INTRODUCTION
G-protein-coupled receptor kinases (GRKs) are key modulators of G-protein-coupled receptor (GPCR) signaling. They constitute a family of seven mammalian serine-threonine protein kinases that phosphorylate agonist-bound receptor. GRKs-mediated receptor phosphorylation rapidly initiates profound impairment of receptor signaling and desensitization. Activity of GRKs and subcellular targeting is tightly regulated by interaction with receptor domains, G protein subunits, lipids, anchoring proteins and calcium-sensitive proteins. Moreover, GRK phosphorylation by several other kinases and autophosphorylation have recently been shown to modulate its functionality. This review summarizes our current knowledge of GRKs-regulatory mechanisms and their physiological function.
GRKs STRUCTURE AND DISTRIBUTION
GRKs comprise a family of seven mammalian serine/threonine protein kinases that phosphorylate and regulate agonist-occupied or constitutively active GPCR[1].There are three sub-groups within the GRK family. GRK1 (rhodopsin kinase) and GRK7 (cone opsin kinase) form one distinct sub-group that is only found in retinal cells. The non-visual GRKs divide into two sub-groups: the GRK2 subfamily, consisting of GRK2 (b-ARK1) and GRK3 (b-ARK2), and the GRK4 subfamily, consisting of GRK4, GRK5 and GRK6. GRK4 is predominantly found in the testes [2], to lesser extent, in some brain regions and the kidney[3,4], whereas GRK2, 3, 5 and 6 are widely expressed. In addition, the different GRKs are highly specific in their receptor preference[5,6].
The basic structure of non-visual GRK family members is similar, with a highly conserved central (263-266 amino acids) catalytic domain. The N-terminal 185-amino acid region displays considerable homology between individual GRKs. The similarity of the N-termini of GRKs has led to speculation that this region might be important in receptor recognition. All non-visual GRKs have a regulator of G-protein signaling (RGS) domain within the N-terminus region, which provides a potential mechanism by which GRKs might regulate GPCR signal transduction via phosphorylation-independent mechanisms. Indeed, growing evidence suggests that this could be the case for GRK2 and GRK3[7-9]. GRK4-6 possess a highly conserved binding site (amino acids 22-29 for GRK5) for phosphatidylinositol (4, 5)-bisphosphate [PtdIns (4, 5) P2], which is thought to enhance catalytic activity[8].The C-terminal of GRK2 and GRK3 is longer than that of the GRK4 subfamily, and contains a 125-amino acid pleckstrin homology (PH) domain. This domain glycine-rich β-globulin (Gbg) plays a role in targeting and translocation of these primarily cytosolic GRKs to membranes following GPCR activation[8]. More recently, a second binding site for Gbg-subunits has been identified within the first 53 amino acids of GRK2[9], which suggests that either the N- or the C-terminal regions might be sufficient to allow GRK2 targeting to the membrane. GRK4 and GRK6 are post-translationally palmitoylated at one or more cysteine residues clustered within the last 15-20 amino acids of the C-terminus, leading to an exclusive membrane-associated localization[8]. GRK5 is also predominantly membrane-associated, and in this case localization is not achieved through lipidation but instead through the PtdIns (4, 5) P2 binding domain of the N-terminus and a polybasic region (amino acids 547-560) close to the C-terminus[11]. Further heterogeneity is possible within the GRK4 subfamily because both GRK4 and 6 are expressed in multiple splice variant forms[10]. Indeed, one splice variant of GRK4 lacks the N-terminal PtdIns (4, 5) P2 binding region, although the physiological significance of isoformic variation is not understood at present. GRK1 and 7 share many structural similarities with the non-visual GRKs, including an N-terminal RGS-like domain and central catalytic domain. Both GRK1 and 7 are membrane-associated; however, unlike GRK4 and 6, this association is via post-translational farnesylation at the C-terminal.
G-PROTEIN-COUPLED RECEPTOR ENDOCYTOSIS: DESENSITIZATION AND SIGNALING
GPCRs represent the largest family of transmembrane signaling molecules in the human genome. As such, they interact with numerous intracellular molecules, which can act either to propagate or curtail signaling from the receptor. Their primary mode of cellular activation occurs through heterotrimeric G proteins, which in turn can activate a wide spectrum of effector molecules, including phosphodiesterases, phospholipases, adenylyl cyclases and ion channels. In the immune system, triggering of GPCR is important for multiple activities, including cellular differentiation/activation, development of lymphoid tissue, and especially, for control of leukocyte chemotaxis. Active GPCRs are also the target of G-protein-coupled receptor kinases, which phosphorylate the receptors culminating in the binding of the protein arrestin. This results in rapid desensitization through inhibition of G protein binding, as well as novel mechanisms of cellular activation that involve the scaffolding of cellular kinases to GPCR-arrestin complexes. Arrestins can also serve to mediate the internalization of certain GPCR, a process which plays an important role in regulating cellular activity both by mediating long-term desensitization through down-regulation (degradation) of receptors and by recycling desensitized receptors back to the cell surface to initiate additional rounds of signaling. The mechanisms that regulate the subsequent intracellular trafficking of GPCR following internalization are largely unknown. Recently, however, it has become clear that the pattern of receptor phosphorylation and subsequent binding of arrestin play a critical role in the intracellular trafficking of internalized receptors, thereby dictating the ultimate fate of the receptor. In addition, arrestins have now been shown to be GPCRs that are capable of internalizing through arrestin-independent mechanisms[11].
GPCR responsiveness is determined by a tightly regulated balance among receptor signaling, desensitization, and resensitization. Receptor desensitization, the waning of GPCR responsiveness to the agonist with time, is an important, physiological “feedback” mechanism that protects against acute and chronic receptor over-stimulation[6]. The protein families of GRKs and arrestins play a pivotal role in the process of desensitization of agonist-activated GPCR[15-17]. There are seven known GRK subtypes, of which four members are expressed ubiquitously (GRK2, 3, 5 and 6)[15,18]. In the arrestin family, two members are restricted to photoreceptors, whereas β-arrestin1 and β-arrestin2 are expressed ubiquitously[15]. Agonist-induced desensitization of GPCR occurs via a multistep process. First, GRKs phosphorylate the intracellular loops and/or carboxyl terminal tail of the receptor, a process that enhances the affinity of the receptor for binding of cytosolic arrestin proteins. Subsequent binding of phosphorylated receptors by arrestins sterically inhibits interaction of the receptor with the G protein. Thus, agonist-induced phosphorylation of GPCR by a GRK, followed by binding of arrestins, efficiently prevents further coupling of the receptor to its G protein, thereby reducing or preventing receptor signaling[12]. Finally, the GRK-arrestin system promotes clathrin-mediated internalization of inactivated receptors to endosomal compartments for subsequent degradation or resensitization[15-17,19].
It is notable that besides its role in desensitization, β-arrestin-mediated receptor internalization can also regulate signal transduction. The internalized GPCR-β-arrestin complex can form a signalosome that activates signaling proteins, such as ERK1/2, p38 MAPK, and JNK. In addition, arrestins act as scaffolds that connect activated GPCR with tyrosine kinase c-Src and the PI-3K-AKT and NF-κB pathways[13,14].
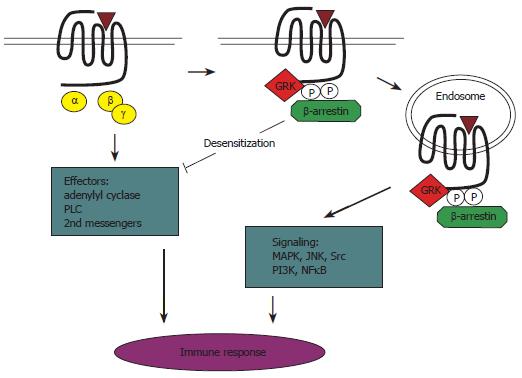
GRKs display activities well beyond their classical role in receptor phosphorylation as well. For example, GRKs have been shown to interact with PI-3Ks and a guanosinetriphosphatase (GTPase)-activating proteins, GIT1, which are involved in regulating receptor trafficking and signaling[15,16]. In addition, GRK2 interacts with a component of the MAPK pathway, as well as with the PI-3K substrate AKT[17,18]. Furthermore, GRK2 and 3 are well-known to bind the Gβγ subunit complex, a process that induces activation of these GRKs. Direct interaction of GRKs with G proteins is suggested by the presence of regulator of RGS-like domains (RH domains) in GRKs[7,22-24]. RGS proteins act as GTPase-activating proteins (GAPs), which induce hydrolysis of guanosine 5'-triphosphate (GTP) and thereby inactivation of GTP-bound Gβγ subunits[19,20]. Selective binding of activated Gαq (and Gα-11) to RH domains of GRK2 and GRK3 (but not to RH domains of GRK1 and 4) was found to selectively inhibit Gq signaling. However, as GRK2/3 were shown not to act as GAPs for Gq[21,27-29], the main role of RH domains in GRK2/3 appears to prevent activated Gq from interacting with downstream effector molecules (Figure 1).
Figure 1 Schematic summary of the role of GRKs/arrestins in activation, signaling, and desensitization of GPCRs in the immune system.
Agonist-activated GPCRs are phosphorylated rapidly by GRKs, leading to recruitment of arrestins. This process, called homologous desensitization, prevents further coupling of the receptor to its G protein, thereby reducing or preventing receptor signaling. In addition, GRKs and arrestins can also act as signal transducers in various signaling pathways. PLC: Phospholipase C.
GRKs and arrestins also interact with non-GPCR. For instance, GRKs and arrestins interact with transforming growth factor -β (TGF-β), epidermal growth factor (EGF), and insulin growth factor receptors[22-28]. In addition, β-arrestin was found to regulate activity of Notch, an important protein in neurogenesis, angiogenesis, and lymphoid development[23]. GRKs and arrestins may directly affect functioning of these non-GPCR or modulate signaling of these receptors indirectly. Transactivation of growth factor receptors, such as the EGF receptor by GPCR, the β2- adrenergic receptor, CXCR4 chemokine receptor, or PGE2 receptor, has been described extensively[24-30]. Hence, GRK/arrestin-mediated regulation of GPCR signaling may indirectly affect signaling of such growth factor receptors. Interestingly, a recent study shows that formation of a PGE/β-arrestin-1/c-Src signaling complex in colorectal carcinoma cells is a crucial step in PGE2-mediated transactivation of the EGF receptor, indicating that arrestins also directly regulate the transactivation of a growth factor receptor by a GPCR agonist[39].
G-PROTEIN-COUPLED RECEPTOR INTERNALIZATION
An important aspect of GPCR activity and regulation is the internalization or sequestration of agonist-activated receptors into the intracellular membrane compartments of the cell. GPCR internalization has become the subject of intensive investigation over the past several years[25-34]. Consequently, a large volume of data has accumulated regarding the mechanisms regulating the endocytosis of a wide variety of different GPCRs. These studies have revealed GPCR domains involved in receptor endocytosis, some of the molecular intermediates that regulate GPCR endocytosis, and the potential for GPCR to internalize by multiple endocytic mechanisms. In addition, although the molecular mechanism involved in the initiation of GPCR endocytosis are best characterized for theβ2AR, recent studies using other GPCRs have revealed an important diversity in the patterns of GPCR endocytosis and intracellular trafficking.
The concept that GPCR are lost from the cell surface following agonist activation originated from the obser-vation that β-adrenergic agonist treatment resulted in a loss of β-adrenergic receptor recognition sites on the surface of frog erythrocytes[26]. Subsequently, cell surface versus internalized β2AR binding sites were discriminated from one another either by differential sedimentation on a sucrose gradient or by using hydrophobic and hydrophilicβ-adrenergic ligands[26-27]. Internalized receptors were found in a “light vesicular” fraction, whereas cell surface receptors were found in a “heavy vesicular” plasma membrane fraction[26]. Similarly, internalized β2AR was accessible to hydrophobic, but not hydrophilic, adrenergic ligands[27]. More recently, the subcellular redistribution of cell surface β2AR in response to agonist activation was demonstrated by immunocytochemical staining of epitope-tagged receptors[28], as well as in real time microscopy in living cells using a green fluorescent protein (GFP)-taggedβ2AR[29]. Similar experiments have now been performed for several GPCR[30-41]. The rate at which GPCR internalize seems to be receptor-specific. For example, the A1 adenosine receptor internalizes quite slowly (t1/2 = 90 min) when compared with the A3 adenosine receptor (t1/2 = 19 min)[31]. These kinetic differences suggest that GPCR internalization can be mediated by multiple endocytic mechanisms and/or that structural heterogeneity between receptor subtypes modulates their relative affinities to bind endocytic adaptor.
G-PROTEIN-COUPLED RECEPTOR KINASES AND DISEASES
GPCRs form the largest family of cell surface receptors, and the defects in GRK function have the potential consequence to affect GPCR-stimulated biological responses in many pathological situations. Furthermore, the regulation of GRK levels in opiate addiction, cancers, psychiatric diseases, cystic fibrosis and cardiac diseases is discussed. Both transgenic mice and human pathologies have demonstrated the importance of GRKs in the signaling pathways of rhodopsin, β-adrenergic and dopamine-1 receptors. The modulation of GRK activity in animal models of cardiac diseases can be effective to restore cardiac function in heart failure and opens a novel therapeutic strategy in diseases with GPCR dysregulation[32].
In human heart failure, impaired βAR signaling compromises cardiac sensitivity to inotropic stimulation[33]. The loss of receptor signaling is associated with an approximate three-fold elevation in myocardial βARK1 expression and GRK activity[34,35]. Myocardial ischemia and hypertension have also been associated with increased expression and activity of βARK1[36,37]. These aspects of human heart disease are similarly evident in animal models, where βARK1 levels are increased in cardiac hypertrophy[38] ischemia[43] and heart failure [39-43]. Given the variety of pathological insults represented in the animal models, βARK1 up-regulation appears to be an early common event in the pathogenesis of heart failure. In fact, βARK1 elevation often precedes the development of clinical heart failure and may represent a novel early marker for cardiac dysfunction. Like βARK1, GRK5 expression and activity are elevated in animal models[40-41], although its role in human heart failure remains unclear. In contrast, GRK3 expression is not increased in human heart failure[42]. At present it seems that for cardiovascular diseases, βAR polymorphisms do not play a role as disease-causing genes; however, they might be risk factors, might modify disease, and/or might influence progression of the disease. Furthermore, βAR polymorphisms might influence drug responses. Thus, evidence has accumulated that a βAR polymorphism (the Arg389GlyβAR) may affect the response to βAR-blocker treatment[42].
GRKs are implicated in the pathophysiology of human diseases, such as arterial hypertension, heart failure and rheumatoid arthritis. While GRK2 and 5 have been shown to be involved in the desensitization of the rat thyrotropin receptor (TSHR), their role in the pathophysiology of hyperfunctioning thyroid nodules (HTNs) is unknown. Therefore, scholars analyzed the expression pattern of the known GRKs in human thyroid tissue and investigated their function in the pathology of HTNs. The expression of different GRKs in human thyroid and HTNs was measured by Western blotting. The influence of GRK expression on TSHR function was analyzed by co-expression experiments in HEK 293 cells. Studies demonstrated that in addition to GRK2, 5 and 6, GRK 3 and 4 were also expressed in the human thyroid. GRK2, 3, 5 and 6 were able to desensitize TSHR in vitro. This GRK-induced desensitization is amplified by the additional over-expression of β-arrestin 1 or 2. No any mutation was found in the GRK2, 3 and 5 from 14 HTNs without TSHR mutations and Gsalpha mutations. The expression of GRK3 and 4 was increased in HTNs independently from the existence of TSHR mutations or Gsalpha mutations. In conclusion, the increased expression of GRK3 in HTNs and the ability of GRK3 to desensitize the TSHR in vitro, suggest a potential role for GRK3 as a negative feedback regulator for the constitutively activated cAMP pathway in HTNs[43].
CONCLUDING REMARKS
Much new information regarding the phosphorylation and regulation of GPCR by GRK2 and GRK3 and their role in GPCR signaling has been revealed during the past few years. More recent studies have started to indicate roles for GRK4, GRK5 and GRK6, both in transfected cell lines and in primary cells. However, it remains to be established whether the multiplicity of GRKs is related to the specificity or differential regulation of GPCR signaling or indeed other, yet to be defined, function. The association of particular GRKs within receptor signaling, trafficking and switching is a key area of current and future investigation
S- Editor Liu Y L- Editor Kumar M E- Editor Liu WF