Published online Dec 21, 2006. doi: 10.3748/wjg.v12.i47.7695
Revised: August 15, 2006
Accepted: August 22, 2006
Published online: December 21, 2006
AIM: To analyze expression of ATP7B in gastric cardiac adenocarcinomas, its clinicopathologic significance, in comparison with distal gastric adenocarcinomas.
METHODS: Immunohistochemical avidin-biotin peroxidase complex method was applied to detect the expression of ATP7B in 49 cases of cardiac carcinomas, the corresponding adjacent non-neoplastic epithelium and 55 cases of distal gastric carcinomas.
RESULTS: The proportion of ATP7B positive samples in gastric cardiac carcinomas (51.0%, 25 of 49) was significantly higher than that in the corresponding adjacent non-neoplastic epithelium (22.4%, 11 of 49) (P = 0.003). ATP7B expression in poorly differentiated gastric cardiac carcinomas was significantly higher than that in well/moderately differentiated gastric cardiac carcinomas (P = 0.030). ATP7B expression in gastric cardiac carcinomas was independent of age, tumor size, nodal stage and metastasis status. ATP7B protein was detected in 30.9% (17/55 cases) of distal gastric carcinomas, markedly lower than that in gastric cardiac carcinomas (P = 0.037).
CONCLUSION: ATP7B protein is frequently overexpressed in gastric cardiac carcinomas, and correlated with the differentiation of cardiac carcinoma. ATP7B expression in gastric cardiac carcinomas is significantly higher than that in distal gastric carcinomas, which might partially explain the difference of chemotherapy response and prognosis between these two gastric carcinomas.
- Citation: Wu DL, Yi HX, Sui FY, Jiang XH, Jiang XM, Zhao YY. Expression of ATP7B in human gastric cardiac carcinomas in comparison with distal gastric carcinomas. World J Gastroenterol 2006; 12(47): 7695-7698
- URL: https://www.wjgnet.com/1007-9327/full/v12/i47/7695.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i47.7695
Gastric carcinoma can be subdivided into distal gastric carcinoma and proximal cardiac carcinoma[1]. Recent studies have suggested that distal gastric carcinoma and proximal cardiac carcinoma have distinct epidemiological, biological and clinical characteristics[1]. For instance, the incidence of carcinoma of the gastric cardia was reported to have increased in most countries during the past few decades, while that of carcinoma of the distal stomach has decreased steadily[2-5]. Carcinoma of the cardia has been reported to form a specific category distinct from carcinoma of the rest of the stomach[6,7].
Drug resistance is a major obstacle of cancer chemotherapy[8,9]. The presence of drug resistance may be prior to exposure to chemotherapy (primary resistance), or may be induced after exposure to chemotherapy (secondary resistance)[8,9]. Platinum containing compounds, such as cisplatin, carboplatin, oxaliplatin, satraplatin, are among the most effective chemotherapeutic agents for cancer. However, their clinical efficacy is often limited by primary or secondary resistance[10]. Several mechanisms have been implicated in cisplatin resistance, including reduced drug accumulation, increased cellular thiol/folate levels and increased DNA repair[10,11]. Among them, reduced intracellular accumulation of cisplatin is a determinant of cisplatin resistance[11]. However, it was not until recently that the mechanism of decrease of cisplatin accumulation in cisplatin-resistant tumor cells was elucidated[11,12]. In 2000, Akiyama and co-workers demonstrated that a copper-transporting P-type adenosine triphosphatase (ATP7B) was associated with cisplatin resistance in vitro[12]. The ATP7B gene was induced by exposure to cisplatin in human prostate cells and ATP7B-transfected cells showed a dramatic decrease in cisplatin accumulation[12]. The above study was supported by subsequent reports[13,14]. ATP7B protein is a member of a class of heavy metal-transporting P-type ATPases that pump copper, cadmium, zinc, silver or lead[15]. ATP7B has been found to be expressed in certain breast carcinomas[16], endometrial carcinomas[17], esophageal carcinomas[18], gastric carcinomas[19], oral squamous cell carcinomas[20] and ovarian carcinomas[21]. Moreover, ATP7B expression status in some carcinomas was correlated with prognosis[17,21] and sensitivity of chemotherapy[18,20].
The aim of this study was to investigate the expression of ATP7B in human gastric cardiac carcinomas, and its clinicopathologic significance, in comparison with distal gastric carcinomas.
It included 49 patients with adenocarcinomas of the gastric cardia, 27 males and 22 females with an average age of 55 ± 12 years, and 55 patients with adenocarcinomas of the distal stomach, 30 males and 25 females with an average age of 58 ± 9.7 years. The 49 patients with adenocarcinomas of the gastric cardia underwent surgery at the Esophageal Carcinoma Hospital of Linzhou in Henan Province, and the 55 patients with distal gastric carcinomas underwent surgery at the First Hospital of Jiaxing in Zhejiang Province. None of the patients had received chemotherapy or radiotherapy before surgery.
All specimens were fixed with formalin and embedded in paraffin. Each block was sectioned serially at 5 μm, one of which was stained with hematoxylin and eosin for histopathological analysis by two pathologists and the others were used for immunostaining[22].
Histopathological diagnoses were made according to previous reports[22].
Anti-ATP7B antibody was a rabbit polyclonal antibody against ATP7B(Boster, China). Avidin-biotin-peroxidase complex (ABC) method was used for ATP7B immunostaining. In brief, after dewaxing of sections, endogenous peroxidase activity was quenched with 3% H2O2, and cross-reactivity was blocked with normal serum. The tissues were incubated overnight at 4°C with primary antibodies (1:100 for anti-ATP7B antibody). Localization of the primary antibodies was achieved by subsequent use of a biotinylated anti-primary antibody, an avidin-biotin complex conjugated with horseradish peroxidase, and 3',5'-diaminobenzidine (Vectasitain Elite Kit). Normal serum blocking and omission of the primary antibody were used as negative controls[22].
The criterion for a positive reaction was clear cytoplasm and cell membrane staining. If more than 10% of the tumor cells were stained, the samples were considered to be ATP7B-positive carcinomas[21].
Pearson Chi-Square and Fisher’s exact tests were performed. The statistical analyses were performed using SPSS 12.0 software. Two-sided P values were calculated and a difference was considered significant if the P value was less than 0.05.
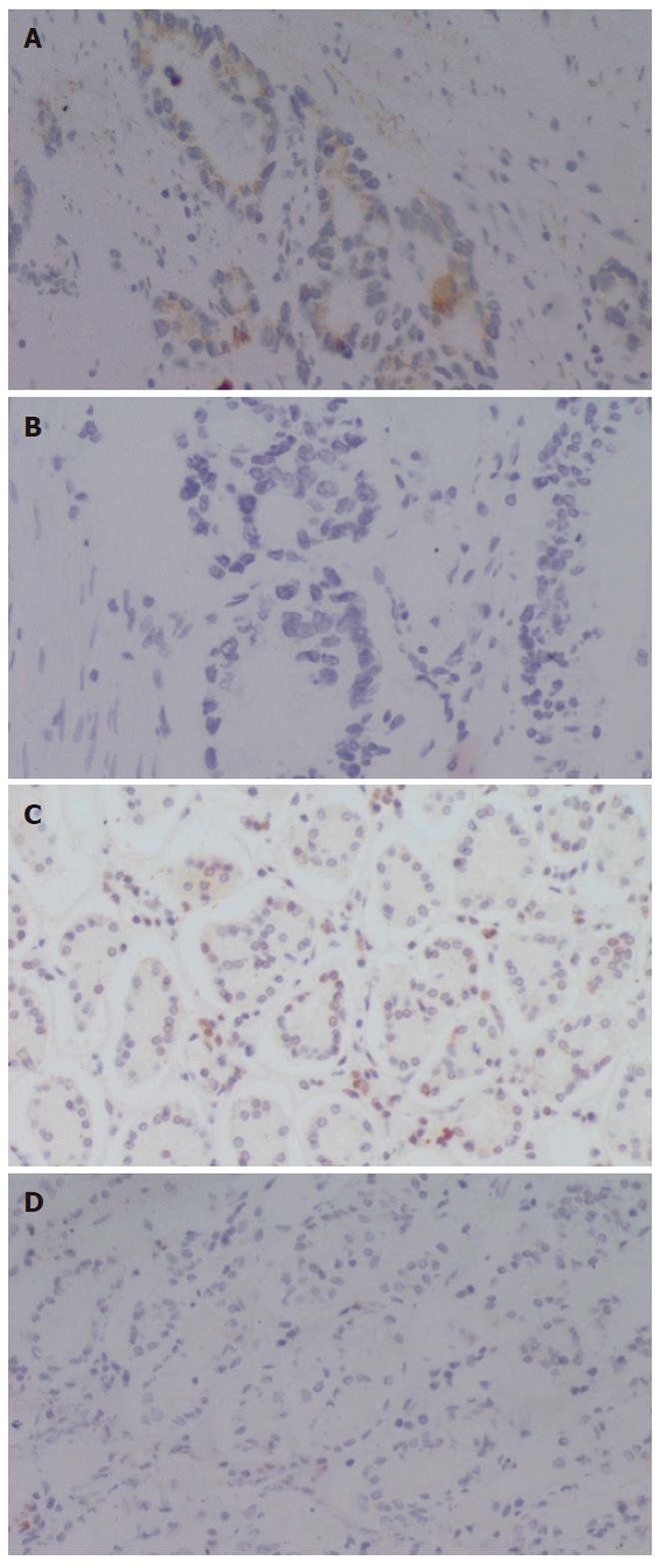
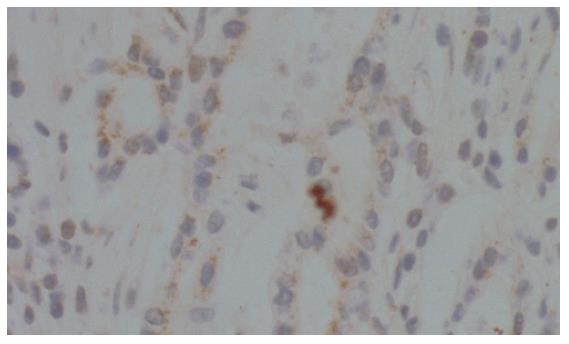
To examine the expression of ATP7B at protein level in human gastric cardia carcinomas, we performed immunohistochemical analysis using a polyclonal antibody against ATP7B. As shown in Figure 1, ATP7B expression was observed in the cytoplasm of gastric cardiac adenocarcinoma cells. A negative control did not reveal ATP7B protein. We used 49 primary gastric cardiac carcinoma specimens for the detection of ATP7B by immunohistochemistry and found that various degrees of cytoplasmic staining of tumor cells were observed in 51.0% (25/49) of the analyzed tumors. We also determined ATP7B expression of the corresponding adjacent non-neoplastic epithelium of all the gastric cardiac carcinomas. ATP7B was identified in 11 of 49 (22.4%) of the examined cases, which was markedly lower than that in gastric cardiac carcinomas (P = 0.003). ATP7B was localized close to the cell membrane of adjacent non-neoplastic epithelium, mainly concentrated at the pit pole of the gastric epithelium[23].
We examined the relationship between clinicopathologic variables and ATP7B expression in human gastric cardiac carcinomas. No significant association was found between ATP7B expression and age, tumor size, nodal stage and metastasis status. Regarding histopathologic type, ATP7B protein positivity in poorly differentiated carcinomas was significantly higher than that in well/moderately differentiated carcinomas (P = 0.030) (Table 1).
| Variables | n | ATP7B | Significance1 | |
| Negative | Positive | |||
| Total | 49 | 24 | 25 (51.0%) | |
| Median age (yr) | 54 | 56 | NS | |
| Degree of differentiation | 0.030 | |||
| Well/moderately differentiated | 13 | 6 | ||
| Poorly differentiated | 11 | 19 | ||
| Tumor size | NS | |||
| T1 + T2 | 10 | 7 | ||
| T3 + T4 | 14 | 18 | ||
| Nodal stage | NS | |||
| N (-) | 3 | 5 | ||
| N (+) | 21 | 20 | ||
| Metastasis status | NS | |||
| M0 | 21 | 19 | ||
| M1 | 3 | 6 | ||
As shown in Figure 2, ATP7B expression was also observed in the cytoplasm of distal gastric carcinoma cells. We used 55 primary distal gastric carcinoma specimens for the detection of ATP7B by immunohistochemistry and found that various degrees of cytoplasmic staining were observed in 30.9% (17/55) of the analyzed tumors, markedly lower than that in gastric cardiac carcinomas (P = 0.037).
Recent research has identified that ATP7B, an energy-dependent copper transporter, is a new resistance marker of tumor cells to platinum containing anticancer drugs[12-14]. The present study provides direct evidence of frequent ATP7B expression in gastric cardiac carcinomas, suggesting the importance of ATP7B protein in primary resistance of gastric cardiac carcinoma cells to platinum containing anticancer drugs. Furthermore, ATP7B expression in poorly differentiated gastric cardiac carcinomas was more frequent than in well/moderately differentiated carcinomas, indicating that ATP7B expression might serve as an independent prognostic factor in these patients.
Several study groups detected no ATP7B protein expression in adjacent non-neoplastic epithelium[16-18]. However, our results are somewhat different from the previous reports. We found that ATP7B protein expression was also seen in some of adjacent non-neoplastic epithelium, although it was much lower than in gastric cardiac carcinomas. This suggests that ATP7B protein in gastrointestinal tract may have physiological function. We suppose that ATP7B might protect gastric cardiac epithelium cells from the harmful heavy metals.
It is well known that gastric cardiac carcinoma is more resistant to chemotherapy and has worse prognosis compared with distal gastric carcinoma, although the underlying mechanisms have not been elucidated[1,24]. Our observations demonstrated that ATP7B expression in gastric cardiac carcinomas was significantly higher than that in distal gastric carcinomas. This might partially explain the difference of chemotherapy response and prognosis between gastric cardiac carcinoma and distal gastric carcinoma. Combined determination of ATP7B and other drug-resistant molecular markers[8], such as Pgp, MRP, LRP, BCRP, could be useful for selection of chemotherapy regimens.
We thank Li-Dong Wang, Professor of Pathology and Oncology, College of Medicine, Zhengzhou University, China for his contribution and help in obtaining esophageal carcinoma specimens.
| 1. | Wang LD, Zheng S, Zheng ZY, Casson AG. Primary adenocarcinomas of lower esophagus, esophagogastric junction and gastric cardia: in special reference to China. World J Gastroenterol. 2003;9:1156-1164. [PubMed] |
| 2. | Blaser MJ, Saito D. Trends in reported adenocarcinomas of the oesophagus and gastric cardia in Japan. Eur J Gastroenterol Hepatol. 2002;14:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Wijnhoven BP, Louwman MW, Tilanus HW, Coebergh JW. Increased incidence of adenocarcinomas at the gastro-oesophageal junction in Dutch males since the 1990s. Eur J Gastroenterol Hepatol. 2002;14:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Newnham A, Quinn MJ, Babb P, Kang JY, Majeed A. Trends in the subsite and morphology of oesophageal and gastric cancer in England and Wales 1971-1998. Aliment Pharmacol Ther. 2003;17:665-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Pera M, Cameron AJ, Trastek VF, Carpenter HA, Zinsmeister AR. Increasing incidence of adenocarcinoma of the esophagus and esophagogastric junction. Gastroenterology. 1993;104:510-513. [PubMed] |
| 6. | Ohno S, Tomisaki S, Oiwa H, Sakaguchi Y, Ichiyoshi Y, Maehara Y, Sugimachi K. Clinicopathologic characteristics and outcome of adenocarcinoma of the human gastric cardia in comparison with carcinoma of other regions of the stomach. J Am Coll Surg. 1995;180:577-582. [PubMed] |
| 7. | Ichikura T, Ogawa T, Kawabata T, Chochi K, Sugasawa H, Mochizuki H. Is adenocarcinoma of the gastric cardia a distinct entity independent of subcardial carcinoma. World J Surg. 2003;27:334-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Leonard GD, Fojo T, Bates SE. The role of ABC transporters in clinical practice. Oncologist. 2003;8:411-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 550] [Cited by in RCA: 589] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 9. | Tulpule A, Sherrod A, Dharmapala D, Young LL, Espina BM, Sanchez MN, Gill PS, Levine AM. Multidrug resistance (MDR-1) expression in AIDS-related lymphomas. Leuk Res. 2002;26:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265-7279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2302] [Cited by in RCA: 2561] [Article Influence: 111.3] [Reference Citation Analysis (1)] |
| 11. | Kartalou M, Essigmann JM. Mechanisms of resistance to cisplatin. Mutat Res. 2001;478:23-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 638] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 12. | Komatsu M, Sumizawa T, Mutoh M, Chen ZS, Terada K, Furukawa T, Yang XL, Gao H, Miura N, Sugiyama T. Copper-transporting P-type adenosine triphosphatase (ATP7B) is associated with cisplatin resistance. Cancer Res. 2000;60:1312-1316. [PubMed] |
| 13. | Samimi G, Howell SB. Modulation of the cellular pharmacology of JM118, the major metabolite of satraplatin, by copper influx and efflux transporters. Cancer Chemother Pharmacol. 2006;57:781-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Katano K, Safaei R, Samimi G, Holzer A, Rochdi M, Howell SB. The copper export pump ATP7B modulates the cellular pharmacology of carboplatin in ovarian carcinoma cells. Mol Pharmacol. 2003;64:466-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Solioz M, Vulpe C. CPx-type ATPases: a class of P-type ATPases that pump heavy metals. Trends Biochem Sci. 1996;21:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 290] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 16. | Kanzaki A, Toi M, Neamati N, Miyashita H, Oubu M, Nakayama K, Bando H, Ogawa K, Mutoh M, Mori S. Copper-transporting P-type adenosine triphosphatase (ATP7B) is expressed in human breast carcinoma. Jpn J Cancer Res. 2002;93:70-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Aida T, Takebayashi Y, Shimizu T, Okamura C, Higasimoto M, Kanzaki A, Nakayama K, Terada K, Sugiyama T, Miyazaki K. Expression of copper-transporting P-type adenosine triphosphatase (ATP7B) as a prognostic factor in human endometrial carcinoma. Gynecol Oncol. 2005;97:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Higashimoto M, Kanzaki A, Shimakawa T, Konno S, Naritaka Y, Nitta Y, Mori S, Shirata S, Yoshida A, Terada K. Expression of copper-transporting P-type adenosine triphosphatase in human esophageal carcinoma. Int J Mol Med. 2003;11:337-341. [PubMed] |
| 19. | Ohbu M, Ogawa K, Konno S, Kanzaki A, Terada K, Sugiyama T, Takebayashi Y. Copper-transporting P-type adenosine triphosphatase (ATP7B) is expressed in human gastric carcinoma. Cancer Lett. 2003;189:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Miyashita H, Nitta Y, Mori S, Kanzaki A, Nakayama K, Terada K, Sugiyama T, Kawamura H, Sato A, Morikawa H. Expression of copper-transporting P-type adenosine triphosphatase (ATP7B) as a chemoresistance marker in human oral squamous cell carcinoma treated with cisplatin. Oral Oncol. 2003;39:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Nakayama K, Kanzaki A, Terada K, Mutoh M, Ogawa K, Sugiyama T, Takenoshita S, Itoh K, Yaegashi N, Miyazaki K. Prognostic value of the Cu-transporting ATPase in ovarian carcinoma patients receiving cisplatin-based chemotherapy. Clin Cancer Res. 2004;10:2804-2811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Song ZB, Gao SS, Yi XN, Li YJ, Wang QM, Zhuang ZH, Wang LD. Expression of MUC1 in esophageal squamous-cell carcinoma and its relationship with prognosis of patients from Linzhou city, a high incidence area of northern China. World J Gastroenterol. 2003;9:404-407. [PubMed] |
| 23. | Fukaya M, Isohata N, Ohta H, Aoyagi K, Ochiya T, Saeki N, Yanagihara K, Nakanishi Y, Taniguchi H, Sakamoto H. Hedgehog signal activation in gastric pit cell and in diffuse-type gastric cancer. Gastroenterology. 2006;131:14-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Safaei R, Holzer AK, Katano K, Samimi G, Howell SB. The role of copper transporters in the development of resistance to Pt drugs. J Inorg Biochem. 2004;98:1607-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
S- Editor Wang J L- Editor Zhu LH E- Editor Liu WF