Published online Dec 14, 2006. doi: 10.3748/wjg.v12.i46.7537
Revised: October 15, 2006
Accepted: October 25, 2006
Published online: December 14, 2006
AIM: To assess the clinical and economical validity of glutamine dipeptide supplemented to parenteral nutrition (PN) in patients undergoing abdominal surgery.
METHODS: A meta-analysis of all the relevant randomized controlled trials (RCTs) was performed. The trials compared the standard PN and PN supplemented with glutamine dipeptide in abdominal surgery. RCTs were identified from the following electronic databases: the Cochrane Library, MEDLINE, EMBASE and ISI web of knowledge (SCI). The search was undertaken in April 2006. Literature references were checked by computer or hand at the same time. Clinical trials were extracted and evaluated by two reviewers independently. Statistical analysis was performed by RevMan4.2 software from Cochrane Collaboration. A P value of < 0.05 was considered statistically significant.
RESULTS: Nine RCTs involving 373 patients were included. The combined results showed that glutamine dipeptide has a positive effect in improving postoperative cumulative nitrogen balance (weighted mean difference (WMD = 8.35, 95% CI [2.98, 13.71], P = 0.002), decreasing postoperative infectious morbidity (OR = 0.24, 95% CI [0.06, 0.93], P = 0.04), shortening the length of hospital stay (WMD= -3.55, 95% CI [-5.26, -1.84], P < 0.00001). No serious adverse effects were found.
CONCLUSION: Postoperative PN supplemented with glutamine dipeptide is effective and safe to decrease the infectious rate, reduce the length of hospital stay and improve nitrogen balance in patients undergoing abdominal surgery. Further high quality trials in children and severe patients are required, and mortality and hospital cost should be considered in future RCTs with sufficient size and rigorous design.
- Citation: Zheng YM, Li F, Zhang MM, Wu XT. Glutamine dipeptide for parenteral nutrition in abdominal surgery: A meta-analysis of randomized controlled trials. World J Gastroenterol 2006; 12(46): 7537-7541
- URL: https://www.wjgnet.com/1007-9327/full/v12/i46/7537.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i46.7537
Glutamine (Gln) is the most abundant free amino acid in the body and plays a vital role in amino acid transport and nitrogen balance. Gln is also a primary fuel for rapidly dividing cells such as enterocytes and lymphocytes, which protect mucosa barricade and enhance immune functions[1]. Patients undergoing elective abdominal surgery usually have malnutrition and Gln concentration is low in blood due to several factors: mechanical obstruction, limitation of food intake, tumor-induced cachexia, obstruction of pancreaticobiliary, malabsorption and ongoing blood loss. Moreover, intravascular and free muscle glutamine pools become depleted in response to perioperative abrosia and operative stress. Free Gln is lack of stability in solution and intravenous adminitration is limited. Glutamine dipeptide (L-alanyl-L-glutamine) can be taken via vein and hydrolyzed into glutamine in circulation as Gln substitution. It was given to patients undergoing abdominal surgery in order to improve their postoperative nitrogen balance and immunonutrition[2]. Therefore, it is worth knowing whether routine supplementation of glutamine dipeptide in parenteral nutrition (PN) can amend clinical outcomes.
Meta-analysis has been applied in medical research to improve statistical efficiency, to evaluate the disadvantage of established studies and to draw reliable conclusions from various potentially relevant studies. It is the most promising approach for future research and a guideline for clinical treatment[3]. This meta-analysis aims to enhance our understanding of the clinical and economical validity of glutamine dipeptide for patients undergoing abdominal surgery.
The meta-analysis included clinical randomized controlled trials (RCTs) of patients undergoing abdominal surgery. The trials compared standard isonitrogen PN and PN supplemented with glutamine dipeptide.
A computerized literature search was applied from the following electronic databases: the Cochrane Library (April 2006), MEDLINE (PubMed) (1966-April 2006), EMBASE (1980-April 2006) and ISI web of knowledge (SCI) (April 2006). The search was undertaken in April 2006. Literature reference proceedings were hand retrieved at the same time. The subject words for the search were glutamine dipeptide in addition to L-alanyl-L-glutamine. Other useful words were glutamine, parenteral nutrition, operation, surgery, postoperation or perioperation, RCT or clinical trials. Study literature includes abstracts at least in the English language and followed by full text.
Data were extracted independently by two reviewers and decided by the research team. Methodological quality of study was evaluated using the Jadad scale[4] and the trial with a score over 2 was included as high quality. Published studies were identified by the following selection criteria: study design-RCT, population-hospitalized adult patients undergoing abdominal surgery, intervention-parenteral nutrition with glutamine dipeptide and standard parenteral nutrition with isonitrogen and isocalorics. The following data were extracted: quantity and group dividing of patients, different doses and days of glutamine dipeptide used, and the baseline of trials. Outcome variables included: nitrogen balance, length of hospital stay, postoperative infection, immune effects, adverse events and mortality.
The statistical analysis was performed by RevMan4.2 software, which was provided by the Cochrane Collaboration. P value < 0.05 was considered statistically significant. Heterogeneity was checked by the Chi-square test Meta-analysis was done with fixed effects model when results of the trials had no heterogeneity. If the results had heterogeneity, random effects model was used and causes were analyzed. The result was expressed with an odds ratio (OR) for the categorical variable and weighted mean difference (WMD) for the continuous variables, and with 95% confidence intervals (CI). The Handbook for Cochrane Reviewer (v 1.8.0) from Cochrane Collaboration was used as a guideline for the meta-analysis.
There were 454 papers relevant to the searching words. Through the steps of screening the title, reading the abstract and the entire article, only nine RCTs[5-13] involving 373 patients were included. Other two studies were excluded because of repeated reports[14-15]. Characteristics of studies included in meta-analysis are presented in Table 1. There were 7 papers published in English and 2 in Chinese. Five studies were done in Europe and 4 in Asia. In the trials, glutamine dipeptide was administered at 0.18-0.5 g/kg per day and lasted 5-7 d.
| Authors | Journals | Year | Study design | Jadad score | Reference | Operation | Patients (Gln/Con) | Gln dipeptide (g/kg) | Days of Gln dipeptide (d) admininistration |
| Stehle P | Lancet | 1989 | RCT | 3 | 5 | Elective resection of carcinoma of colon or rectum | 12 (6/6) | 0.28 | 5 (postoperative 1-5) |
| O’Riordain | Annals of Surgery | 1994 | RCT | 3 | 6 | Colorectal Resection | 22 (11/11) | 0.18 | 5 (postoperative 1-5) |
| Morlion BJ | Ann Surg | 1998 | Double-blind RCT | 4 | 7 | Major abdominal surgery | 28 (13/15) | 0.3 | 5 (postoperative 1-5) |
| Metes | Clinical Nutrition | 2000 | RCT | 4 | 8 | Major abdominal surgery | 30 (15/15) | 0.5 | 5 (postoperative 1-5) |
| Jiang Z | Zhong guo Yi Xue Ke Xue Yuan Xue Bao | 2000 | Prospective double-blind RCT | 5 | 9 | Gastrointestinal operations | 120 (60/60) | 0.36 | 6 (postoperative 1-6) |
| Neri | Nutrition | 2001 | Multiple centers prospective double-blind RCT | 3 | 10 | Major abdominal surgery | 33 (16/17) | 0.18 | 5 (postoperative 1-5) |
| Fan YP | Zhonghua Wai Ke Za Zhi | 2005 | RCT | 3 | 11 | Abdominal surgery | 40 (20/20) | 0.16 | 7 (postoperative 1-7) |
| Lin MT | World J Gastroenterol | 2005 | RCT | 4 | 12 | Abdominal surgery | 48 (25/23) | 0.417 | 6 (postoperative 1-6) |
| Yao GX | Clin Nutr | 2005 | RCT | 3 | 13 | Gastrointestinal operations | 40 (20/20) | 0.5 | 5 (preoperative 1-postoperative 3) |
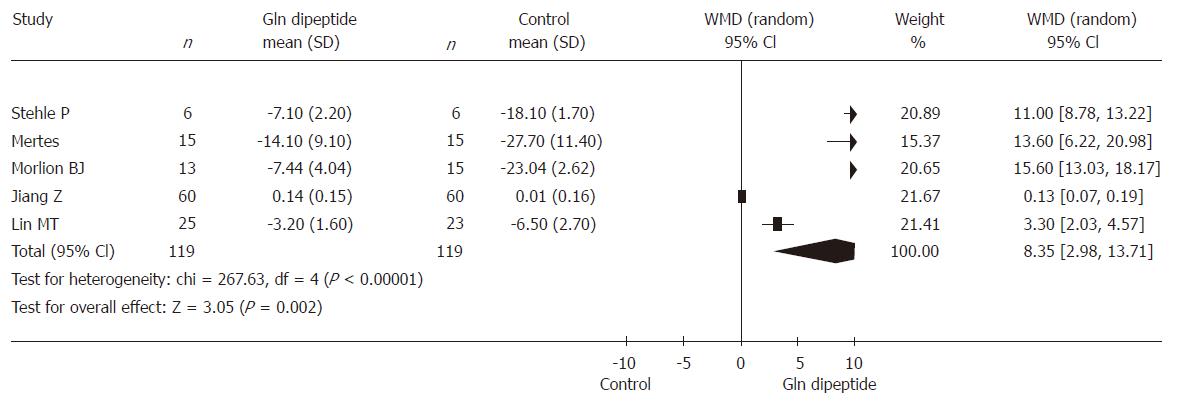
Six RCTs (involving 238 patients) reported postoperative cumulative nitrogen balance. The result was heterogeneous (P < 0.00001) and the random effects model was used because of different preoperative general nutrition, different PN design and different doses and days of glutamine dipeptide supplementation. Combined analysis indicated that the use of glutamine dipeptide had a positive effect in improving the postoperative cumulative nitrogen balance (WMD = 8.35, 95% CI [2.98, 13.71], P = 0.002) (Figure 1).
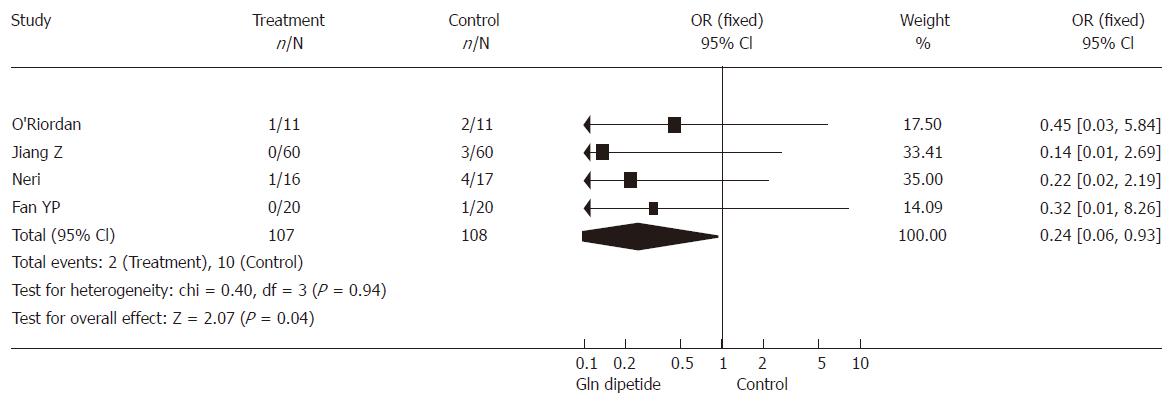
Five RCTs (involving 215 patients) reported the postoperative morbidity of infection. There was no heterogeneity (P = 0.94). Combined analysis indicated that the use of glutamine dipeptide reduced infective events (OR = 0.24, 95% CI [0.06, 0.93], P = 0.04) (Figure 2).
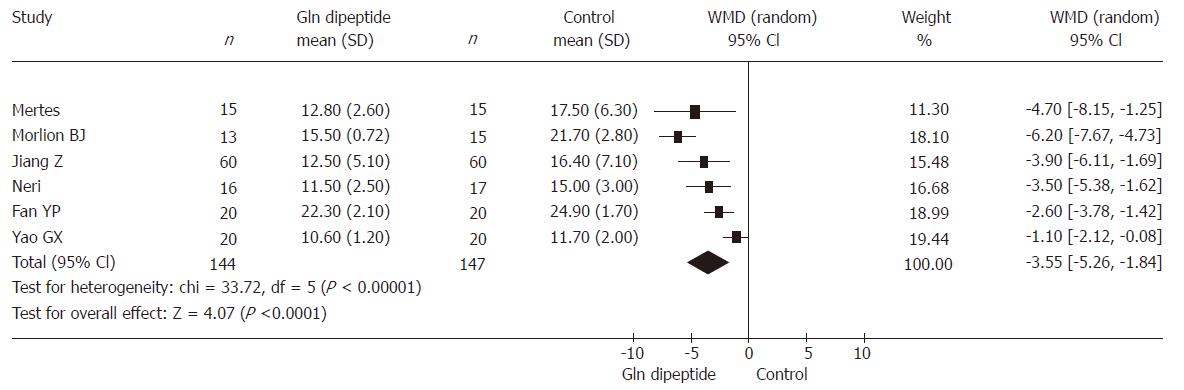
Six RCTs (involving 291 patients) reported length of hospital stay. The result was heterogeneous (P < 0.00001) and the random effects model was used because of different severity of primary diseases, different levels of operational injury, different preoperative general nutrition and APACHEII scores. Comprehensive analysis indicated that the use of glutamine dipeptide had a positive effect in shortening the length of hospital stay (WMD = -3.55, 95% CI [-5.26, -1.84], P < 0.00001) (Figure 3).
Before operation, abdominal diseases often led to lack of Gln, following malnutrition and immune dysfunctions. After operation, demand for Gln exceeded the supply from diet and from muscle as a response to injury. And it has been shown that intravascular and free muscle glutamine pools become deficient or depleted in response to operative stress[5,16,17]. The deficiency of glutamine is the main cause for protein metabolism disorder, intestinal mucosal injury, enteral wall permeability destruction, bacterial translocation and immunosuppression. All these increase the perioperative infection risk and hinder the postoperative recovery.
Meta-analysis indicates that the use of glutamine dipeptide could improve the postoperative nitrogen balance better than standard PN. Gln, accounting for approximately one third of the translocated nitrogen, plays a major role in supporting vital organ function and repairing wound[18]. Full supplementary glutamine dipeptide not only prevents protein lost, but also makes nutrition metabolism effective.
The study also gives us evidence that use of glutamine dipeptide decreases infective events. Gln is utilized at a high rate by cells of the immune system. It supports optimal lymphocyte proliferation, and production of cytokines by lymphocytes and macrophages. Macrophage-mediated phagocytosis is influenced by glutamine availability[1,19]. Hydrolysable glutamine dipeptide can substitute glutamine to support lymphocyte and macrophage functions in vitro. Animal studies have shown that intake of glutamine increases survival to bacterial challenge[19]. After operation, immunosuppression resulted in lowered plasma glutamine concentrations. If Gln or its precursors are provided to patients at risk of immunosuppression following surgery, sufficient maintenance of plasma glutamine concentration is of benefit to maintaining immune function. In clinical trials, glutamine dipeptide ameliorates restoration of plasma CD14 levels, improves lymphocyte recovery[6,9] and attenuates plasma IL-6 to relieve immunodepression[12,15]. Moreover, glutamine in circulation can also improve barricade function of gastroenteral mucosa to prevent bacterial translocation[9].
In those studies, mortality and hospital cost were seldomly reported. Length of hospital stay becomes a clinical end point as a surrogate for both clinical and economic efficiency. Meta-analysis shows that the use of glutamine dipeptide has a positive effect on shortening the length of hospital stay. Factors influencing hospital stay by Gln include accelerating wound repair and recovery of intestinal mucosal integrity, preventing potential delay of infection. It implies that such an approach is optimal in clinical practice, especially for severe surgeries[15]. At the same time, no serious adverse effects were found in all the included studies.
In conclusion, postoperative PN supplemented glutamine dipeptide is effective and safe to decrease the infectious rate, reduce length of hospital stay and improve nitrogen balance in patients undergoing abdominal surgery. Further high quality trials in children and severe patients are required; Mortality and hospital cost should be considered in future RCTs with sufficient size and rigorous design.
We thank Dr. Jin Wen from Chinese Evidence-Based Medicine/Cochrane Center for his technological support and Dr. Yong Zhou for providing the research references.
| 1. | Hall JC, Heel K, McCauley R. Glutamine. Br J Surg. 1996;83:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 116] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Ward N. Nutrition support to patients undergoing gastrointestinal surgery. Nutr J. 2003;2:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Sheldon TA. Systematic reviews and meta-analyses: the value for surgery. Br J Surg. 1999;86:977-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary. Control Clin Trials. 1996;17:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12275] [Cited by in RCA: 13051] [Article Influence: 435.0] [Reference Citation Analysis (3)] |
| 5. | Stehle P, Zander J, Mertes N, Albers S, Puchstein C, Lawin P, Fürst P. Effect of parenteral glutamine peptide supplements on muscle glutamine loss and nitrogen balance after major surgery. Lancet. 1989;1:231-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 269] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | O'Riordain MG, Fearon KC, Ross JA, Rogers P, Falconer JS, Bartolo DC, Garden OJ, Carter DC. Glutamine-supplemented total parenteral nutrition enhances T-lymphocyte response in surgical patients undergoing colorectal resection. Ann Surg. 1994;220:212-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 139] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Morlion BJ, Stehle P, Wachtler P, Siedhoff HP, Köller M, König W, Fürst P, Puchstein C. Total parenteral nutrition with glutamine dipeptide after major abdominal surgery: a randomized, double-blind, controlled study. Ann Surg. 1998;227:302-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 159] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Mertes N, Schulzki C, Goeters C, Winde G, Benzing S, Kuhn KS, Van Aken H, Stehle P, Fürst P. Cost containment through L-alanyl-L-glutamine supplemented total parenteral nutrition after major abdominal surgery: a prospective randomized double-blind controlled study. Clin Nutr. 2000;19:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Jiang Z, Huang Y, Li Z, Li D, Yu K. [Principle of evidence-based medicine and its application in clinical trial practice for glutamine and gut permeability]. Zhongguo Yixue Kexueyuan Xuebao. 2000;22:407-410. [PubMed] |
| 10. | Neri A, Mariani F, Piccolomini A, Testa M, Vuolo G, Di Cosmo L. Glutamine-supplemented total parenteral nutrition in major abdominal surgery. Nutrition. 2001;17:968-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Fan YP, Yu JC, Kang WM, Zhang Q. [Effects on glutathione of patients received glutamine dipeptide enriched parenteral nutrition post abdominal surgery]. Zhonghua Waike Zazhi. 2005;43:1383-1386. [PubMed] |
| 12. | Lin MT, Kung SP, Yeh SL, Liaw KY, Wang MY, Kuo ML, Lee PH, Chen WJ. Glutamine-supplemented total parenteral nutrition attenuates plasma interleukin-6 in surgical patients with lower disease severity. World J Gastroenterol. 2005;11:6197-6201. [PubMed] |
| 13. | Yao GX, Xue XB, Jiang ZM, Yang NF, Wilmore DW. Effects of perioperative parenteral glutamine-dipeptide supplementation on plasma endotoxin level, plasma endotoxin inactivation capacity and clinical outcome. Clin Nutr. 2005;24:510-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Powell-Tuck J. Total parenteral nutrition with glutamine dipeptide shortened hospital stays and improved immune status and nitrogen economy after major abdominal surgery. Gut. 1999;44:155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Lin MT, Kung SP, Yeh SL, Lin C, Lin TH, Chen KH, Liaw KY, Lee PH, Chang KJ, Chen WJ. The effect of glutamine-supplemented total parenteral nutrition on nitrogen economy depends on severity of diseases in surgical patients. Clin Nutr. 2002;21:213-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Hammarqvist F, Wernerman J, Ali R, von der Decken A, Vinnars E. Addition of glutamine to total parenteral nutrition after elective abdominal surgery spares free glutamine in muscle, counteracts the fall in muscle protein synthesis, and improves nitrogen balance. Ann Surg. 1989;209:455-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 285] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 17. | Fürst P, Pogan K, Stehle P. Glutamine dipeptides in clinical nutrition. Nutrition. 1997;13:731-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 93] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Wilmore DW. The effect of glutamine supplementation in patients following elective surgery and accidental injury. J Nutr. 2001;131:2543S-2549S; discussion 2550S-2551S. [PubMed] |
| 19. | Calder PC, Yaqoob P. Glutamine and the immune system. Amino Acids. 1999;17:227-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 140] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
S- Editor Wang GP L- Editor Ma JY E- Editor Lu W