Published online Feb 21, 2006. doi: 10.3748/wjg.v12.i7.1013
Revised: July 2, 2005
Accepted: September 16, 2005
Published online: February 21, 2006
AIM: To study the relationship between Survivin and PTEN expression and lymph node metastasis, depth of invasion and prognosis of gastric cancer patients in China.
METHODS: Specimens of gastric cancer tissue were collected from the Affiliated Hospital of Jianghan University. All the 140 patients had complete examination data. All lymph nodes were found by the fat-clearing method. The interrupted serial 4-micron sections, routine hematoxylin and eosin staining and immunohistochemical methods were used to detect the lymph node metastases. Gastric cancer tissue microarray was performed to test the expression of Survivin and PTEN (17A) in gastric cancer by immunohistochemical method. All data were processed using χ2 test, Fisher’s exact test, Kaplan-Meyer Log-rank method and Cox multivariate analysis (SPSS 12.0 software).
RESULTS: One hundred and eighteen specimens were used in our tissue microarray (utilization rate was 82.4%). A total of 7580 lymph nodes were found. Metastases were found in 90 specimens and 1618 lymph nodes were detected. The positive rate of Survivin and PTEN expression was 52.5% (62/118) and 76.2% (90/118), respectively. A highly positive correlation was found between Survivin and PTEN expression (χ2 = 4.17, P = 0.04). Survivin expression was positively correlated with UICC N stage (χ2=8.69, P=0.03) and histological classification (χ2=4.41, P=0.04) by χ2 tests. PTEN expression was positively correlated with depth of invasion (P=0.02) and histological classification (χ2=5.47, P=0.02). But Survivin and PTEN expressions were not related with prognosis of gastric cancer patients. A significant correlation between lymph node metastasis and prognosis was demonstrated by Cox multivariate analysis (χ2=4.85, P=0.028).
CONCLUSION: Survivin is positively correlated with PTEN expression in gastric cancer and is a molecular marker of lymph node metastasis while PTEN expression is a molecular marker of advanced gastric cancer. UICC N stage is the most important prognostic factor of gastric cancer in China.
- Citation: Deng H, Wu RL, Zhou HY, Huang X, Chen Y, Liu LJ. Significance of Survivin and PTEN expression in full lymph node-examined gastric cancer. World J Gastroenterol 2006; 12(7): 1013-1017
- URL: https://www.wjgnet.com/1007-9327/full/v12/i7/1013.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i7.1013
Gastric carcinoma is one of the commonest malignancies and the first killer among all tumors in China[1]. When the disease is diagnosed, it has been in its advanced stage with lymph node metastasis. Looking for molecular markers to predict the depth of invasion and lymph node metastasis of gastric cancer has important significance.
Repression of apoptosis has been observed in gastric cancer[2]. Survivin, a new member of IAP family, is a powerful apoptosis repression factor. It has been implicated in the control of cell cycle kinetics and cell proliferation[3]. Survivin can be found during embryonic and fetal development but is completely down-regulated and undetectable in normal adult tissues and is prominently expressed in many human malignancies[4]. PTEN/MMAC1/TEP1, a novel tumor suppressor gene, is located on chromosome band 10q 23.3. This gene has three different names: PTEN (phosphatase and tensin homologue deleted on chromosome ten)[5], MMAC1 (mutated in multiple advanced cancer 1)[6] and TEP1 (TGF-β-regulated and epithelial cell-enriched phosphatase 1)[7], and codes for the 403 amino acid protein with tyrosine phosphatase activity. It contains a region of homology to tensin and auxillin, cytoskeletal proteins that interact with adhesion molecules. PTEN encoding products could dephosphorate PIP3 that could inhibit cell growth and increase cell apoptosis. It also could inhibit cell invasion and metastasis by dephosphorating focal adhesion kinase (FAK) and restrain cell differentiation by inhibiting mitogen-activated protein kinase (MAPK) signal pathway[8-10].
It is still controversial about the correlation between Survivin and PTEN expression and depth of invasion and lymph node metastasis as well as the prognosis significance of Survivin and PTEN expression in gastric cancer. In this study, we used tissue microarray and immunohistochemical technologies to study the relationship between Survivin and PTEN expression in gastric cancer and their relation with the depth of invasion, lymph node metastasis of gastric cancer and the prognosis of gastric cancer patients.
Specimens of gastric cancer tissue were collected from the Affiliated Hospital of Jianghan University. All the 140 patients had complete examination data (99 males and 41 females, median age 58 years, ranging 26 - 77 years). No patients received radiotherapy and chemotherapy before surgery. Depth of invasion and lymph node metastasis were staged by the standards of UICC. No patients were diagnosed as having gastric adenocarcinoma which was classified into well-differentiated and poorly-differentiated adenocarcinoma. Size was calculated according to the biggest diameter of tumor.
All the 140 specimens of gastric cancer tissue were taken at the distance of 0.5-1 cm. Routine pathological examination was performed to determine the depth of invasion and histological classification of gastric cancer. All lymph nodes were found by the fat-clearing fat method (>15/case)[11]. The interrupted 4-micron sections, routine hematoxylin and eosin staining and immunohistochemical SP methods (CK18 + EMA, Vector Laboratory Co.) were used to detect the lymph node metastasis.
We took core needle biopsies with a diameter of 0.6 mm from donor paraffin-embedded cancer tissue blocks (2-4 dots per specimen) using a dedicated tissue array instrument (Beecher Instruments, New Jersey, USA). Slides for tissue microarray were read as previously described[12]. Standard dots which could not be analyzed, included dots having no tumor tissue, the area of defection being higher than 50% of dots, the area of tumor being less than 10% of dots, location of dots being unclear.
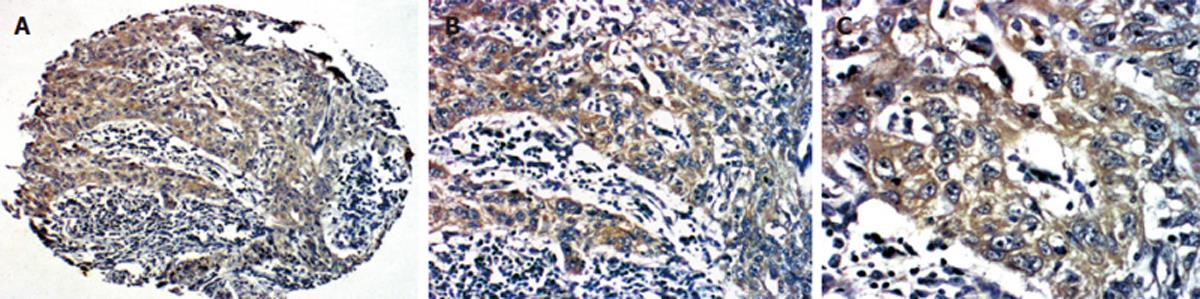
The primary polyclonal antibody to Survivin (RAB-0536, NeoMarkers; ready-to-use), monoclonal antibody to PTEN (clone 17A, NeoMarkers; ready-to-use) and SP kit were purchased from Fujian Maxin Ltd (China). Immunohistochemical staining of Survivin and PTEN was performed. Diaminobenzedin (DAB) was used for color development. The positive result of Survivin and PTEN showed brown color of cytoplasma.
Statistical software package SPSS 12.0 was used. Depth of invasion and lymph node metastasis associated with Survivin expression were analyzed by χ2 test or Fisher’s exact test. Survival analysis was conducted by the Kaplan-Meier method and survival characteristics were compared using the log rank test. The Cox proportional hazard regression model was used to compare the relative influences of different prognostic factors. P < 0.05 was considered statistically significant.
All the 140 specimens were embedded for two tissue microarrays. One included 288 cores, the other included 420 cores. According to the standard dots that could not be analyzed, 118 specimens could be analyzed in our study (Figure 1). The utilization rate of tissue microarray was 84.3%.
All the 118 patients had complete examination data (82 males and 36 females, median age 58 years, ranging 26 - 77 years). The median size of tumor was 5.4 cm (ranging 0.8 - 15 cm). The depth of invasion was T1 in 11, T2 in 18, T3 in 38, and T4 in 51 patients, respectively. A total of 7580 lymph nodes were found in 118 cases (64.2/case, ranging 17 - 157 nodes, median number being 62 nodes) and 1618 lymph node metastases were found in 90 cases (UICC N stage, N0 in 28, N1 in 27, N3 in 29, and N4 in 34 cases, respectively) by the fat-clearing method. Well-differentiated gastric adenocarcinoma was found in 43 cases and poorly-differentiated gastric adenocarcinoma was found in 85 cases.
Among the 118 patients, accumulation of Survivin in cytoplasma was found in 62 (52.5%, 62/118) cases and PTEN was detected in 90 cases (76.3%, 90/118) (Figure 2). There was a positive correlation between expressions of Survivin and PTEN (χ2 = 4.17, P = 0.04) (Figure 3).
The positive rate of Survivin expression in UICC N stage was 65.5% (18/28) for N0, 67.9% (18/27) for N1, 48.3% (14/29) for N2, 36.1% (12/34) for N3, respectively. There was a significant correlation between Survivin and N stages of UICC (χ2 = 8.69, P = 0.03) by χ2 tests. The positive rate of Survivin expression was 67.5% (28/43) in well-differentiated adenocarcinoma and 46.2% (34/75) in poorly-differentiated adenocarcinoma. There was a significant correlation between Survivin expression and histological classification (χ2=4.41, P = 0.04) by χ2 test. No correlation was found between Survivin expression and other pathological features (Table 1).
| Survivin expression | PTEN expression | |||||
| (n=118) | (n=118) | |||||
| + | - | P | + | - | P | |
| Gender | ||||||
| Male | 46 | 36 | 59 | 23 | ||
| Female | 16 | 20 | 31 | 5 | ||
| Age(yr) | ||||||
| <60 | 33 | 31 | 44 | 20 | ||
| ≥60 | 29 | 25 | 46 | 8 | ||
| Size | ||||||
| D<5cm | 26 | 25 | 41 | 10 | ||
| D≥5cm | 36 | 31 | 49 | 18 | ||
| Depth of invasion | 0.02 | |||||
| T1 | 4 | 7 | 5 | 6 | ||
| T2 | 12 | 6 | 14 | 4 | ||
| T3 | 19 | 19 | 34 | 4 | ||
| T4 | 27 | 24 | 37 | 14 | ||
| Histological type | 0.04 | 0.02 | ||||
| Well-differentiated | 28 | 15 | 38 | 5 | ||
| Poorly-differentiated | 34 | 41 | 52 | 23 | ||
| Lymph node metastasis | 0.03 | |||||
| N0 | 18 | 10 | 23 | 5 | ||
| N1 | 18 | 9 | 19 | 8 | ||
| N2 | 14 | 15 | 21 | 8 | ||
| N3 | 12 | 22 | 27 | 7 | ||
The positive rate of PTEN expression was 45.5% (5/11) for T1, 77.8% (14/18) for T2, 89.5% (34/38) for T3, 72.6% (37/51) for T4, respectively. A highly positive correlation was found between depth of invasion and PTEN expression (P = 0.02) by Fisher’s exact test. The positive rate of PTEN expression was 88.4% (38/43) in well-differentiated adenocarcinoma and 69.3% (52/75) in poorly-differentiated adenocarcinoma. There was a significant correlation between PTEN expression and histological classification (χ2 = 5.47, P = 0.02) by χ2 test. No correlation was found between PTEN expression and other pathological features (Table 1).
Univariate analysis showed that depth of invasion (χ2 = 12.25, P < 0.0001) and UICC N stage (χ2 = 20.23, P < 0.0001) were significantly related with survival time. Multivariate analysis of these predictive variables revealed that only the UICC N stage (χ2 = 4.85, P = 0.028) was not related with survival time.
The expression of Survivin and PTEN was not related with survival time. But when the survival time was longer than 7 mo, Survivin positive group had a worse prognosis than Survivin positive group (P = 0.047).
Survivin, a structurally unique member of IAP family, is expressed in mitosis in a cell cycle-dependent fashion and localized in components of the mitotic apparatus[3]. It is potentially involved both in inhibition of apoptosis and in control of cell division[13,14]. Survivin is found in most human cancers but undetectable at a very low level in differentiated adult tissues[4]. In most cancers, expression of Survivin is correlated with reduced apoptotic index, poor prognosis, and increased risk of recurrence[15-18]. The relation between Survivin expression, depth of invasion and lymph node metastasis in gastric cancer is not well known. Miyachi et al[19] found that Survivin expression is related with lymph node metastasis but not with depth of invasion. Tsuburaya et al[20] showed that Survinin mRNA expression is related with depth of invasion but not with lymph node metastasis. The concordance of mRNA and protein is controversial. Our results were based on tissue microarray, immunohistochemistry, complete pathological data and complete lymph node examination data. We found that Survivin expression was higher in well-differentiated gastric adenocarcinoma than in poorly-differentiated gastric adenocarcinoma and was correlated with lymph node metastasis. The prognosis significance of Survivin expression is not well known. Wang et al[21] found that Survivin positive expression in nuclei is correlated survival time, but its positive expression in cytoplasm is not correlated with survival time. We did not find any relation between Survivin expression and survival time. But when the survival time was longer than 7 mo, Survivin positive group had a worse prognosis than PTEN positive group.
PTEN, a tumor suppressor gene, is located on chromosome band 10q 23.3. PTEN encoding products could inhibit cell growth and increase cell apoptosis by dephosphorating PIP3. It also could inhibit cell invasion and metastasis by dephosphorating focal adhesion kinase (FAK) and restrain cell differentiation by inhibiting mitogen-activated protein kinase (MAPK) signal pathway[8-10]. The relation between PTEN expression, depth of invasion and lymph node metastasis is still unknown. Zheng et al[22] found that PTEN expression is related with lymph node metastasis and depth of invasion. Dong et al[22] found that PTEN expression is related with lymph node metastasis but not with depth of invasion. Our results showed that PTEN expression was higher in advanced gastric cancer than in early gastric cancer and was not related with lymph node metastasis. The prognosis significance of PTEN expression in gastric cancer is still unknown. Li et al[23] found that PTEN expression is not related with survival time. We did not find any correlation between PTEN expression and survival time using tissue microarray and PTEN monoclonal antibody (17A)
Survivin and PTEN can regulate cell cycle and apoptosis, but they have adverse biological effects. Dong et al[22] showed that PTEN expression is negatively related with Survivin expression. However, we found a positive relation between Survivin and PTEN expression.
Lymph node metastasis is the most important marker of prognosis of gastric cancer patients[4,14]. Our study has confirmed that lymph node stage of gastric cancer is an independent prognosis marker of gastric cancer.
In conclusion, Survivin expression is positively related with PTEN expression in gastric cancer. They are not related with prognosis of gastric cancer. Survivin and PTEN expression is a molecular marker of advanced gastric cancer but not a molecular marker of lymph node metastasis in gastric cancer. UICC N stage is the most important prognostic factor in gastric caner.
| 1. | Liu T, Wang XY, Song WJ, Zhu CZ, Li Y. Incidence of gastric malignant tumors during the past 20 years in Tianjin [J]. Shijie Huaren Xiaohua Zazhi. 2004;12:20-22. |
| 2. | Lauwers GY, Scott GV, Karpeh MS. Immunohistochemical evaluation of bcl-2 protein expression in gastric adenocarcinomas. Cancer. 1995;75:2209-2213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 3. | Li F, Ambrosini G, Chu EY, Plescia J, Tognin S, Marchisio PC, Altieri DC. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature. 1998;396:580-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1451] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 4. | Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2342] [Cited by in RCA: 2399] [Article Influence: 82.7] [Reference Citation Analysis (1)] |
| 5. | Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3525] [Cited by in RCA: 3610] [Article Influence: 124.5] [Reference Citation Analysis (0)] |
| 6. | Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2004] [Cited by in RCA: 2054] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 7. | Li DM, Sun H. TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res. 1997;57:2124-2129. [PubMed] |
| 8. | Maehama T, Taylor GS, Dixon JE. PTEN and myotubularin: novel phosphoinositide phosphatases. Annu Rev Biochem. 2001;70:247-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 367] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 9. | Besson A, Robbins SM, Yong VW. PTEN/MMAC1/TEP1 in signal transduction and tumorigenesis. Eur J Biochem. 1999;263:605-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Waite KA, Eng C. Protean PTEN: form and function. Am J Hum Genet. 2002;70:829-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 343] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 11. | Liu LJ, Zhang YT. The clinical research of lymph node metastasis in gastric cancer. Zhonghua Shiyan Waike Zazhi. 1995;12:91-92. |
| 12. | Kang JY, Dolled-Filhart M, Ocal IT, Singh B, Lin CY, Dickson RB, Rimm DL, Camp RL. Tissue microarray analysis of hepatocyte growth factor/Met pathway components reveals a role for Met, matriptase, and hepatocyte growth factor activator inhibitor 1 in the progression of node-negative breast cancer. Cancer Res. 2003;63:1101-1105. [PubMed] |
| 13. | Reed JC, Bischoff JR. BIRinging chromosomes through cell division--and survivin' the experience. Cell. 2000;102:545-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 105] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Giodini A, Kallio MJ, Wall NR, Gorbsky GJ, Tognin S, Marchisio PC, Symons M, Altieri DC. Regulation of microtubule stability and mitotic progression by survivin. Cancer Res. 2002;62:2462-2467. [PubMed] |
| 15. | Monzó M, Rosell R, Felip E, Astudillo J, Sánchez JJ, Maestre J, Martín C, Font A, Barnadas A, Abad A. A novel anti-apoptosis gene: Re-expression of survivin messenger RNA as a prognosis marker in non-small-cell lung cancers. J Clin Oncol. 1999;17:2100-2104. [PubMed] |
| 16. | Swana HS, Grossman D, Anthony JN, Weiss RM, Altieri DC. Tumor content of the antiapoptosis molecule survivin and recurrence of bladder cancer. N Engl J Med. 1999;341:452-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 217] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Adida C, Haioun C, Gaulard P, Lepage E, Morel P, Briere J, Dombret H, Reyes F, Diebold J, Gisselbrecht C. Prognostic significance of survivin expression in diffuse large B-cell lymphomas. Blood. 2000;96:1921-1925. [PubMed] |
| 18. | Kato J, Kuwabara Y, Mitani M, Shinoda N, Sato A, Toyama T, Mitsui A, Nishiwaki T, Moriyama S, Kudo J. Expression of survivin in esophageal cancer: correlation with the prognosis and response to chemotherapy. Int J Cancer. 2001;95:92-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 19. | Miyachi K, Fujita M, Tanaka N, Sasaki K, Sunagawa M. Correlation between telomerase activity and telomeric-repeat binding factors in gastric cancer. J Exp Clin Cancer Res. 2002;21:269-275. [PubMed] |
| 20. | Tsuburaya A, Noguchi Y, Yoshikawa T, Saito A, Doi C, Okamoto T, Fukuzawa K. An anti-apoptosis gene, survivin and telomerase expression in gastric cancer. Hepatogastroenterology. 2002;49:1150-1152. [PubMed] |
| 21. | Wang ZN, Xu HM, Jiang L, Zhou X, Lu C, Zhang X. Expression of survivin in primary and metastatic gastric cancer cells obtained by laser capture microdissection. World J Gastroenterol. 2004;10:3094-3098. [PubMed] |
| 22. | Zheng HC, Chen Y, Kuang LG, Yang L, Li JY, Wu DY, Zhang SM, Xin Y. [Expression of PTEN-encoding product in different stages of carcinogenesis and progression of gastric carcinoma]. Zhonghua Zhong Liu Za Zhi. 2003;25:13-16. [PubMed] |
| 23. | Li H, Zhang XF, Wang C, Wu LM, Cai QF. Expression of tumor suppressor gene PTEN in human gastric carcinoma and its clinical significance. Shi Yong Zhong Liu Zazhi. 2002;17:301-303. |
S- Editor Guo SY L- Editor Wang XL E- Editor Liu WF