INTRODUCTION
P120ctn is a catenin family member[1,2]. Studies indicated that p120ctn plays an important role in E-cadherin-mediated epithelial cell adhesion and cell signaling[3-6]. Recent studies demonstrated that p120ctn plays a critical role in certain cancerous diseases[7-13]. Nuclear translocation of β-catenin is correlated with tumor progression and has been defined as an oncogene[14]. P120ctn acts as a modulator in E-cadherin-mediated cell-cell adhesion and signaling including β-catenin turnover[11,15]. However, the exact role of p120ctn in cell biological function remains unclear. Tyrosine phosphorylation of p120ctn enhances its nuclear translocation and displays hepatoma cell malignant features[16,17]. In order to further understand the role of p120ctn in E-cadherin-mediated cell biological function and its mechanism, we studied the effects of p120ctn overexpression on β-catenin subcellular localization, regulation of cell cycle and apoptotic proteins as well as cell proliferation in hepatoma cells.
MATERIALS AND METHODS
Cell culture
BEL-7404 human hepatoma cell line was purchased from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences in Shanghai. Cells were maintained in RPMI 1640 medium (Gibcol BRL, Grand Island, NY, USA) supplemented with 100 mL/L of fetal bovine serum, 10 000 U/L of penicillin and 10 000 μg/L of streptomycin, in humidified atmosphere containing
50 mL/L of CO2 at 37 °C.
DNA transfection
P120ctn isoform 3A plasmid and empty vector were a generous gift from Dr. Albert B. Reynolds in the department of Cancer Cell Biology, Vanderbilt University, USA. Plasmid DNA was propagated using a conventional protocol. Lipofectamine transfection reagent (Gibcol BRL) was used following the manufacturer’s instructions. G418 (Gibcol BRL) was used as a selection antibiotic.
Immunoprecipitation and Western blot
Cells were lysed in lysis buffer containing 40 mmol/L Na3PO4 (pH 7.2), 250 mmol/L NaCl, 50 mmol/L NaF, 5 mmol/L EDTA, 10 mL/L Triton X-100, 10 mL/L deoxycholate (Sigma-Aldrich, St. Louis, MO, USA)) for 20 min on ice. Cellular debris and nuclei were removed by centrifugation at 13 000 r/min for 15 min at 4 °C. Protein concentration was determined using Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA), 1 000 μg of total protein from each sample was immunoprecipitated with 10 μg of anti-E-cadherin monoclonal antibody (BD Pharmingen, San Diego, CA, USA), and 50 μL of protein G agarose (Gibcol BRL) was added and samples were mixed by rotation at 4 °C for 1 h. The beads were pelleted and washed four times, then 50 μL of 2×SDS sample buffer was added and boiled for 5 min, and 30 μL of supernatant was loaded to SDS-PAGE gel for protein resolving. Proteins were then transferred to PVDF membrane (Bio-Rad) and incubated with anti-p120ctn (BD Pharmingen), anti-β-catenin (BD Pharmingen) or anti-survivin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-cyclin D1 (Santa Cruz) for Western blotting at 4 °C overnight. Membranes were incubated with HRP-conjugated secondary antibody at room temperature for 1 h, and illuminated by ECL solution (Amersham Biosciences, Piscataway, NJ, USA). Protein bands were visualized using Kodak X-ray film and processed by Kodak film processor (Kodak, Rochester, NY, USA).
Confocal microscopy
Cells were grown on coverslips coated with poly lysine or laminin. The cover slips were rinsed with PBS, then fixed with 12 g/L of formaldehyde at room temperature for 15 min, followed by blocking with 30 mL/L BSA at room temperature for 30 min, the cover slips then were incubated with primary antibodies for 1 h at room temperature and were washed with TBS-T and incubated with FITC-conjugated secondary antibody for 1 h at room temperature. The cover slips were mounted on slides and examined under a Leica laser confocal microscope.
Cell proliferation assay
A total of 200 000 of different types of cells were seeded into 60 mm dishes and grown for 3 d. Cells were trypsinized and trypan blue exclusion assay was used in cell counting. Experiment was performed in triplicate.
RESULTS
P120ctn expression after transfection
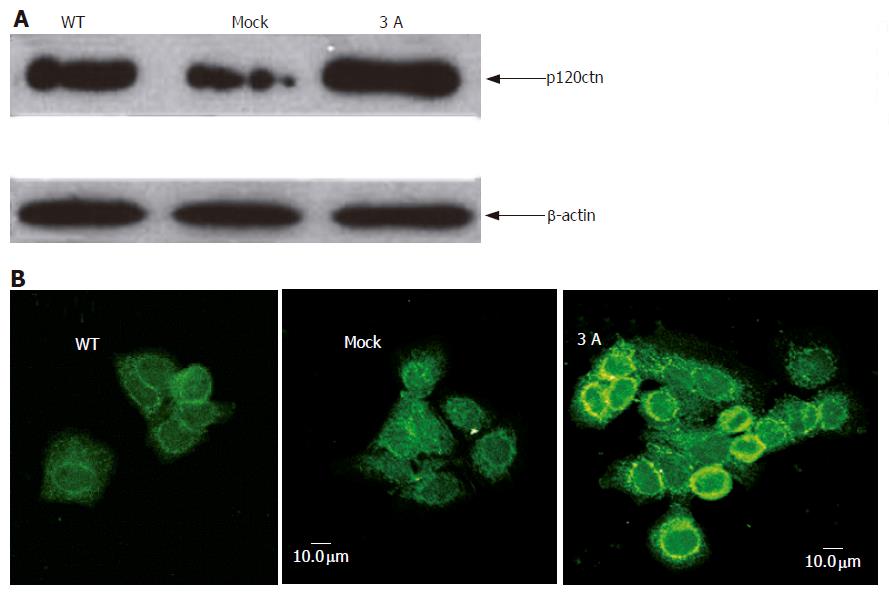
BEL-7404 cells were stably transfected with p120ctn isoform 3A, and the transfection efficiency was determined by Western blotting using mouse anti-p120ctn antibody. The result showed that the expression of p120ctn increased after transfection (Figure 1A). Confocal microscopy result indicated that membranous and cytoplasmic expression of the protein increased mainly at cell-cell contact region (Figure 1B).
Figure 1 Western blotting (A) and confocal microscopy (B) showing increased expression of p120ctn and its subcellular localization after cells transfected with p120ctn isoform 3A.
Effect of transfection on the binding of p120ctn to E-cadherin
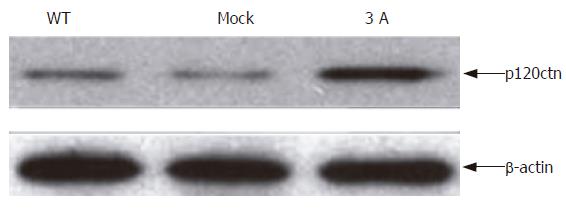
In order to understand the effect of p120ctn overexpression on the binding of p120ctn to E-cadherin, we immunoprecipitated E-cadherin and immunoblotted it with anti-p120ctn. The result showed that the level of p120ctn in E-cadherin-p120ctn complex was increased (Figure 2).
Figure 2 Binding of p120ctn to E-cadherin after cells transfected with p120ctn isoform 3A.
Subcellular relocalization of β-catenin after overexpression of p120ctn
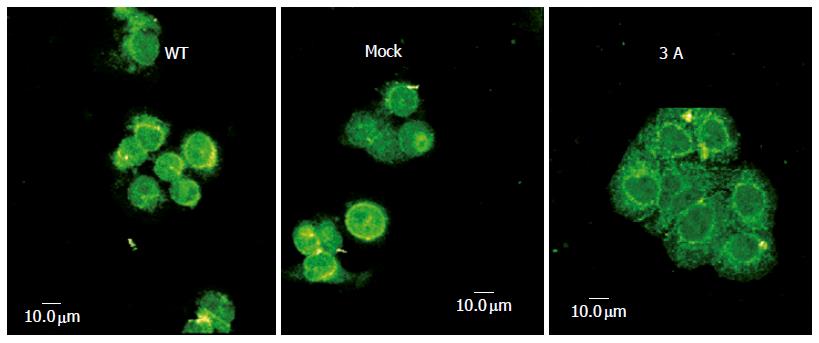
In order to understand the relationship between p120ctn and β-catenin in E-cadherin-mediated cell adhesion and signaling, we examined the alteration of β-catenin subcellular localization after overexpression of p120ctn isoform 3A under confocal microscope. Figure 3 shows the changes of β-catenin expression pattern, namely the obvious expression of membranous protein and the reduction of nuclear expression.
Figure 3 Changes of β-catenin subcellular localization after cells transfected with p120ctn isoform 3A.
Effect of p120ctn overexpression on the binding of β-catenin to E-cadherin
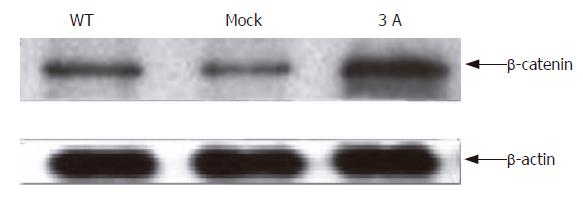
Since overexpression of p120ctn reduced the nucleic but increased membranous β-catenin expression, we immunoprecipitated E-cadherin and immunoblotted it with anti-β-catenin. The result showed that the expression level of β-catenin in E-cadherin and β-catenin complex increased after p120ctn isoform 3A transfection (Figure 4).
Figure 4 Binding of β-catenin to E-cadherin after cells transfected with p120ctn isoform 3A.
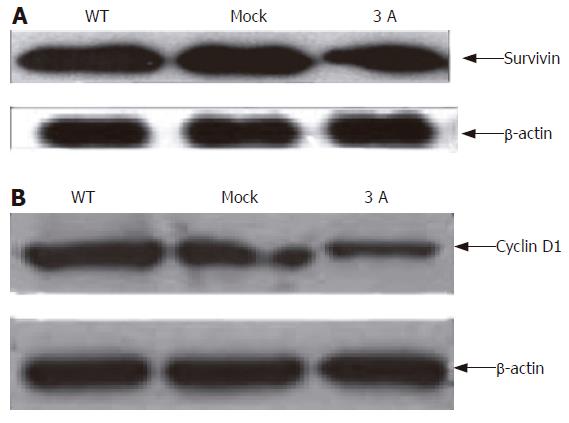
P120ctn transfection down regulated survivin expression
Survivin could be highly expressed in cancer but undetectable in nonproliferating normal adult tissues[18-20]. Our Western blot result showed that overexpression of p120ctn reduced survivin expression, in some extent, in the cells (Figure 5A).
Figure 5 Down regulation of survinin (A) and cyclin D1 (B) expression after transfection of p120ctn.
P120ctn expression down regulated cyclin D1 expression
We performed Western blotting to detect cyclin D1 expression after cells were transfected with p120ctn isoform 3A. The result indicated that cyclin D1 was down regulated after transfection (Figure 5B).
Effect of p120ctn transfection on cell proliferation
Our above results showed that overexpression of p120ctn could reduce β-catenin nuclear expression, enhance binding of p120ctn and β-catenin to E-cadherin. Moreover, overexpression of p120ctn isoform 3A could down regulate survivin and cyclin D1 expression in cells. Thus, the effect of p120ctn overexpression on the proliferation of BEL-7404 hepatoma cells was examined. Using trypan blue exclusion cell counting method, we found that transfection of p120ctn decreased cell proliferation (Figure 6).
Figure 6 Effect of p120ctn transfection on cell proliferation.
DISCUSSION
P120ctn binds to the cytoplasmic tail of E-cadherin in epithelial cells[1,4]. Studies indicated that perturbing p120ctn-E-cadherin binding can lead to nuclear translocation and affect cell biological behavior[21-23], suggesting that p120ctn acts as an oncogenic protein like β-catenin. On the other hand, alterations in E-cadherin and its cytoplasmic regulators, catenins, have been implicated as central to this process, and p120-catenin is frequently altered and/or lost in tumors of the colon, bladder, stomach, breast, prostate, lung, and pancreas[5,11]. Moreover, loss of p120ctn appears to be an early event in tumor progression, possibly preceding loss of E-cadherin, suggesting that p120 plays a role as a “dual modulator” in different cell types[5,7].
In our study, overexpression of p120ctn isoform 3A could enhance its binding to E-cadherin in BEL-7404 human hepatoma cell line, the expression was mainly found at membrane, and cytoplasmic expression could be seen as a minor event. Our results are consistent with a previous report[17], indicating that p120ctn binds to E-cadherin, forming an E-cadherin-p120ctn adhesion complex.
In order to better understand the relationship between p120ctn and β-catenin in E-cadherin-mediated cell-cell adhesion and signaling in liver cancer cells, we examined β-catenin subcellular localization and its binding to E-cadherin after transfection of p120ctn isoform 3A. Our results showed that β-catenin translocation could be seen after transfection of p120ctn. β-catenin became nuclear “exporting”, namely its nuclear expression was reduced while membranous and cytoplasmic expression was increased. Transfection of p120ctn also increased β-catenin binding to E-cadherin, demonstrating that p120ctn can recruit β-catenin from nucleus to cytoplasm and strengthen the formation of E-cadherin-catenin complex. Since β-catenin is an oncoprotein, it binds to the TCF/LEF transcription factor and triggers cell cycle progression, thus influencing cell biological function. Our results are consistent with a previous report[22]. We were unable to detect other pathways of the turnover of nuclei-exported β-catenin, such as APC or ubiquitous degradation pathway due to certain study limitations.
It was reported that β-catenin and p120ctn play an important role in cell proliferation, and TCF/beta-catenin signaling participates in regulation of survivin transcription in colon cancer[23,24]. Monoclonal anti-Wnt-2 antibody induces melanoma cell apoptosis by inactivating survivin[25]. Pizem et al[26] reported that the expression of survivin is associated with aberrant activation of the WNT (wingless) pathway in medulloblastoma patients. Our results indicated that overexpression of p120ctn could down regulate survivin expression, which could be explained by the fact that survivin is reduced due to the recruitment of β-catenin to E-cadherin-β-catenin complex and the free portion of β-catenin could be degradated by APC and ubiquitization after p120ctn overexpression, leading to decrease of nuclear β-catenin and transcription factor inactivation.
β-catenin plays an important role in cell biological function[27,28]. It was reported that cyclin D1 transcription involves Wnt signaling in gastric cancer cell line[29]. In skeletal myocytes, beta-catenin overexpression increases proliferation and cyclin D1 expression while decreases apoptosis and induces hypertrophy[30]. It has been shown that in HeLa and squamous cells, differentiation-inducing factor-1 inhibits tumor cell proliferation and reduces the expression of cyclin D1 mRNA and the amount of beta-catenin[31], suggesting that there is a close correlation between β-catenin and cyclin D1 in tumor cell biology. In our study, transfection of p120ctn isoform 3A could reduce cyclin D1 expression, resulting in the decrease of hepatoma cell proliferation.
In conclusion, overexpression of p120ctn in hepatoma cells can recruit β-catenin from nucleus to membrane and cytoplasm, enhanced E-cadherin-catenin adhesion complex, down regulated apoptosis related protein survivin and cell cycle related protein cyclin D1 expression, and finally, inhibited cell proliferation.