasma and cerebrospinal fluid protein and liver enzymes concentrations were determined. Trypan and Evans blue dyes, systemically injected, were investigated in hippocampus to study blood-brain barrier integrity. Portal pressure was periodically recorded.
RESULTS: Forty days after stricture, portal pressure was normalized, plasma ammonia was moderately high, and both dyes were absent in central nervous system parenchyma. All other parameters were reestablished. When portal pressure was normalized and ammonia level was lowered, but not normal, the altered integrity of blood-brain barrier becomes reestablished.
CONCLUSION: The impairment of blood-brain barrier and subsequent normalization could be a mechanism involved in hepatic encephalopathy reversibility. Hemodynamic changes and ammonia could trigger blood-brain barrier alterations and its reestablishment.
- Citation: Eizayaga F, Scorticati C, Prestifilippo JP, Romay S, Fernandez MA, Castro JL, Lemberg A, Perazzo JC. Altered blood-brain barrier permeability in rats with prehepatic portal hypertension turns to normal when portal pressure is lowered. World J Gastroenterol 2006; 12(9): 1367-1372
- URL: https://www.wjgnet.com/1007-9327/full/v12/i9/1367.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i9.1367
Portal hypertension (PH) and hepatic encephalopathy are major complications in human cirrhosis or portal vein thrombosis. Hyperdynamic splanchnic circulation and hyperaemia associated to systemic circulatory alterations are usually present[1,2].
Our laboratory found in the last years, the presence of derangement in the CNS in a prehepatic portal hy-pertensive rat model. Increases in the uptake and release of norepinephrine in diencephalic and telencephalic dis-crete brain regions and significant increments in tyrosine hydroxylase activity in these nuclei were described in previous publications[3-5].
Finally, morphologic alterations in astrocytes (AS) and endothelial cells (EC) located in the hippocampal region (CA1 and CA4) were also found in this experimental model[6].
The morphological cell components of BBB are basically AS, EC and pericytes. Two of this, AS processes and EC, were found altered in this model and in acute acetaminophen intoxication fourteen days after portal vein ligation[7,16,17].
Beside this, forty days after portal vein stricture, portal pressure spontaneously turns to normality. The aim of the present experiment was to investigate if normalization of PP was accompanied by the reestablishment of BBB properties and relate these findings with plasma ammonia concentration.
Male Wistar rats, with an average weight of 240 g were utilized and animal welfare was in accordance with the guidelines of the Faculty of Pharmacy and Biochemistry and approved by the Ethical Committee of the Faculty accordingly with Helsinki’s Declaration. The animals were placed in individual cages, with free access to food (standard laboratory rat chow) and water, and 12 h light cycle: 8 a.m. - 8 P.M. Special care for perfect air renewal was taken.
Four groups of rats were used: Sham14d- sham operated rats; PH14d - Portal vein stenosis; both groups were used 14 d post-surgery. Sham40d- sham operated; PH40d- Portal vein stenosis; used 40 d after surgery.
Each group had separated subgroups for portal pressure determination, serum and CSF fluid determinations, Trypan blue and Evans blue injection respectively.
Portal hypertension was obtained by calibrated stenosis of the portal vein (PH) according to Chojkier et al[1]. Rats were lightly anesthetized with ether and then a midline abdominal incision was made. The portal vein was located and isolated from the surrounding tissues. A ligature of 3.0 silk sutures was placed around the vein, and snugly tied to a 20-gauge blunt-end needle placed along side the portal vein. The needle was subsequently removed to yield a calibrated stenosis of the portal vein. Operations were performed at 2 PM to obey circadian rhythm. Fourteen days after portal vein ligation, animals exhibit an increase in portal pressure. After 20 d portal pressure begins to fall down to normal values approximately after 30 d.
Sham operated rats underwent the same experimental procedure except that the portal vein was isolated but not stenosed. Animals were placed in individual cages and allowed to recover from surgery. Portal pressure was measured the 14th d and the 40th d after surgery in the corresponding group, by puncture of the splenic pulp. Animals were sacrificed by decapitation between 2 and 4 PM to avoid circadian variations.
Portal pressure measurement Fourteen and forty days after the corresponding operation, the rats were anaesthetized with sodium pentobarbital (40 mg/kg), intraperitoneally (ip). Portal pressure was measured through a needle placed in the splenic pulp, and maintained in place by cyanoacrylate gel. The needle was cannulated to a polyethylene catheter (50) filled with a heparinized saline solution (25 U/mL) and connected to a Statham Gould P23ID pressure transducer (Statham, Hato Rey, Puerto Rico) coupled to a Grass 79D polygraph (Grass Instruments, Quincy, MA).
Biochemical determinations Plasma was obtained from blood drained from aorta artery puncture. Under anesthesia, samples of cerebrospinal fluid (CSF) were obtained by cisternal puncture for pro-tein determination according to Bradford[8]. Plasma ammonia concentrations were determined using Ammoniac Enzyimatic UV Kits ( Biomerieux, France). Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were determined in plasma using a commercial standardized and optimized Boehringer-Mannheim (Germany) kits.
Brain water content Cortical brain zones were utilized for the determination of water content in order to quantify possible brain edema. Gravimetric method was employed according to Marmorou et al[9].
Trypan blue transcardial perfusion Rats were perfused with Trypan blue (TB, Sigma Chemical Co. St. Louis MO. USA.) solution and then fixed with paraformaldehyde. TB solution (0.5 %) was prepared by dissolving 1 g of TB in 200 mL of PBS, with gentle heat. The solution was allowed to cool at room temperature, added to the filtrate and placed on ice for immediate use. The temperature of TB solution was 10-12 °C at the time of perfusion. Rats were anaesthetized with ethyl urethane (1mg/kg) and perfused transcardially with 200 ml of TB solution; followed by 300 mL of ice-cooled paraformaldehyde (2% in PBS). The flow rate of perfusate was maintained at 25 mL/min. Brains were dissected and post-fixed overnight in 30 % sucrose for 2 d. Subsequently, the brains were placed in powdered dry ice and stored at -80 °C until processed for microscopic studies. Slices of brain tissue were obtained with cryostat in section of 300 microns according to Paxinos and Watson[10]. Hippocampal slices were evaluated under light microscope and expressed as positive (+) or negative (-) for TB staining. Medial eminence and choroids plexus staining were used as control of TB adequate perfusion. This method was adapted from Ikeda et al[11].
Evans blue test Evans blue (EB, Sigma Chemical Co. St. Louis MO. USA.) dye (25% in 0.9% NaCl solution) was intravenously injected at dose of 25 mg/kg in rats under ether anesthesia. One hour after the injection, animals were sacrificed by decapitation. Brains were weighed, clipped and individually placed within formamide p.a. (2mL/brain). These tubes were kept at 37°C for 48 h. The content of dye extracted from each brain was determined by spectrophotometer (Photometer 4010, Boehringer) at 620 nm and compared to standard graph created through the recording of optical densities from serial dilutions of EB in 0.9% NaCl solution[11,12].
Results were expressed as mean ± SE. For multiple com-parisons ANOVA, followed by Newman-Keuls or Student Newman Keuls tests were used. A P < 0.05 was considered significant.
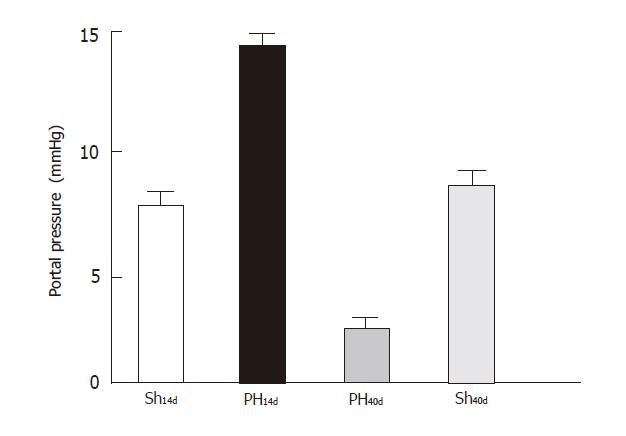
The portal pressure (Figure 1) in Group Sh14d was 7.6 ±1.90 (n = 6), in Group PH14d 14±1.80 (n = 6), in Group PH40d was 2.52 ±1.35 (n = 3), Sh40d 7.7 ± 1.98 (n = 5). Differences between the following groups were significant: Sh14dvs PH14d (P < 0.001); PH14d vs. PH40d, (P < 0.001); Sh14d vs PH40d (P < 0.01). When sham groups (Sh14dvs Sh40d) were compared differences were not significant.
Group PH14d presented 87.40 ± 8.00 mg/mL (n = 4); Group PH40d, 107.60 ± 2.10 mg/mL (n = 5); Group Sh14d, 108.80±7.60 mg/mL (n = 4) and Group Sh40d, 113.00 ± 2.10 mg/mL (n = 4) (PH14dvs. Sham40d, P < 0.05). Results showed a plasma protein concentration decrease tendency in portal hypertensive animals of day 14, which was only significant when compared with sham operated group after 40 d. This tendency was opposite to that observed in CSF.
Protein content in cerebrospinal fluid was 40.60±6.80 µg/mL in Group PH14d (n = 4), 20.62±0.69 µg/mL (n = 5) in Group PH40d, 19.52±0.80 µg/mL (n = 4) in Group Sh14d, and 21.70 ±2.27 µg/mL (n = 4) in Group Sh40d. Statistical analysis indicated that there were significant differences between Sham14d and Group PH14d (P < 0.05) and between Group PH14d and Group Sh40d (P < 0.01) (Figure 1). According to these results PH14d rats had increased protein permeability through the BBB. This increment was reverted when CSF protein was measured 40 days after portal vein stricture (Figure 2). These changes were not related to the above-cited variations in plasma protein values.
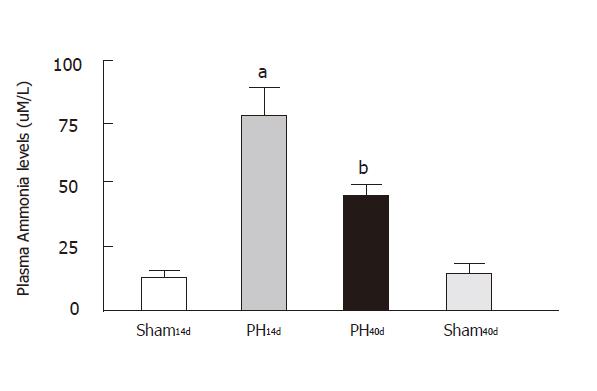
Portal hypertensive rats showed that ammonia plasma level was 79.00 ± 15.00 µm/L in Group PH14d, (n = 8), 44.76±4.51µm/L in Group PH40d (n = 5), 12.99 ± 3.94 µm/L in Group Sh14d (n = 11) and 11.00 ± 4.20µm/L in Group Sh40d (n = 9) (Figure 2). This graphic shows a clear increase of plasma ammonia in PH14, and a decrease in plasma ammonia levels when portal pressure is normalized. Levels in Group PH40d are still higher than in Group Sh14d, with statistical significance between groups: Sh14 vs. PH14 (P < 0.001); Sh40vs PH40 (P < 0.01) and Sh14vs Sh40 (NS). Group I14d and Group IV40d showed a very similar and low plasma concentration. When comparing groups PH14 with PH40, P value is < 0.01, thus indicating a better ammonia metabolism.
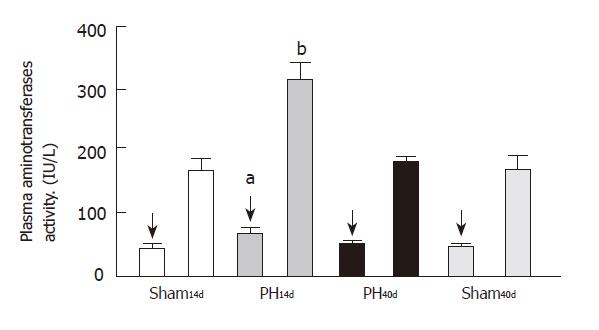
Plasma ALT activity was 63.00 ± 9.00 IU/L in Group PH14d (n = 6), 44.00±4.50 IU/L in Group PH40d (n = 7), 39.00±4.00 IU/L in Group Sh14d (n = 6) and 39.00±3.50 IU/L in Group Sh40d (n = 6). Statistical analysis shows an increase of PH14d levels when compared to groups Sh14d and Sh40d (P < 0.05).
The activity of plasma AST was 316.0 ± 23.0 IU/L in Group PH14d (n = 6), 167.0±14.0 IU/L in Group PH40d (n = 7), 155.0 ± 25.0 IU/L in Group Sh14d (n = 6) and 155.0 ± 32.0 IU/L in Group Sh40d (n = 6). Statistical calculations outline an increment of the enzyme in PH14d group, when compared with groups of Sh14d (P < 0.001), PH40d (P < 0.01), and Sh40d (P < 0.001). The elevation of their activity usually indicates some level of rat liver alteration, which is observed in this model of prehepatic portal hypertension.
Water content in cortical brain areas (H2O/gr %) was 79.21±0.17 in Group PH14d (n = 6), 79.21 ± 0.17 in Group PH40d (n = 7), 78.95 ± 0.18 in Group Sh14d (n = 6) and 77.26 ± 0.29 in Group Sh40d (n = 6). No significant differences were found between the groups.
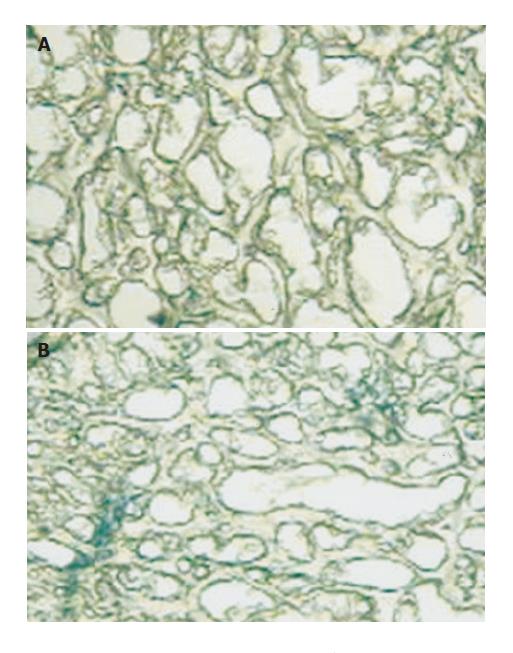
Trypan blue test showed the presence of the dye limited to the vascular area, not involving other areas in Groups Sh14d and Sh40d. Meanwhile, EB appeared positively in vascular area and diffusely in perivascular area in Group PH14d. In Group PH40d both were detected in vascular area and very slightly in perivascular areas. These findings demonstrate the existence of an increased BBB permeability to the dye in PH14d rats, which turns to normal when pressure levels are normalized 40 d later (Figures 6A and B).
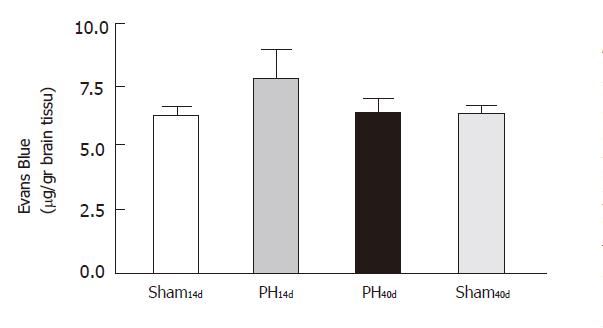
The systemic injection of EB confirmed quantitatively the results found with TB. The results, expressed in µg/g of brain tissue, were 6.170±0.380 in Group Sh14d (n = 12), 8.680±0.700 in Group PH14d (n = 9), 6.300 ± 0.590 in Group PH40d (n = 6) and 6.250 ± 0.450 in Group Sh40d (n = 6). Statistical comparison showed that there was significant difference between Group PH14d and Group PH40d (P <0.01) and between Group Sh14d and Group PH14d (P <0.01). There was no significant difference between Group Sh14d and Group PH40d; Group Sh40d and Group Sh14d shared almost identical values (Figure 5).
The BBB is formed by complex tight junctions of the brain capillary endothelial cells in close relation with the astrocyte processes. These morphological and functional structures make possible a selective transport across BBB and a non-selective brain distribution of drugs(13). Three cellular components characterize BBB: Endothelial cells, astrocyte end feet and pericyte(14). Tight junctions present between cerebral endothelial cells form a diffusion barrier that restricts the influx of most blood-borne substances from entering the central nervous system (CNS). Dysfunction of BBB complicates a number of neurological diseases[15].
Alterations in astrocytes architecture as well as changes in its metabolism due to ammonia detoxification, osmotic balance and cell homeostasis are a well-known feature of hepatic or porto-systemic encephalopathy[16,17].
In previous publications, we described the alteration of BBB in this model of prehepatic portal hypertension, with or without acute acetaminophen intoxication[18,19]. In this paper, the addition of Evans blue technique confirms previous results and adds a quantitative measurement to morphologic findings.
No difference in brain water content was found in this model of partial portal vein ligation, opposed to changes in brain water content found in rats with acetaminophen intoxication as previously described[19]. The presence, however, of EEG modifications[18] and rota rod test modifications[19], led us to classify it as a probable sub-clinic encephalopathy. The lack of a rise in brain water content observed in this model is probably due to the fact that the chronic nature of the portal pressure rise and porto-systemic circulation probably allows some kind of compensation mechanism[16].
Alterations of BBB permeability have been studied in different models of cirrhosis and porto-caval shunting, observing different kind of BBB changes like for some aminoacids but not others[20-22], Evans blue[23] , but not for 14C mannitol or 3H-glutamate[22].
Our results show an altered BBB permeability, qualitative and quantitative, in portal vein ligated rats 14 d after surgery (PH14d) and posterior normalization after 40 d of portal vein stricture (PH40d). This alteration is shown by significant differences observed in protein concentration in CSF and concentration of Evans blue dye in CNS and confirmed by microscopic observation of Trypan blue diffusion. It is interesting to stress out that in PH14d group, protein concentration in CSF is elevated despite a tendency to fall in plasma protein when compared to control (Sh14d). There were no differences in plasma or CSF protein content between Sh14d and PH40d or Sh40d, nor in the same groups when comparing Evans Blue concentration.
In this study, a decrease in portal pressure accompanies normalization of BBB permeability expressed as CSF protein content and diffusion of Evans blue and Trypan blue dyes. Despite these results, ammonia remains moderately elevated after 40 d, indicating the presence of some amount of portal systemic shunting and that some levels of increased ammonia may not interfere with BBB normal function. This presence of portal systemic shunting could also explain the fact that portal pressure is lower in PH40d, when compared to the sham operated group. In spleen-portographies done in the past, we observed in rats of more than 30 d of portal ligation, some cases of porto-portal collateral circulation shunted the silk ligature (personal observation, unpublished).
The fact that BBB altered permeability is resolved but plasma ammonia concentration is not completely normal remains unexplained. Further studies should be conducted to clarify the responsibility of vasoactive substances released in portal hypertension in BBB alterations in this rat model.
The elevated AST and ALT may indicate some kind of liver alteration present in this model with an otherwise almost normal liver function. Astrocyte changes and ammonia detoxification in the brain are probably some of the mechanisms implicated in BBB alteration and posterior normalization. Other factors have been implicated like cytokines and carbon monoxide[23-25].
The normalization of BBB permeability could be a necessary part of hepatic encephalopathy reversibility. This could be a simple model for further studies in BBB function and reversibility of permeability mechanisms useful for a faster recovery of hepatic encephalopathy in patients.
We confirm the spontaneous tendency of this model to normalize portal pressure. No changes in cortical brain water content were found. Following the spontaneous decrease of portal pressure at 40 d, the BBB altered permeability reestablished its properties. Despite BBB permeability normalization, ammonia concentration remained moderately high, but significantly lower than the portal hypertension group 14 d after surgery. This could be a useful model to study BBB alterations and posterior normalization in portal hypertension with a low grade of encephalopathy.
We thank Dr. C. Taira and Dr. C. Ghanem for their kind advise.
| 1. | Chojkier M, Groszmann RJ. Measurement of portal-systemic shunting in the rat by using gamma-labeled microspheres. Am J Physiol. 1981;240:G371-G375. [PubMed] |
| 2. | Genecin P, Groszmann R J. The Biology of Portal Hypertension. Arias, J L. Boyer, N Fausto, W B Jakpby, D Schachter, D A. Shafritz, 3th ed. The Liver Biology and Pathobiology. New York: Raven Press Ltd 1994; 1327-1342. |
| 3. | Lemberg A, Eizayaga FX, Vatta M, Dominguez A, Romay S, Bianciotti LG, Sansó G, Fernández B. Prehepatic portal hypertension in rats modifies norepinephrine metabolism in hypothalamus, medulla oblongata and portal vein. Dig Dis Sci. 1993;38:1259-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Lemberg A, Perazzo J, Romay S, Eizayaga F, Vatta M, Rodriguez-Fermepin M, Bianciotti L, Monserrat A, Fernandez B. Norepinephrine uptake is enhanced in discrete telencephalic and diencephalic areas and nuclei in prehepatic portal hypertensive rats. Brain Res Bull. 1998;45:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Lemberg A, Rubio M, Bengochea L, Romay S, Eizayaga F, Diez A, Perazzo JC. Tyrosine hydroxilase activity in discrete brain regions from prehepatic portal hypertensive rats. Hepatogastroenterology. 1998;45:547-550. [PubMed] |
| 6. | Perazzo J C, Canessa O, Ferrini M, Franchino M A, Bengochea A, Ghanem C, Lemberg A. Alteraciones morfol�gicas de Astrocitos en cerebros de ratas hipertensas portales. Medicina (Buenos Aires). 1998;58:582 (Abst). |
| 7. | Ruibal A, Grinspon D, Scorticati C, Eizayaga F X, Iacono R, Lemberg A, Perazzo J. Cambios en la barrera hematoencefalica del hipocampo, en ratas hipertensas portales. Medicina (Buenos Aires). 2000;60:133 (Abst). |
| 8. | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189576] [Cited by in RCA: 159181] [Article Influence: 3183.6] [Reference Citation Analysis (1)] |
| 9. | Marmorou A, Poll W, Shulman K, Bhagavan H. A rapid and sensitive method for the quantification of micrograms quantities of proteins utilizing the principle of protein-dye building. J. Neurosurg. 1978;49:530-537. |
| 10. | Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, 4th edition. Ed: Academic Press; 1998; . |
| 11. | Ikeda Y, Wang M, Nakazawa S. Simple quantitative evaluation of blood-brain barrier disruption in vasogenic brain edema. Acta Neurochir Suppl (Wien). 1994;60:119-120. |
| 12. | Ohtsuki S. New aspects of the blood-brain barrier transporters; its physiological roles in the central nervous system. Biol Pharm Bull. 2004;27:1489-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Cancilla PA, Frommes S, Kahn LE, DeBault LE. Regeneration of Cerebral microvessels. A Morphological and histochemical Study after local freeze-injury. Lab Invest. 1979;40:79-82. |
| 14. | Belayev L, Busto R, Zhao W, Ginsberg MD. Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res. 1996;739:88-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 412] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 15. | Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1431] [Cited by in RCA: 1633] [Article Influence: 74.2] [Reference Citation Analysis (0)] |
| 16. | von Dahl S, Gerald Kircheis, Dieter Haussinger. Hepatic encephalopathy as a complication of liver disease. World J Gastroenterol. 2001;7:152-156. |
| 17. | Suárez I, Bodega G, Fernández B. Glutamine synthetase in brain: effect of ammonia. Neurochem Int. 2002;41:123-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 278] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 18. | Scorticati C, Prestifilippo JP, Murer G, Lemberg A, Perazzo JC, Functional alterations in central nervous system of prehepatic portal hypertensive rats. Medicina (Buenos Aires). 2001;61:673-675. |
| 19. | Scorticati C, Prestifilippo JP, Eizayaga FX, Castro JL, Romay S, Fernández MA, Lemberg A, Perazzo JC. Hyperammonemia, brain edema and blood-brain barrier alterations in prehepatic portal hypertensive rats and paracetamol intoxication. World J Gastroenterol. 2004;10:1321-1324. [PubMed] |
| 20. | Zanchin G, Rigotti P, Bettineschi F, Vassanelli P, Battistin L. [In vivo aminoacid transport in the brain of the rat with porto-caval anastomosis (author's transl)]. Riv Patol Nerv Ment. 1978;99:178-188. [PubMed] |
| 21. | Dussini N, Zanchin G, Rigotti P, Chiandetti L, Vassanelli P, Battistin L. [Aromatic and branched chain amino acids after porto-caval anastomosis: plasma and brain regional levels in the rat (author's transl)]. Riv Patol Nerv Ment. 1978;99:235-243. [PubMed] |
| 22. | Alexander B, Li X, Benjamin IS, Segal MB, Sherwood R, Preston JE. A quantitative evaluation of the permeability of the blood brain barrier of portacaval shunted rats. Metab Brain Dis. 2000;15:93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Yang S, Wang J, He B, Fang G, Fu R, Chen X. [Relationship between plasma carbon monoxide and blood-brain barrier permeability in cirrhotic rats]. Zhonghua Gan Zang Bing Za Zhi. 2002;10:129-131. [PubMed] |
| 24. | Liberto CM, Albrecht PJ, Herx LM, Yong VW, Levison SW. Pro-regenerative properties of cytokine-activated astrocytes. J Neurochem. 2004;89:1092-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 341] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 25. | Warskulat U, Görg B, Bidmon HJ, Müller HW, Schliess F, Häussinger D. Ammonia-induced heme oxygenase-1 expression in cultured rat astrocytes and rat brain in vivo. Glia. 2002;40:324-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
Co-first-author: Camila Scorticati and Juan P Prestifilippo
S- Editor Wang J L- Editor Zhang JZ E- Editor Wu M