Published online Dec 28, 2006. doi: 10.3748/wjg.v12.i48.7774
Revised: August 28, 2005
Accepted: November 18, 2005
Published online: December 28, 2006
AIM: To develop hepatitis C virus (HCV) vaccine using HBcAg as the immuno-carrier to express HCV T epitope and to investigate its immunogenicity in mice.
METHODS: We constructed the plasmid pTrc-coreNheI using gene engineering technique, constructed the pcDNA3.1-coreNheI-GFP plasmid with GFP as the reporter gene, and transfected them into Hela cells. The expression of GFP was observed under confocal microscopy and the feasibility of using HBcAg as an immuno-carrier vaccine was studied. pTrc-core gene with a synthetic T epitope antigen gene of HCV (35-44aa) was fused and expressed in the plasmid pTrc-core-HCV (T). For the fusion of the HBcAg-T protein, sucrose, density gradient centrifugation was used, and its molecular weight and purity were analyzed by SDS-PAGE. Then balb/c mice were immunized by the plasmid with the HBcAg (expressed by pTrc-core) protein as control. The tumor regression potential was investigated in mice and evaluated at appropriate time. After three times of immunization, the peripheral blood and spleen of vaccinated mice were collected. HBcAb was detected by ELISA, and nonspecific T lymphocyte proliferation and response of splenocytes were respectively examined by MTT assay. T cell subset of blood and spleen were detected by FACS.
RESULTS: GFP was successfully expressed. Tumor regression trial showed that no tumor formation was found in the group receiving immunization, while tumor xenograft progression was not changed in the control group. Strong nonspecific lymphocyte proliferation response was induced. FACS also showed that the ratio of CD8+ T cells in the experimental group was higher than the controls, but the serum HBcAb in experimental group was similar to the control.
CONCLUSION: HBcAg can be used as an immuno-carrier of vaccine, the fusion of HBcAg-T protein could induce stronger cellular immune responses and it might be a candidate for therapeutic vaccines specific for HCV.
- Citation: Chen JY, Li F. Development of hepatitis C virus vaccine using hepatitis B core antigen as immuno-carrier. World J Gastroenterol 2006; 12(48): 7774-7778
- URL: https://www.wjgnet.com/1007-9327/full/v12/i48/7774.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i48.7774
Hepatitis C virus (HCV) is a global public health problem, infecting 170 million people now, accounting for approximately 3% of the world population[1]. More than 50 million are infected with HCV in China, and the number has a tendency to rise. Persistent HCV infection has a high risk of progressing to chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma, usually more than a decade after initial infection[2]. Thus, it is important to develop adequate treatment for HCV infection. Polyethylene glycol plus Ribavirin treatment is effective, while new infections are continuously emerging from blood transfusion, needle sharing, close contact of HCV infected patients and other unidentified sources[3]. Thus to control the spread of HCV by developing new methods, such as therapeutic vaccines becomes an urgent task, especially in developing countries including China, where there is a large infected population.
In this study, we used HBcAg as immuno-carrier, inserted HCV T epitope into HBcAg el-loop, fused the protein particles of HBcAg-T via density gradient centrifugation, and immunized them in Balb/C mice with suitable dosage, then observed the immunogenicity of this fused protein in order to find a candidate for HCV therapeutic vaccine.
The E. coli strain DH5α, JM109 and Hela cell line were conserved in our laboratory; Vector pTrc-core which carries a HBc gene was a kind gift of Dr. Li Jingli (Changchun, China); and Vector pcDNA-GFP was constructed in our laboratory which carries a GFP gene.
Restriction enzymes including NheI, HindIII and BamHI, T4 DNA ligase, Taq DNA polymerase, CIAP and RNase were purchased from TaKaRa Biotechnology (Dalian, China); Lipofectamine™ 2000 was purchased from Gibco Corporation; and other reagents were analytically pure reagents produced in China.
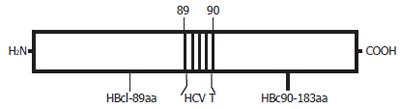
The HBcAg-T recombinant protein (Figure 1) was expressed in E. coli JM109 transfected with the expression of plasmid pTrc-core-HCV(T). This plasmid encodes a HBc gene (aa 1 to 183) with the HCV T epitope inserted into the HBcAg loop region between aa 79 and 80.
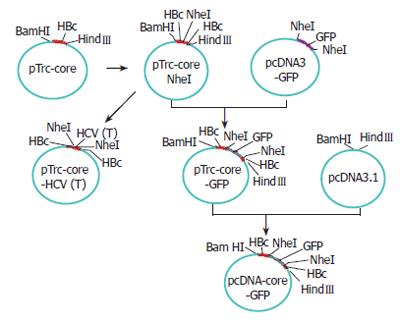
To construct this hybrid vaccine, a plasmid (pTrc-coreNheI) containing HBc gene was first digested at the NheI restriction sites, which had been strategically introduced into plasmid pTrc-core by PCR between the codons for P79 and A80 of the loop region. A synthetic double-stranded DNA fragment CTAGCgccgacctcatggggt acataccgctcgtcG encoding the sequence of HCV T epitope, modified by addition of 5’ NheI and 3’ NheI overhangs, was then inserted to yield plasmid pTrc-core-HCV(T) (Figure 2). This plasmid was used to direct the expression of a particle containing HCV T epitope. The positive clone of recombinant plasmid pTrc-core-HCV (T) was identified by PCR, restriction endonuclease cleavage and sequencing.
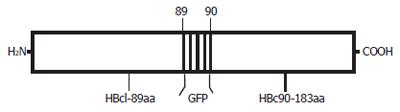
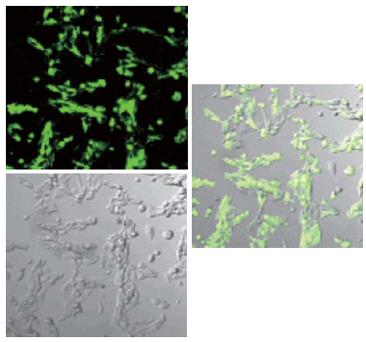
A second plasmid, pcDNA3.1-core-GFP (Figure 2), was used to direct the expression of a particle containing the GFP sequence fused in the 79-80aa loop region of the HBcAg (Figure 3). The plasmid pTrc-coreNheI was first digested with NheI restriction enzyme and dephosphorylated, then, a double stranded DNA fragment encoding the GFP sequence obtained from plasmid pcDNA3-GFP by the NheI restriction enzyme was inserted to produce plasmid pTrc-core -GFP. To yield the final expression vector pcDNA3.1-core-GFP, this plasmid pTrc-core-GFP was cut with BamHI and HindIII and then cloned into the pcDNA3.1 vector, which had been prepared with the same two restriction enzymes, then transfected the plasmid pcDNA3.1-core -GFP into the Hela cell line. Forty-eight hours after transfection, GFP fusion protein expression cells were directly observed under Olympus fluorescence microscope (Japan).
E. coli strain JM109 was transformed with either pTrc-core or pTrc-core-HCV (T) and selected on Luria-Bertani plates containing ampicillin (100 μg/mL). After 16-24 h of incubation at 37°C, a single colony was picked, expanded overnight, and used to inoculate a 500 mL culture (tryptone-yeast extract -NaCl [TYN] medium supplemented with 1 g glucose/L and 50 μg/mL ampicillin). After 16-20 h, cells were harvested by centrifugation. The pellets were resuspended in 5 mL of 25% sucrose in 50 mmol/L Tris pH8.0, added with 150 μL freshly prepared lysozyme (50 g/L), 100 μL of RNAse (10 g/L) and 100 μL of DNAse (10 g/L), mixed and incubated in a 37°C water bath for 30 min. Samples were placed on ice for 5 min, added with 5 mL of lysis solution (10% Triton X-100, 0.4% sodium deoxycholate, 50 mmol/L Tris pH8.0 and 62.5 mmol/L EDTA) and incubated with occasional shaking at 37°C for 30 min. The solution was incubated for a further 30 min at 37°C and centrifuged at 4000 r/min for 15 min in a benchtop centrifuge. Supernatant was removed to a new tube. Lysate was vortexed at high speed for 30 s, and added with 2 mL of a 5 mol/L urea stock to create a 1 mol/L concentration. Three mL of this lysate was loaded onto the top of a sucrose gradient (60%, 50%, 40%, 30%, 20%) followed by density gradient centrifugation. After centrifugation at 4°C, 32 000 r/min for 22 h, samples (450 mL/tube) were collected to detect the ELISA response intensity using HBeAg diagnosis kit. Molecular weight and purity were identified by sodium dodecyl sulfate -polyacrylamide gel electrophoresis (SDS-PAGE), and ultraviolet spectrophotometer to measure its concentration. The purified proteins were stored at -20°C prior to use.
BALB/c mice were obtained from Jilin University. Mice were immunized subcutaneouly with 2 μg HBcAg-T or HBcAg in Freund’s incomplete adjuvant on d 0, 14, and 28. Fourteen days after the immunizing, half of the mice from each team were simultaneously inoculated with 200 μL (1 × 109/L) H22 cell, 10 d after the last protein immunizing, the mice were sacrificed to get the tumor mass. Serum samples were collected 30 d after immunizing. Five days after the booster inoculation, mice were sacrificed and spleen were removed for T-cell analysis. Untreated mice served as controls.
Anti-HBcAg antibody (HBcAb) was measured by ELISA using commercial kit (Rong Sheng, Shanghai, China), the endpoint titer was determined as the dilution of immune sera giving an optical density greater than the mean + 2 standard deviations obtained with normal sera.
The CD3+, CD4+ and CD8+ T lymphocyte of blood and spleen were assayed by flow cytometry (Becton Dickson Company, USA).
Triplicate wells containing spleen cells of naïve or immunized BALB/c mice were stimulated with HBcAg-T or HBcAg particles (2 g/L). Proliferation in 2-d cultures was measured by MTT to evaluate the cellular immune responses of the vaccinated animals, and results were expressed as stimulation indices (SI).
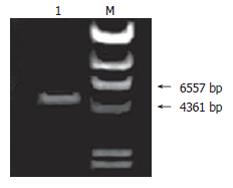
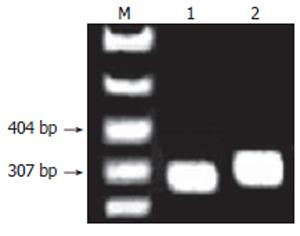
On agarose gel electrophoresis, the product of pTrc-coreNheI digested by NheI formed a band of expected size of 5223 bp (Figure 4), The sequencing of plasmid pTrc-coreNheI PCR product was in agreement with expected (Figure 5). These proved that the NheI site was added successfully into the plasmid pTrc-core.
Under confocal microscopy, GFP was expressed successfully after plasmid pcDNA3.1-HBc-GFP was transfected into Hela cells (Figure 6). This suggested that GFP fragment was well exposed on the surface of chimeric VLPs, and the foreign gene inserted could be expressed correctly.
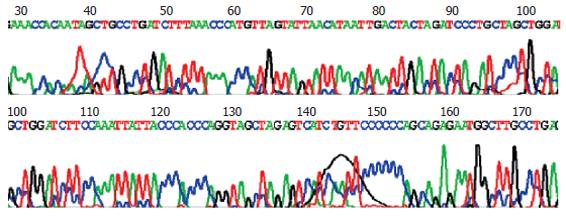
A 336 bp specific band was obtained by PCR amplification with pTrc-core-HCV (T) as template (Figure 7), and DNA sequencing confirmed that the sequence was completely concordant with what had been expected. These showed that the construction of plasmid pTrc-core-HCV(T) was successful. Sequencing result: (HCV T epitope underlined, NheI site italic) ctagacaccgcctcagctctgtat
cgagaagccttagagtctcctgag
cattgctcacctcaccatactgca
ctcaggcaagccattctctgctgg
ggggaattgatgacctagctacct
gggtgggtaataatttggaagatc
cagctagcgccgacctcatggggt
acataccgctcgtcgctagcaggg
atctagtagtcaattatgttaata
ctaacatgggtttaaagacaggca
actattgtggtttcatatatcttg
ccttacttttggaagagagactgt
acttgaatatttggtctctttcgg
agtgtggattcgcactcctcca.
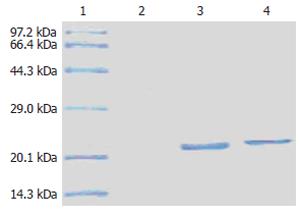
Protein HBcAg-T and HBcAg were examined by SDS-PAGE. On the gel, a single protein band was displayed at about 2.1 kDa (Figure 8). It was consistent with the molecular weight of the two proteins. It suggested that the HBcAg-T and HBcAg was pure, and we could use them directly to immunize the animals.
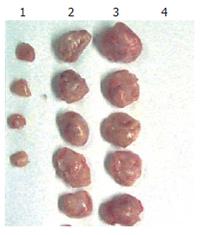
Four mice in HBcAg immunized group formed tumor masses (Figure 9), while none in the mice in HBcAg-T immunized group, the difference being obvious between the blank and HBcAg immunized groups (P < 0.05).
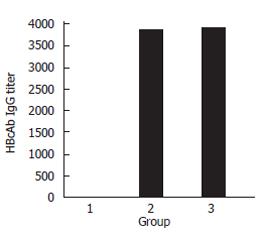
ELISA showed that the difference of HBcAb titer between the control and HBcAg-T immunize groups was not significant (1:3880 vs 1:3900). This proved that T epitope inserted could not enhance the humoral immunity of mice obviously (Figure 10).
FACS showed that the ratio of CD8+ T cell in blood and spleen of HBcAg or HBcAg-T immunized mice was higher than the blank, and the ratio of HBcAg-T immunized group was the highest, showing that HBcAg-T could accelerate the proliferation of CTL, and induce stronger cellular immunity (Table 1).
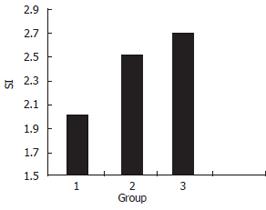
MTT showed that (Figure 11) HCV T epitope can accelerate unspecific T cell proliferative reaction (P < 0.05).
The HCV is a highly variable virus, various attempts have been made to develop a vaccine against HCV infection, such as protein vaccine[4-6], oral vaccine[7] and the new RNAi technique[8]. Although some encouraging results were obtained, no effort on vaccine development has been successful. Recent studies about HCV epitopes showed that epitope vaccines might be a feasible strategy for HCV vaccine designs. Using the epitopes but not the whole HCV protein as the antigen could avoid HCV antigen protein to induce cancer, and its immunosuppressive action at the same time could refrain the patents from promoting HCV immune escape strain formation or aggravating infection degree induced by immune response for unsuitable sites. In this study, we chose a highly conservative T epitope (HLA-A2 type) of HCV core region (35-44aa)[9] to design HCV therapeutic vaccine.
Only one epitope can not induce strong immune response because the immunogenicity is weak, we therefore used HBcAg as the immuno-carrier to develop HCV vaccines. Being a carrier of foreign epitopes, HBcAg has more advantages than other proposed particulate carriers. There are more powerful immunogenic epitopes[10], such as: Th epitopes which lie in 1-20aa, 28-47aa, 50-69aa, 81-105aa, 126-146aa and 141-165aa; HLA-A2,A31,A11 restricted CTL epitopes which lie in 18-27aa, 141-151aa and 88-96aa; and so on. So HBcAg carrier can provide a high level of T cell immunogenicity for the inserted HCV T epitope, its immune response may play an important role in HCV therapy. HBcAg can show exogenous epitopes with multicopy pattern, keep their original conformation, raise the epitope avidity, offer Th signal to exogenous epitopes and strikingly enhance immune responses. Experiments proved that HBC possesses Ti antigen property[11], which can induce immune response without adjuvant. Since Clarke[12] first reported on the virus-like particle (VLP) of HBcAg fusion in 1987, and constructed the first VLPs[13], HBcAg was often used as an immuno-carrier to develop immunogens and vaccines and had been used in many pathogens such as Hantaan virus, malarial parasite and HIV[14-16]. Now some investigators mentioned the features of HBcAg VLPs[17,18], and pointed out that the region between position 78 and 83 of HBcAg (maily immunodominant region, MIR) is surface accessible[19]. It was shown that HBcAg e1loop (78-83aa) was not necessary for assembling, without it HBcAg could auto-assemble into VLPs in vitro itself. In this study, we also showed that after GFP was introduced into this position, the assembly of HBcAg is unaffected and the inserted GFP could be exposed on the surface of VLPs. Thus, the position is an ideal site for HCV epitope inserting[20], and the foreign gene inserted here could be expressed correctly. Based on these findings, we inserted HCV T epitope into HBc e1loop, constructed fusion expression of plasmid pTrc-core-HCV (T), its expression product HBcAg-T was extracted, purified and quantitated, then immunized into Balb/c mice. We investigated its immunogenicity by some tests. Tumor regression trial showed that no tumor from the experimental group was found, being different from the control group. It means that the fusion antigens could stimulate mice to produce high-level CTL, which inhibits the tumor cells to survive; HBcAb was almost similar in the serum of the mice from HBcAg-T or HBcAg injected group, stronger nonspecific lymphocytes proliferation response was induced in experimental group; FACS also showed the ratio of CD8+ T cell in the experimental group was higher than the control. All these findings demonstrated that HBcAg-T had induced stronger cellular immune response.
Since cytotoxic T lymphocytes (CTL) play a critical role in preventing the spread of HCV, and it has been suggested that cell-mediated immune responses played an important role in protection against HCV chronic infection[21], so vaccine-based HCV CTL induction could be a promising strategy to treat HCV-infected patients. In this study, the fused protein has been found to have the capacity to induce CTL in mice, so the success of this experiment undoubtedly could serve as a basis for developing HCV therapeutic vaccine.
| 1. | Xu K, Deng XY, Yue Y, Guo ZM, Huang B, Hong X, Xiao D, Chen XG. Generation of the regulatory protein rtTA transgenic mice. World J Gastroenterol. 2005;11:2885-2891. [PubMed] |
| 2. | El-Serag HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology. 2002;36:S74-S83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 184] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 3. | Zhu LX, Liu J, Ye Y, Xie YH, Kong YY, Li GD, Wang Y. A candidate DNA vaccine elicits HCV specific humoral and cellular immune responses. World J Gastroenterol. 2004;10:2488-2492. [PubMed] |
| 4. | Heile JM, Fong YL, Rosa D, Berger K, Saletti G, Campagnoli S, Bensi G, Capo S, Coates S, Crawford K. Evaluation of hepatitis C virus glycoprotein E2 for vaccine design: an endoplasmic reticulum-retained recombinant protein is superior to secreted recombinant protein and DNA-based vaccine candidates. J Virol. 2000;74:6885-6892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Inchauspé G, Feinstone S. Development of a hepatitis C virus vaccine. Clin Liver Dis. 2003;7:243-59, xi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Nevens F, Roskams T, Van Vlierberghe H, Horsmans Y, Sprengers D, Elewaut A, Desmet V, Leroux-Roels G, Quinaux E, Depla E. A pilot study of therapeutic vaccination with envelope protein E1 in 35 patients with chronic hepatitis C. Hepatology. 2003;38:1289-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 87] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Schödel F, Peterson D, Milich D. Hepatitis B virus core and e antigen: immune recognition and use as a vaccine carrier moiety. Intervirology. 1996;39:104-110. [PubMed] |
| 8. | Randall G, Grakoui A, Rice CM. Clearance of replicating hepatitis C virus replicon RNAs in cell culture by small interfering RNAs. Proc Natl Acad Sci USA. 2003;100:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 255] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 9. | Battegay M, Fikes J, Di Bisceglie AM, Wentworth PA, Sette A, Celis E, Ching WM, Grakoui A, Rice CM, Kurokohchi K. Patients with chronic hepatitis C have circulating cytotoxic T cells which recognize hepatitis C virus-encoded peptides binding to HLA-A2.1 molecules. J Virol. 1995;69:2462-2470. [PubMed] |
| 10. | Pumpens P, Grens E. HBV core particles as a carrier for B cell/T cell epitopes. Intervirology. 2001;44:98-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 217] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Fehr T, Skrastina D, Pumpens P, Zinkernagel RM. T cell-independent type I antibody response against B cell epitopes expressed repetitively on recombinant virus particles. Proc Natl Acad Sci USA. 1998;95:9477-9481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 137] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Clarke BE, Brown AL, Grace KG, Hastings GZ, Brown F, Rowlands DJ, Francis MJ. Presentation and immunogenicity of viral epitopes on the surface of hybrid hepatitis B virus core particles produced in bacteria. J Gen Virol. 1990;71:1109-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Clarke BE, Newton SE, Carroll AR, Francis MJ, Appleyard G, Syred AD, Highfield PE, Rowlands DJ, Brown F. Improved immunogenicity of a peptide epitope after fusion to hepatitis B core protein. Nature. 1987;330:381-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 247] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Milich DR, Hughes J, Jones J, Sällberg M, Phillips TR. Conversion of poorly immunogenic malaria repeat sequences into a highly immunogenic vaccine candidate. Vaccine. 2001;20:771-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Chen X, Li M, Le X, Ma W, Zhou B. Recombinant hepatitis B core antigen carrying preS1 epitopes induce immune response against chronic HBV infection. Vaccine. 2004;22:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Takeda S, Shiosaki K, Kaneda Y, Nakasatomi T, Yoshizaki H, Someya K, Konno Y, Eda Y, Kino Y, Yamamoto N. Hemagglutinating virus of Japan protein is efficient for induction of CD4+ T-cell response by a hepatitis B core particle-based HIV vaccine. Clin Immunol. 2004;112:92-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Pumpens P, Grens E. Hepatitis B core particles as a universal display model: a structure-function basis for development. FEBS Lett. 1999;442:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 92] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Zlotnick A, Cheng N, Conway JF, Booy FP, Steven AC, Stahl SJ, Wingfield PT. Dimorphism of hepatitis B virus capsids is strongly influenced by the C-terminus of the capsid protein. Biochemistry. 1996;35:7412-7421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 235] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Conway JF, Cheng N, Zlotnick A, Stahl SJ, Wingfield PT, Belnap DM, Kanngiesser U, Noah M, Steven AC. Hepatitis B virus capsid: localization of the putative immunodominant loop (residues 78 to 83) on the capsid surface, and implications for the distinction between c and e-antigens. J Mol Biol. 1998;279:1111-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Pumpens P, Razanskas R, Pushko P, Renhof R, Gusars I, Skrastina D, Ose V, Borisova G, Sominskaya I, Petrovskis I. Evaluation of HBs, HBc, and frCP virus-like particles for expression of human papillomavirus 16 E7 oncoprotein epitopes. Intervirology. 2002;45:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Youn JW, Park SH, Cho JH, Sung YC. Optimal induction of T-cell responses against hepatitis C virus E2 by antigen engineering in DNA immunization. J Virol. 2003;77:11596-11602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
S- Editor Wang J L- Editor Ma JY E- Editor Liu WF