Published online Feb 21, 2006. doi: 10.3748/wjg.v12.i7.1056
Revised: July 2, 2005
Accepted: July 28, 2005
Published online: February 21, 2006
AIM: Pouchitis develops in ileoanal pouches in up to 50% of patients with ulcerative colitis during the first 10 years after pouch surgery while being rare in patients after proctocolectomy for familial adenomatous polyposis coli (FAP) syndrome. Defensins are major components of the innate immune system and play a significant role in gastrointestinal microbial homeostasis. Pouch defensin and cytokine expression were correlated with states of pouch inflammation to study their role in pouchitis.
METHODS: Patients with ulcerative colitis and FAP syndrome were stratified into groups with pouches after surgery, pouches without or with pouchitis. Biopsies from terminal ileum from a healthy intestine or from normal terminal ileum of patients with ulcerative colitis served as controls. mRNA from pouches and controls was analysed for defensin and cytokine expression.
RESULTS: Expression of defensins was increased in all pouches immediately after surgery, compared to ileum of controls. Initially, pouches in ulcerative colitis revealed higher defensin expression than FAP pouches. Defensin expression declined in both patient groups and increased again slightly in pouchitis in patients with ulcerative colitis. FAP pouches without pouchitis had strong expression of β-defensin hBD-1, while all other defensins remained at low levels. Cytokine expression in ulcerative colitis pouches was high, while FAP pouches showed moderately elevated cytokines only after surgery.
CONCLUSION: Development of pouchitis correlates with decreased defensin expression in ulcerative colitis in addition to high expression of cytokines. The low incidence of pouchitis in FAP pouches correlates with increased expression of hBD-1 β- defensin in association with low cytokine levels.
- Citation: Kiehne K, Brunke G, Wegner F, Banasiewicz T, Fölsch UR, Herzig KH. Relationships between mucinous gastric carcinoma, MUC2 expression and survival. World J Gastroenterol 2006; 12(7): 1056-1062
- URL: https://www.wjgnet.com/1007-9327/full/v12/i7/1056.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i7.1056
Pouchitis is a major long term complication after proctocolectomy and ileoanal pouch anastomosis. Up to 50% of patients with ulcerative colitis experience at least one episode during the first ten years after surgical pouch construction while pouches in patients with familial adenomatous polypous (FAP) syndrome have pouchitis rates below 5%[1,2]. The causes of primary pouchitis remain uncertain, while secondary pouchitis is usually being caused by conditions that respond to surgical treatment (e.g. fistulas, local ischemia). Pouchitis develops when the faecal stream is re-diverted towards the pouch after closure of a protective ileostomy indicating the involvement of bacteria in the pathomechanism of pouchitis[3] which is further substantiated by the effective treatment of pouchitis with antibiotics[4].
Ulcerative colitis is a chronic inflammatory disorder of the intestine of unknown aetiology. Immunologic and genetic determinants appear to be major disease factors, but environmental factors and impaired host defense mechanisms emerge to be of significant importance[5,6,7]. The epithelial defense lines against microbial insults comprise co-ordinated actions of the innate and the acquired immune responses. Thus, defects of these mechanisms might result in an overgrowth of normal colonic flora with increases in aerobic bacteria in addition to the appearance of atypical bacteria and fungi[8]. Finally, local invasion of microbials and induction of inflammatory responses causes self-destructive injury.
The FAP syndrome is caused by genetic changes leading to growth of colonic polyps. For both disorders, ulcerative colitis and FAP syndrome, ileoanal pouch procedure is a valuable therapeutic option to prevent development of colorectal cancer.
α-and β-defensins are major components of the epithelial mammalian innate immune system[9]. Defensins are small cationic peptides with high activity against a variety of microbials, encoded by genes and some are regulated in response to challenge with bacterial antigens. Gastrointestinal α-defensins (HD5 and HD6) are almost exclusively expressed in and secreted from Paneth cells of the small intestine, while β-defensins (hBD-1, hBD-2, hBD-3) are secreted by virtually all gastrointestinal epithelial cells to a varying extent[10,11]. Defensins are envisaged to play significant roles in intestinal microbial homeostasis and in the primary defence against enteral and systemic infections[9,12]. In the present study we analysed defensin expression in ileoanal pouches of patients with ulcerative colitis and FAP syndrome. Pouches were stratified into groups with ileostoma- protected pouches after surgery, and pouches (after closure of the ileostomy) without or with pouchitis. Expression data of antimicrobial peptides were related with expression of cytokines and the pouch groups to analyse the potential role of defensins as protectors for the development of pouchitis.
Pouch patients with ulcerative colitis or FAP syndrome as the underlying disease were included. After construction of ileoanal pouches the patients were followed by pouchoscopy and biopsies were collected for histology and RNA analysis. Pouches from patients with ulceraticve colitis or FAP syndrome were further stratified into the following groups (Table 1): 1) pouches soon after surgery (without faecal load and with protective ileostoma), 2) pouches after closure of the ileostoma, no signs of pouchitis, 3) pouches with pouchitis. Severity of pouchitis was scored by the pouchitis disease activity index (PDAI), comprising clinical, endoscopic and histologic features[13] and the Moskowitz score to classify histology of pouch biopsies[14].
| UCPouch before closure of ileostomy | UCPouch in use, no pouchitis | UCpouchitis | FAPpouch before closure of ileostomy | FAPpouch in use, no pouchitis | FAPpouchitis | Healthy control | UC control term.ileurn without inflammation | |
| Patients (n) | 10 | 15 | 11 | 6 | 14 | 7 | 23 | 35 |
| Age(yr) | 17-36 | 19-45 | 27-50 | 17-27 | 19-45 | 22-36 | 17-53 | 18-59 |
| Sex(f/m) | 5/5 | 8/7 | 4/7 | 4/2 | 10/4 | 4/3 | 12/11 | 23/12 |
| Pouch Classification: | ||||||||
| Moskowitz | 2-4 | <4 | >4 | 2-4 | <4 | >4 | - | - |
Biopsies from control patients or from control patients with ulcerative colitis were collected from a macroscopically and histologically healthy terminal ileum. Patients characteristics were comparable between controls, ulcerative colitis and FAP groups, as shown in Table 1.
Two biopsies were examined by histology and two others were immediately snap-frozen in liquid nitrogen for RNA analysis. Miroscopy (not shown) of pouch biopsies showed features of metaplasia in all pouches when pouches had been exposed to faeces for several month. There was an increase of mucin containing cells and with a reduction of vili in the ileal mucosa as previously reported[15,16,17]. Electron microscopy also demonstrated an increase of mucin containing cells while Paneth cells remained stable in number and morphology (not shown). Changes were most pronounced in biopsies from pouches with the most severe signs of pouchitis in endoscopy.
The study was approved by the Ethics Committees of the University of Kiel, Germany and the University of Poznan, Poland (Ref.No. A158/01). All patients gave their written informed consent prior to investigation.
Total RNA was extracted from frozen biopsies using silica gel-based spin columns (RNeasy Kit, QIAGEN, Hilden, Germany). Genomic DNA was digested by thorough treatment with deoxyribonuclease I (QIAGEN, Hilden, Germany). Reverse transcription of 2µg RNA was performed using 0,5µg oligo(dT)15- primers (You prime First strand cDNA synthesis kit; Amersham Biosciences, Freiburg, Germany) following the manufacturers instructions.
Specific intron spanning primers were designed based on the published mRNA sequences using the Primer3 software[18] (also available under http://www-genome.wi.mit.edu/genome-software/other/primer3.html]. Oligonucleotides were obtained from Sigma ARK (Darmstadt, Germany), sequences and product sizes for each primer pair used are depicted in Table 2.
| Primer | Orientation | Sequence | PCR product size(bp) | GeneBank accession |
| hBD-1 | Sense | 5`-TTG TCT GAG ATG GCC TCA GGT GGT AAC | 253 | NM005218 |
| Antisense | 5`-ATA CTT CAA AAG CAA TTT TCC TTT AT | |||
| hBD-2 | Sense | 5`-ATC AGC CAT GAG GGT CTT GT | 173 | AF448141 |
| Antisense | 5`-GAG ACC ACA GGT GCC AAT TT | |||
| hBD-3 | Sense | 5`-AGC CTA GCA GCT ATG AGG ATC | 206 | NM018661 |
| Antisense | 5`-CTT CGG CAG CAT TTT CGG CCA | |||
| HD5 | Sens | 5`-GCC ATC CCT GCT GCC ATT C | 241 | NM 021010 |
| Antisense | 5`-AGA TTT CAC ACC CCG GAG A | |||
| HD6 | Sense | 5`-CCT CAC CAT CCT CAC TGC TGT TC | 266 | NM001926 |
| Antisense | 5`-CCA TGA CAG TGC AGG TCC CAT A | |||
| TNFα | Sense | 5`-TCA GCT TGA GGG TTT GCT ACA A | 100 | NM000594 |
| Antisense | 5`-TCT GGC CCA GGC AGT CAG ATC | |||
| IL-1β | Sense | 5`-CCA GCT ACG AAT CTC CGA CCA CCA CTA C | 600 | NM000576 |
| Antisense | 5`-TGC TTG AGA GGT GCT GAT GTA CCA GTT G | |||
| IL-4 | Sense | 5`-AAC ACA ACT GAG AAG GAA ACC TTC T | 276 | NM000589 |
| Antisense | 5`-GCT CGA ACA CTT TGA ATA TTT CTC | |||
| IL-6 | Sense | 5`-AGG AGC CCA GCT ATG AAC TCC TTC | 120 | NM000600 |
| Antisense | 5`-TGG AAT CTT CTC CTG GGG GTA CTG | |||
| IL-8 | Sense | 5`-ATG ACT TCC AAG CTG GCC GTG GC | 307 | NM000584 |
| Antisense | 5`-TCT CAG CCC TCT TCA AAA ACT TC |
Real- time RT-PCR analyses were performed in a fluorescence temperature cycler (LightCycler, Roche Molecular Biochemicals) according to the manufacturer’s instructions. Standard curves for each specific defensin were constructed by cloning the purified PCR-products containing the target sequence into pCR-Blunt II-TOPO vector with 3519 bp (Invitrogen). Clones were sequenced on ALP Express (Pharmacia, Uppsala, Sweden). Concentration of the reference plasmid was measured spectrophotometrically and converted into number of copies / µL by using the formula: number of copies/µL = (X g/µL plasmid DNA / Y × 660) × 6.023 × 1023, where X is the measured plasmid concentration, Y is the number of nucleotide pairs for the plasmid plus the size of the insert, 660 g/mol is the average molecular weight of a nucleotide pair, and 6.023 × 1023 is Avogadro´s number (number of copies/mol). To generate standard curves, the plasmids were serially diluted (1: 10) over the appropriate concentration range. The solvent included unrelated, defensin- sequence free cDNA in a final concentration of 10 ng/µL. To achieve a reliable standard curve for each measured defensin, the plasmids were PCR- amplified in three replicates for each standard dilution point over the complete range (107 to 100). Standard curves were measured repeatedly to calculate averages. The reverse transcribed cDNA from the biopsies corresponding to 20 ng of RNA served as template in a 10 µL PCR reaction containing 4 mmol/L MgCl2, 0,5 µmol/L of each primer and 1x LightCycler-Fast Start DNA Master SYBR Green I mix (Roche Molecular Biochemicals, Mannheim, Germany). Samples were loaded into capillary tubes and incubated in the fluorescence thermocycler (LightCycler) with initial denaturing at 95 °C for 10 min, followed by 45 cycles, each cycle consisting of 95 °C for 15 s for denaturation, primer specific annealing temperature for 10 s, and 72 °C for 10s for elongation. SYBR Green I fluorescence was detected at the end of each cycle to monitor the amount of PCR product formed during the cycle. At the end of each run, melting curve profiles were produced (cooling the samples to 65 °C for 15 s and then heating slowly at 0.2 °C/s to 95 °C with continuous measurement of fluorescence) to exclude non-specific amplification of transcripts. The absolute mRNA transcript number in each sample was calculated by use of calibration curves. Identical results were obtained in control experiments when the standard plasmids were mixed with cDNA obtained from a variety of sources including cDNA from cultured intestinal mouse STC-1 cells which do not contain defensin transcripts (not shown).
Reverse transcribed cDNA from biopsies corresponding to 20 ng RNA was used as template in a 10 µL PCR reaction using Taq polymerase in a Thermal cycler (Gene Amp PCR System 2400, Perkin Elmer). PCR started with initial denaturation at 95 °C for 10 min, followed by 30 cycles each consisting of denaturation at 95 oC for 45 s, annealing at 60 oC for 45 s and elongation at 72 °C for 60 s. Elongation in the final PCR cycle was for 7 min. PCR products were resolved by 8% agarose gel electrophoresis
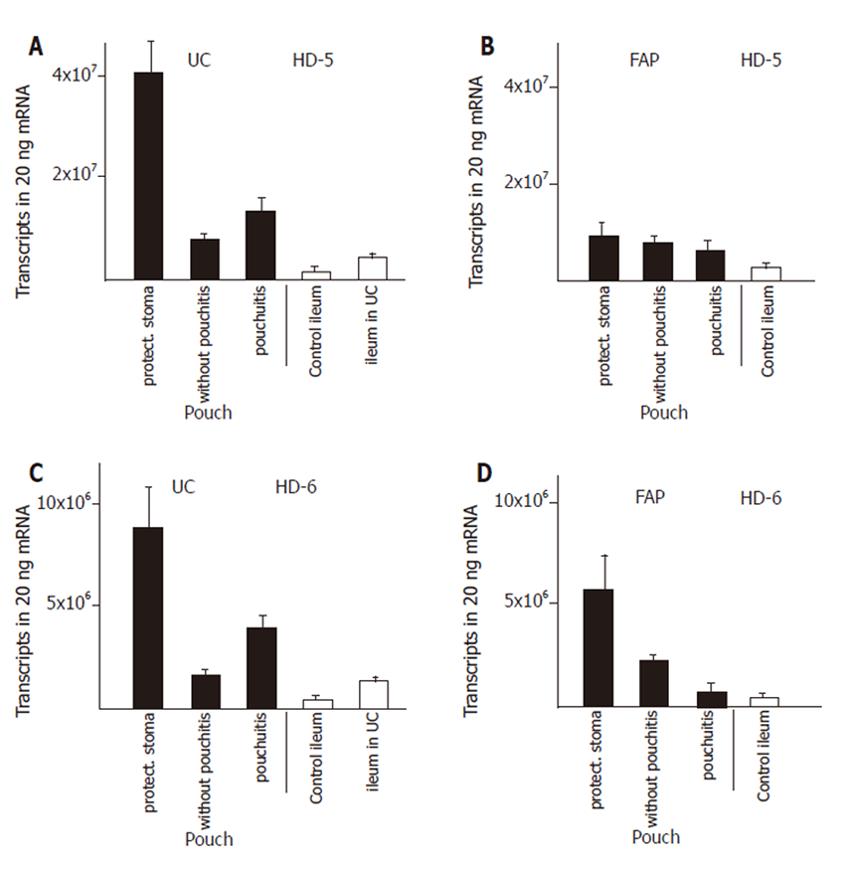
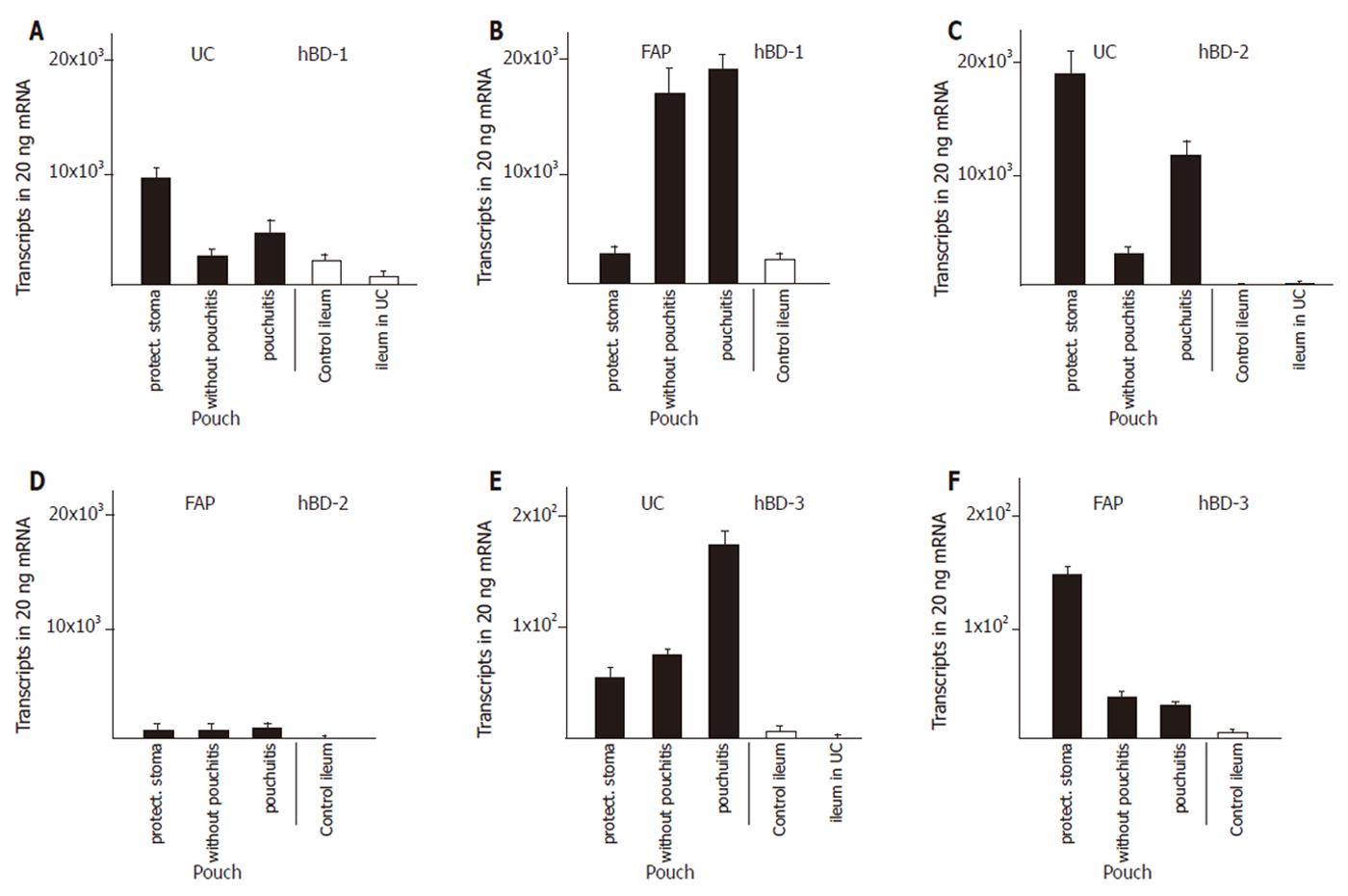
We established control groups of normal ileum from healthy patients and ileum from patients with active ulcerative colitis but without macroscopic or microscopic involvement in order to obtain parameters to which defensin expression in diseases could be correlated. The expression of α-defensins HD5 (Figure 1A) and HD6 (Figure 1C) in ileum of patients with ulcerative colitis was increased by two to three fold, resp, compared to ileum of healthy controls. The β-defensins HBD-2 (Figure 2C) or hBD-3 (Figure 2E) were present at very low levels in the healthy ileum. HBD-2 expression was increased (from 3 ± 1 to 149 ± 110 transcripts per 20 ng mRNA) in the ileum in patients with ulcerative colits but remained still at insignificant levels, and hBD-3 remained unchanged. Surprisingly, hBD1 (Figure 2A) expression in the not affected ileum of patients with ulcerative colitis is reduced to levels below 30% of hBD-1 expression in a normal ileum.
Expression of both α- and β- defensins in pouches of ulcerative colitis and FAP patients was most pronounced during the first three to nine months after surgical construction. Pouches at that time were not exposed to the faecal stream because of the presence of an upstream protective ileostoma. α-Defensin expression was increased by 5- fold (HD6), and 10- fold (HD5), compared with the expression in the not affected ileum in patients with ulcerative colitis (Figure 1). In pouches of ulcerative colitis patients, hBD-1 showed a 15- fold increase compared to the ileum in patients with ulcerative colitis (Figure 2A). HBD-2 is virtually absent in the normal ileum, but in pouches after surgery hBD-2 is expressed at 18 × 103 copies per 20 ng mRNA which is a strong increase compared to normal ileum mucosa or to the ileum of patients with ulcerative colitis (Figure 2C). HBD-3 is expressed at very low levels in the ileum or in healthy or inflamed pouches while a slight increased expression is found in pouches after surgery (Figure 2F).
When pouches in ulcerative colitis patients are in use after closure of the protective ileostoma and pouchitis was not present, HD5 (Figure 1A), HD6 (Figure 1C), hBD-1 (Figure 2A), and hBD-2 (Figure 2C) expression levels declined from the initially present high levels to levels similar to or slightly increased above (2- to 4- fold ) the defensin expression level in ileum of patients with ulcerative colitis. When pouchitis develops, a rise in defensin expression is observed between 2- fold (HD5, Figure 1A; HD6, Figure 1C; hBD-1, Figure 2A; hBD-3, Figure 2E) or 3- fold (hBD-2, Figure 2C) compared with the expression levels present in pouches without pouchitis.
Pouches of FAP patients after pouch surgery showed only minor changes of defensin expression, compared to normal ileum. Levels of HD5 and HD6 expression was increased 3- and 5 fold, respectively. (Figure 1B and D), while hBD-1 expression was unchanged (Figure 2B). HBD-2 and hBD-3 expression was increased, but total expression levels remained at very low transcript numbers (Figure 2D, F). When pouches were exposed to the faecal stream, the initial increased HD5 (Figure 1B) and HD6 (Figure 1D) defensin expression levels declined, and hBD-2 expression remained stable (Figure 2D). Pouchitis in FAP pouches did not cause an increase of HD5 (Figure 1B) and HD6 (Figure 1D) expression as pouchitis in ulcerative colitis patients, and hBD-2 (Figure 2D) expression levels remained unchanged. In contrast to all other defensins, hBD-1 (Figure 2B) expression levels in FAP pouches after surgery remained identical to hBD-1 expression levels in the normal ileum. Furthermore, hBD-1 expression increased markedly (6- fold) in the group of FAP pouches without pouchitis compared with normal ileum and hBD1 expression slightly increased further in FAP pouches under pouchitis.
Cytokine expression in pouch biopsies showed significant differences between the various pouch groups. Pouches from ulcerative colitis patients after surgery had strong expression of the proinflammatory cytokines TNF-α, IL-1β and the immunomodulatory cytokine IL-10, when compared with unaffected ileum from ulcerative colitis patients (Table 3). Expression of IL-8 was intermediate while IL-4 and IL-6 were poorly detectable. The expression of TNF-α, IL-1β, IL-8 and IL-10 remained largely unchanged in pouches from patients with ulcerative colitis, irrespective of the presence or absence of pouchitis and indicating a predominant TH1 response in the pouch patients with ulcerative colitis. Pouches from FAP patients immediately after surgery showed a similar pattern of cytokine expression as measured in pouches in ulcerative colitis, although cytokine expression levels were lower in the FAP patients. Cytokine expression declined in FAP pouches exposed to faeces. Interestingly, cytokine expression was undetectable for IL-4, IL-6, IL-8, and IL-10 while very minor expression was found for TNF-α and IL-1β in FAP pouches with pouchitis.
| Cytokine | Controls | Pouches | ||||||
| Ileum control | Ileum in UC | UC ileostoma | UC without pouchitis | UC pouchitis | FAP ileostoma | FAP without pouchitis | FAP pouchitis | |
| TNFα | + | (+) | +++ | ++ | ++ | + | + | + |
| IL-1β | - | ++ | +++ | +++ | +++ | +++ | ++ | + |
| IL-4 | - | - | (+) | (+) | (+) | + | (+) | (+) |
| Il-6 | - | - | + | + | (+) | (+) | (+) | (+) |
| IL-8 | (+) | ++ | ++ | ++ | ++ | ++ | + | (+) |
Surgical construction of an ileoanal pouch results in massive increases of α- and β-defensin levels in the ileum type pouch mucosa. In general, the expression of defensins in pouches is significantly higher than in the normal ileum. Furthermore, pouches in ulcerative colitis initially show much higher α- and β-defensin levels than pouches in FAP. When pouches are in use and exposed to faeces, they undergo metaplastic changes[15,16,17] and show reduced defensin expression compared to pouches early after surgery which are protected by an upstream ileostomy. In the presence of pouchitis, there is an up regulation of α- and β-defensins in the inflamed pouches of ulcerative colitis patients. In contrast, FAP pouches show a down regulation of the initially elevated α- defensin expression in pouches without or with pouchitis. The most prominent changes are observed in the expression of the β-defensin hBD-1, which is markedly upregulated in FAP pouches after closure of the protective ileostoma and expressed at low levels in pouches of patients with ulcerative colitis. Furthermore, cytokines in pouches of patients with ulcerative colitis are strong expressed in all stages while FAP pouches show a transient and weaker cytokine expression.
The pathomechanisms of pouchitis remain undetermined with potential genetic, microbial, and immunologic causes[2,5,6,7]. The most obvious difference between ileoanal pouch mucosa and the normal ileum is metaplasia of the typical ileum mucosa to a more or less colonic epithelial type[15,16,17]. Pouch mucosa has immunohistochemical features and enzymatic markers of both ileum and colon, but complete metaplasia does not occur. Paneth cells remain stable in cell number over several months after pouch construction[17]. The observed changes in defensin expression in the present study can only partially be correlated to histologic changes or colonic metaplasia. The healthy ileum has a much higher expression of HD5 and HD6 than a healthy colon and an identical expression of the β-defensins (unpublished data). The initial extreme upregulation of α- defensins and to a lesser degree of β defensins after pouch construction could be due to Paneth and epithelial cell stimulation but can not be correlated to metaplasia which occurs at later times. Furthermore, the decrease in α-defensin expression upon time in older pouches or pouchitis cannot be correlated with a decrease of Paneth cell number. Thus, α-defensin transcription appears to be regulated after pouch surgery by unknown mechanisms, which cause multiplication of defensin expression during this time.
Pouches in ulcerative colitis develop pouchitis at a much higher incidence than pouches in FAP[1] which has been related to the elevated cytokine levels in ulcerative colitis[19,20]. Except for TNF-α and IL-10, cytokine expression pattern is largely similar in the colon and in pouches of patients with ulcerative colitis[21].The expression of cytokines (TNF-α, IL-1β, IL-8 and IL-10) in pouches of ulcerative colitis patients is steadily upregulated independent of the presence or absence of pouchitis, which further contrasts to FAP pouches, where cytokines are expressed only during the initial time after pouch construction but not in older pouches or in pouchitis. Regulation of defensin expression by TNFα, IL-1α and IL-1β cytokines has been described for hBD-2, hBD-3, and HD5 in cell culture systems, while expressional changes of hBD-1, hBD-4 and HD6 have not been observed in these model systems[11,22,23,24,25]. In inflammatory bowel disease, reduced expression of hBD-2 and hBD-3 has been described in Crohn´s disease, while increases have been found in the colon of ulcerative colitis in the inflamed areas[26]. Together, a modulating effect of cytokines on the up- or down regulation of defensins in pouchitis can be postulated. The β- defensin hBD-1 expression shows the most striking difference between ulcerative colitis pouches and FAP pouches with strong increases in the non inflamed FAP pouches and relatively low expression in UC pouches. The inverse correlation of hBD-1 and cytokine expression is obvious. Thus, the increased levels of hBD-1 and the low cytokine levels are correlated with low incidence of pouchitis.
Microbials or their products could be involved in defensin regulation in pouches after surgery and in pouchitis. β-defensins are upregulated in response to bacterial antigens or in the presence of cytokines [22]. Thus, the rise of hBD-2 and hBD-3 in pouchitis reflects mucosal damage and infection. The gene regulatory mechanisms that control hBD-1 expression remain speculative, since hBD-1 was previously classified as being steadily expressed by previous in vitro studies[11]. Fehlbaum et al [27] described the induction of β-defensin expression by L-isoleucin, which as an essential amino acid must arise from external sources or from degradation of host proteins liberated by bacterial proteases. In this context, L-isoleucin could serve as a marker for microbial presence or for host tissue degradation. Presence of L-isoleucin in turn would then signal to the host the need to increase defence mechanisms.
Faecal stream and faecal stasis in pouches have been postulated to be involved in the pathomechanisms of primary pouchitis[3,28]. As long as the faecal stream is diverted from the pouch through a protective ileostomy, pouches do not develop primary pouchitis. In addition, the ileum of the ileostomy does not react with inflammation, although faeces pass through the ileostomy. A variety of pathogenic bacteria have been found in patients with pouchitis, e.g. Clostridium perfringens[29]. A shift from the normal enteral microbial spectrum towards a pronounced presence of anaerobes, a reduction of bacterial diversity and an increase in fungal diversity have been described in ulcerative colitis[8]. Since each individual defensin has a characteristic pattern of antimicrobial activity, an alteration of defensin expression or a general reduction of defensins is likely to result in marked quantitative and qualitative changes of the luminal microbial contents. Future studies have to focus on the interaction of various protective mucosal factors and the intestinal contents.
In conclusion, the present data correlate the development of pouchitis in ulcerative colitis with changes of defensin expression pattern and cytokine expression. Surgical construction of an ileoanal pouch resulted in massive increases of α- and β- defensin levels in the ileum type pouch mucosa. In general, the expression of defensins in pouches was significantly higher than in the normal ileum. The initial high levels of α- defensins after pouch surgery in ulcerative colitis appear to be protective for the pouch mucosa, since the decrease of α- defensins upon time correlates with the incidence of pouchitis in ulcerative colitis. Especially for the FAP pouches, hBD-1 could be another protector for the development of pouchitis. Cytokines, metaplastic changes of the ileum mucosa and inappropriate expression of defensin antimicrobial peptides appear to play major roles in the pathomechanism of pouchitis.
| 1. | Heuschen UA, Autschbach F, Allemeyer EH, Zöllinger AM, Heuschen G, Uehlein T, Herfarth C, Stern J. Long-term follow-up after ileoanal pouch procedure: algorithm for diagnosis, classification, and management of pouchitis. Dis Colon Rectum. 2001;44:487-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 92] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Kühbacher T, Schreiber S, Runkel N. Pouchitis: pathophysiology and treatment. Int J Colorectal Dis. 1998;13:196-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | de Silva HJ, Millard PR, Soper N, Kettlewell M, Mortensen N, Jewell DP. Effects of the faecal stream and stasis on the ileal pouch mucosa. Gut. 1991;32:1166-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Madden MV, McIntyre AS, Nicholls RJ. Double-blind crossover trial of metronidazole versus placebo in chronic unremitting pouchitis. Dig Dis Sci. 1994;39:1193-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 187] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1309] [Cited by in RCA: 1357] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 6. | Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2693] [Cited by in RCA: 2772] [Article Influence: 115.5] [Reference Citation Analysis (3)] |
| 7. | Tamboli CP, Neut C, Desreumaux P, Colombel JF. Dysbiosis in inflammatory bowel disease. Gut. 2004;53:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 529] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 8. | Ott SJ, Musfeldt M, Wenderoth DF, Hampe J, Brant O, Fölsch UR, Timmis KN, Schreiber S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53:685-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 875] [Cited by in RCA: 956] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 9. | Otte JM, Kiehne K, Herzig KH. Antimicrobial peptides in innate immunity of the human intestine. J Gastroenterol. 2003;38:717-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Cunliffe RN. Alpha-defensins in the gastrointestinal tract. Mol Immunol. 2003;40:463-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 108] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | O'Neil DA, Porter EM, Elewaut D, Anderson GM, Eckmann L, Ganz T, Kagnoff MF. Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. J Immunol. 1999;163:6718-6724. [PubMed] |
| 12. | Cunliffe RN, Mahida YR. Expression and regulation of antimicrobial peptides in the gastrointestinal tract. J Leukoc Biol. 2004;75:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Sandborn WJ, Tremaine WJ, Batts KP, Pemberton JH, Phillips SF. Pouchitis after ileal pouch-anal anastomosis: a Pouchitis Disease Activity Index. Mayo Clin Proc. 1994;69:409-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 550] [Cited by in RCA: 525] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 14. | Moskowitz RL, Shepherd NA, Nicholls RJ. An assessment of inflammation in the reservoir after restorative proctocolectomy with ileoanal ileal reservoir. Int J Colorectal Dis. 1986;1:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 245] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Apel R, Cohen Z, Andrews CW, McLeod R, Steinhart H, Odze RD. Prospective evaluation of early morphological changes in pelvic ileal pouches. Gastroenterology. 1994;107:435-443. [PubMed] |
| 16. | Reinholt FP, Veress B, Lindquist K, Liljeqvist L. Qualitative assessment and morphometry in the study of the ileal reservoir after restorative proctocolectomy. APMIS. 1989;97:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Shepherd NA, Healey CJ, Warren BF, Richman PI, Thomson WH, Wilkinson SP. Distribution of mucosal pathology and an assessment of colonic phenotypic change in the pelvic ileal reservoir. Gut. 1993;34:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 93] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365-386. [PubMed] |
| 19. | Gionchetti P, Campieri M, Belluzzi A, Bertinelli E, Ferretti M, Brignola C, Poggioli G, Miglioli M, Barbara L. Mucosal concentrations of interleukin-1 beta, interleukin-6, interleukin-8, and tumor necrosis factor-alpha in pelvic ileal pouches. Dig Dis Sci. 1994;39:1525-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Reinecker HC, Steffen M, Witthoeft T, Pflueger I, Schreiber S, MacDermott RP, Raedler A. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1993;94:174-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 654] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 21. | Patel RT, Bain I, Youngs D, Keighley MR. Cytokine production in pouchitis is similar to that in ulcerative colitis. Dis Colon Rectum. 1995;38:831-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Harder J, Meyer-Hoffert U, Teran LM, Schwichtenberg L, Bartels J, Maune S, Schröder JM. Mucoid Pseudomonas aeruginosa, TNF-alpha, and IL-1beta, but not IL-6, induce human beta-defensin-2 in respiratory epithelia. Am J Respir Cell Mol Biol. 2000;22:714-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 319] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 23. | Harder J, Meyer-Hoffert U, Wehkamp K, Schwichtenberg L, Schröder JM. Differential gene induction of human beta-defensins (hBD-1, -2, -3, and -4) in keratinocytes is inhibited by retinoic acid. J Invest Dermatol. 2004;123:522-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 166] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 24. | García JR, Jaumann F, Schulz S, Krause A, Rodríguez-Jiménez J, Forssmann U, Adermann K, Klüver E, Vogelmeier C, Becker D. Identification of a novel, multifunctional beta-defensin (human beta-defensin 3) with specific antimicrobial activity. Its interaction with plasma membranes of Xenopus oocytes and the induction of macrophage chemoattraction. Cell Tissue Res. 2001;306:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 311] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 25. | Wehkamp J, Schwind B, Herrlinger KR, Baxmann S, Schmidt K, Duchrow M, Wohlschläger C, Feller AC, Stange EF, Fellermann K. Innate immunity and colonic inflammation: enhanced expression of epithelial alpha-defensins. Dig Dis Sci. 2002;47:1349-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 63] [Reference Citation Analysis (1)] |
| 26. | Wehkamp J, Harder J, Weichenthal M, Mueller O, Herrlinger KR, Fellermann K, Schroeder JM, Stange EF. Inducible and constitutive beta-defensins are differentially expressed in Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2003;9:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 223] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 27. | Fehlbaum P, Rao M, Zasloff M, Anderson GM. An essential amino acid induces epithelial beta -defensin expression. Proc Natl Acad Sci U S A. 2000;97:12723-12728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Rutgeerts P, Goboes K, Peeters M, Hiele M, Penninckx F, Aerts R, Kerremans R, Vantrappen G. Effect of faecal stream diversion on recurrence of Crohn's disease in the neoterminal ileum. Lancet. 1991;338:771-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 517] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 29. | Ruseler-van Embden JG, Schouten WR, van Lieshout LM. Pouchitis: result of microbial imbalance. Gut. 1994;35:658-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 169] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
S- Editor Wang J L- Editor Kumar M E- Editor Ma WH