Published online Dec 14, 2006. doi: 10.3748/wjg.v12.i46.7440
Revised: August 6, 2006
Accepted: August 11, 2006
Published online: December 14, 2006
Members of the receptor tyrosine kinase family, that include EGFR, ErbB-2/HER-2, ErbB-3/HER-3 and ErbB-4/HER-4, are frequently implicated in experimental models of epithelial cell neoplasia as well as in human cancers. Therefore, interference with the activation of these growth factor receptors represents a promising strategy for development of novel and selective anticancer therapies. Indeed, a number of inhibitors that target either EGFR or HER-2, with the exception of a few that target both; have been developed for treatment of epithelial cancers. Since most solid tumors express different ErbB receptors and/or their ligands, identification of inhibitor(s), targeting multiple EGFR family members may provide a therapeutic benefit to a broader patient population. Here we describe the significance of an ErbB family of receptors in epithelial cancers, and summarize different available therapeutics targeting these receptors. It also emphasizes the need to develop pan-ErbB inhibitors and discusses EGF-Receptor Related Protein, a recently isolated negative regulator of EGFR as a potential pan-ErbB therapeutic for a wide variety of epithelial cancers.
- Citation: Nautiyal J, Rishi AK, Majumdar AP. Emerging therapies in gastrointestinal cancers. World J Gastroenterol 2006; 12(46): 7440-7450
- URL: https://www.wjgnet.com/1007-9327/full/v12/i46/7440.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i46.7440
Gastrointestinal cancers account for 21% of all cancers incidences and 25% of the cancer mortality in the United States[1]. Despite recent advances in diagnosis and treatment, gastric carcinoma, ductal carcinoma of the pancreas and colorectal cancer are the leading causes of cancer-related deaths worldwide. The pathogenesis of these cancers remains elusive and the available treatment options are limited. Among the GI cancer-related deaths, carcinoma of the stomach ranks seventh in the USA. However, stomach cancer is the leading cause of cancer-associated mortality in Japan, China and India[2]. Gastric cancer ranks number 2 worldwide, second to lung cancer and thus is a major international health concern[2]. Colorectal cancer is the third most common malignancy among men and women in the USA and ranks second for cancer related deaths[3]. In terms of global incidence, colorectal cancer ranks third in frequency, but fourth in cancer related mortality[2]. Adenocarcinoma of the pancreas is the second most common gastrointestinal malignancy in the USA[4]. However, pancreatic cancer is the fourth leading cause of cancer-related mortality among American men and women[5]. GastroIntestinal Stromal Tumor (GIST) is a rare stomach and intestinal cancer with unknown global incidence that spreads rapidly and the survival rate for patients is quite low. GIST is resistant to most known therapies and the available ones have much harsher side effects.
Different treatments are available for gastrointestinal (GI) cancers. These may be employed alone or in combin-ation with other therapeutic/adjuvant therapy. The available treatments for GI tract cancers are (1) Surgery: resection of the solid tumor whenever possible (2) Chemotherapy: employing cytotoxic drugs to kill cancers cells (3) Radiation therapy: to treat localized solid tumors, (4) Hormonal therapy: systemic treatment that targets cancer cells through out the body. Different analogs of GI hormonal peptides and endogenous growth factors are utilized to inhibit the progression of tumor. Such analogs target gastric releasing peptide (GRP), bombesin, somatostatin, as well as peptide receptors and antagonists of growth hormone releasing hormone (GH-RH)[6]. However, the successful treatment of cancer often requires the combination and coordination of several different treatment approaches. This is referred to as multi-modality treatment and may consist of surgery, chemotherapy, radiation therapy, and/or hormonal therapy. It is important to emphasize that surgery is a local treatment and is only capable of removing cancer cells from a defined area. By the time a cancer is diagnosed, many patients will already have experienced spread of cancer cells through the blood and lymph system to other locations in the body. They are referred to as micrometastases. Currently available tests cannot always detect micrometastases. Information obtained during surgery and from other tests determines the likelihood of the cancer having spread and whether additional treatments with chemotherapy, radiation, or hormonal therapy is necessary.
With the recent advances towards understanding of the molecular basis of carcinogenesis, different cell signaling pathways have been implicated in aberrant growth of cells. Direct or indirect interference with signaling pathways often results in modulation of cellular growth. The modalities that target single or multiple signaling pathways, referred to as “Targeted therapies” have been developed and are currently being utilized in clinics as anti-cancer therapeutics. A targeted therapy is designed to treat the cancer cells and minimize damage to normal, healthy cells. Treatments that “target” cancer cells specifically offer the advantage of reduced treatment-related side effects and improved outcome. Conventional cancer treatments such as radiation therapy, do not distinguish between cancer cells and healthy cells. Consequently, healthy cells are commonly damaged in the process of treating the cancer, which results in side effects. Chemotherapy damages rapidly dividing cells, a hallmark trait of cancer cells. In the process, healthy cells that are also rapidly dividing (such as blood cells and the cells lining the mouth and GI tract) are also damaged. Many chemotherapy drugs, which damage DNA of both malignant cells and normal cells, frequently cause toxicity. Treatment-related damage to healthy cells leads to complications of treatment, or side effects. These side effects may be severe, reducing a patient’s quality of life, compromising their ability to receive their full, prescribed treatment, and sometimes, limiting their chance for an optimal outcome from treatment.
A number of genes/proteins have been identified to be abnormally expressed in tumors and thus serve as targets for therapeutic intervention. Among the known targets for treatment of GI cancers, Epidermal Growth Factor Receptor and its family members (EGFRs) form the most attractive candidate. EGFR sits across the outer membrane of the cell, receiving and transmitting growth signals from the cell surface to the nucleus. EGFRs have been implicated in variety of epithelial cancers and are generally overexpressed or aberrantly activated in one third of all the cancers. The overexpression of the receptor has been reported in cancers of the stomach (33%-74%), colorectum (25%-77%), esophagus (43%-89%), and pancreas (30%-50%)[7]. Increased EGFR or some of its family members expression is normally associated with poor prognosis with more advanced disease, increased metastasis and decreased survival[8-10]. For the sake of simplicity, the current article will focus on different clinically approved and ongoing developing therapeutics that target EGFR or its family members.
The EGFR family of RTKs is comprised of four members in mammals but containing one member of the EGFR family in C.elegans and D. melanogaster. The four members are called: EGFR (also ErbB-1 or HER-1), ErbB-2/HER-2, ErbB-3/HER-3 and ErbB-4/HER-4[11,12]. The signaling transduced by these members is crucial for the development of mice, fruitflies and nematodes[13-17]. The best-characterized functions of the ErbB family of receptors are regulating aspects of replication, migration and survival of cells. In this regard, these receptors interact with multiple signaling molecules and pathways, transmitting and receiving both stimulatory and inhibitory signals[18-20].
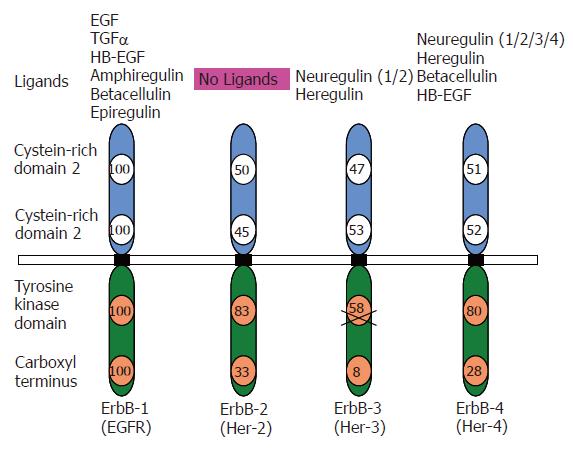
All members of the ErbB family are cell surface allosteric enzymes consisting of a single transmembrane domain that separates the extracellular ligand binding domain from the intracellular kinase domain (Figure 1). Under normal physiological conditions, activation of ErbB receptors is regulated by the specific spatial and temporal expression of their ligands which are members of the EGF family of growth factors[11,20]. Ligand binding initiates homo/hetero dimerization of the receptors, leading to auto and trans- tyrosine phosphorylation of the receptors. Tyrosine phosphorylation/activation of the kinase domain leads to recruitment of different proteins initiating a downstream signal cascade. Protein phosphorylation and dephosphorylation, catalyzed by protein tyrosine kinases and protein phosphatases respectively represent the two fundamental biochemical events for downstream intracellular signal transduction[21]. Autophosphorylation of tyrosine residues within the C-tail terminus of EGFR in the cytoplasm following activation of the receptor initiates a cascade of intracellular signaling pathways[22,23]. The receptor tyrosine kinase signaling may be terminated by the endocytosis of the receptor-ligand complex[24]. The downstream signaling by ErbB receptors results in transcriptional regulation of various genes including proto-oncogenes like jun, fos and myc in addition to some zinc-finger containing transcription factors[25].
Signaling by ErbB receptors is quite diversified and finely tuned at two levels of regulation. These include the specific binding of the ligand to the receptor(s) and the ability of each receptor to form homo/hetero dimers[20,26]. The peptide ligands are produced as transmembrane precursors and their ectodomains are processed by proteolysis, which leads to shedding/secretion of the soluble form of growth factors[27]. There are several ErbB specific ligands, all sharing an EGF-like motif of 45-55 amino acids, including six cysteine residues that interact covalently to form three loops. Depending on the binding specificity conferred by this region, the ligands may be categorized into three groups (Figure 1). The first group includes EGF, amphiregulin and transforming growth factor α (TGF-α) that bind specifically to EGFR/ErbB-1. The second group includes betacellulin (BTC), heparin binding EGF (HB-EGF) and epiregulin that exhibit dual specificity for ErbB-1 and ErbB-4[24]. The third group includes neuregulins (Neu also called Neu differentiation factors or Heregulins). This group is further divided into two sub-groups depending on their ability to bind to ErbB-3 and ErbB-4 or only ErbB-4[28,29]. The second level of regulation depends on the homo-hetero dimerizing partners. Although, a total of nine possible homo- and hetero-dimeric receptor combinations can occur, EGFRs often display preference for their dimeric partners. In this network, ErbB-2 is the most preferred partner and thus plays a co-ordinating role[30,31]. The ErbB-2 containing dimers are known for their high signaling potency as ErbB-2 drastically reduces the rate of ligand receptor dissociation and allows for strong and prolonged activation of the downstream signaling pathways[32,33]. Also, each homo/hetero dimer has been shown to possess unique specificity for the ligand that would stimulate the ErbB activation[34-39]. Within the same heterodimer, the signaling properties of a receptor can be significantly modulated by specific ligand binding. In context of EGFR/ErbB-4 heterodimer, EGF induction is quite fast and recruits both Grb2 and p85 for downstream signaling. On the other hand, addition of NDF stimulation of the receptor heterodimer is relatively slow and recruits only p85 to activate downstream pathways.
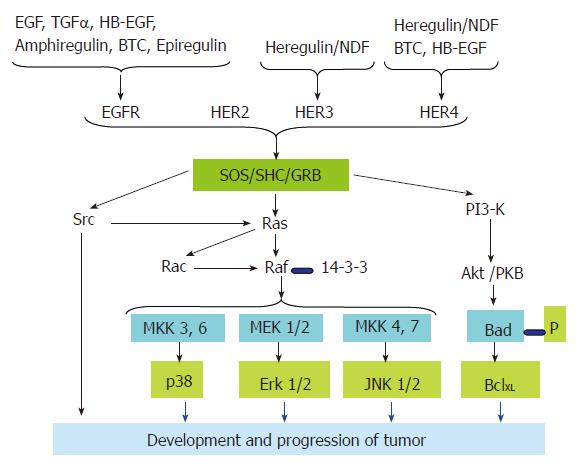
Each of the seven ligands (Figure 2) has a preferred receptor homo/hetero dimer. The ligand-bound receptor homo/hetero dimer, in turn, has a different set of tyrosine phosphorylation sites, which serve as docking sites for specific SH2 containing proteins and recruit different combinations of intracellular signaling molecules[20,40,41]. This is despite the fact that there is a considerable overlap in the molecules recruited to the active receptors. For example, tumor cells that over express EGFR with kinase domain mutations preferentially activate the pro-survival PI3K-Akt pathway and Signal Transducer and Activator of Transcription (STAT) pathways. EGFR has no consensus sequence for the p85 adaptor subunit of PI3K; it couples to this pathway via GAB1. GAB1 in turn binds the Growth Factor Receptor Bound protein 2 (GRB2), which docks at the phosphorylated tyrosine of the kinase domain of the activated EGFR. Similarly, there is no evidence for direct binding of STAT to the EGFR. But it is proposed that this coupling is mediated via tyrosine 1068 and tyrosine 1086 of the EGFR kinase domain[42]. ErbB-2 couples to the Mitogen-Activated Protein Kinase (MAPK) pathway through GRB2, SHC, downstream of Kinase Related (DOK-R)[43] and CRK. PhosphoLipase C γ (PLCγ) binding has recently been implicated in transducing signals by EGFR[44]. Although ErbB-3 is able to bind neuregulins (NRGs), it has impaired kinase activity owing to substitutions in crucial residues in the tyrosine-kinase domain. Therefore, ErbB-3 gets phosphorylated and functions as a signaling entity only when it heterodimerizes with another ErbB receptor[45], ErbB-2 being the preferred partner. ErbB-3 contains six docking sites for the p85 adaptor subunit of PI3K[37,46] and couples very efficiently to this pathway[41].
The ErbB family of RTK plays a crucial role in the development of the cardiovascular system, nervous system, mammary gland, and probably others[47]. Expression patterns of ErbB receptors and their ligands, as well as targeted inactivation of components of the ErbB signaling network highlight the importance of short-range ligand-receptor interactions, especially in mid-gestation processes. ErbB receptors regulate different developmental processes by fine-tuning of apoptosis and proliferation. In light of data from in vivo and in vitro studies, it may be concluded that the primary role of ErbB receptor signaling is to promote growth and proliferation. This is achieved in two ways (1) suppressing apoptotic signals and (2) promoting pro- survival signals. ErbB receptor signaling has the ability to antagonize the activation of extrinsic apoptosis signals by Fas receptor and TNFR-1[48]. On the other hand, ErbB-1 signaling can increase Bcl-XL transcription through STAT-3[49,50] or the mitogen-activated protein kinase kinase (MEK) protein of the mitogenic Ras pathway[51]. Thus, ErbB receptors function at both the transcriptional and post-transcriptional levels to modulate the expression and localization of Bcl family proteins to promote cell survival.
ErbB receptors and clinical studies: In head and neck cancer, the vast majority of tumors are strongly EGFR-positive[10]. Studies have also reported EGFR overexpression in the following cancers: bladder, brain, breast, cervical, uterine, colon, esophageal, glioma, non-small-cell lung cancer (NSCLC), ovarian, pancreatic and renal cell[52-54]. ErbB-2 overexpression, generally attributable to gene amplification, occurs in 25%-30% of breast cancers and correlates with shorter time to relapse and lower overall survival[55]. Overall, about 30% of invasive ductal carcinomas overexpress ErbB-2, but no ErbB overexpression is seen in benign breast disease[56]. ErbB-3 overexpression was linked to several negative prognostic factors, including lymph node involvement, invasion, and patient survival[57]. Involvement of ErbB-4 in breast cancer remains controversial. Some reports indicate that increased ErbB-4 expression or signaling is associated with tumorigenesis. ErbB-4 overexpression has been observed in a variety of cancers, including tumors of the thyroid, breast, and gastrointestinal tract[58-61]. However, other reports indicate that increased ErbB-4 expression or signaling correlates with tumor cell differentiation and reduced tumor aggressiveness. ErbB-4 overexpression in breast tumors is associated with progesterone receptor and estrogen receptor expression and is often a favorable factor in prognosis[62,63]. In one study of common solid human cancers, the loss of ErbB-4 expression was seen in a significant percentage of breast, prostate, and head and neck malignancies[64,65]. These findings raise the intriguing possibility that ErbB-4 is unique to the ErbB family of receptors in that ErbB-4 expression and signaling may couple to reduced tumorigenesis or tumor cell proliferation. However, due to presence of the conflicting evidence, it remains unclear what general or specific roles ErbB-4 plays in differentiation, tumor suppression, or proliferation.
Elevated Co-expression of partners and ligands: In many cases EGFR is co-expressed with other members of the ErbB family, leading to the formation of highly transforming dimers such as EGFR//ErbB-2 and EGFR//ErbB-3. It is also well known that other dimers such as ErbB-2//ErbB-3 play a key role in various cancers such as breast carcinoma. ErbB-3 is frequently overexpressed than ErbB-2 in gastric cancers and was widely detectable, making it a potential marker for postgastrectomy recurrence. It was further demonstrated that ErbB-3 functions as an indispensable ErbB-2 dimerization partner and is required for proliferation of ErbB-2-overexpressing tumor cells in ErbB-2 overexpressing breast tumor cell lines[66]. There was a correlation between ErbB-3 expression and sensitivity to ErbB-2 directed inhibitors. For above reasons, the prognostic significance of any ErbB expression in tumors also depends on the expression of other ErbB members being co-expressed. For instance, in childhood medulloblastoma patients with tumors overexpressing both ErbB-2 and ErbB-4 have worse prognosis than patients with tumors that express either receptor alone[67]. Moreover, the levels of ligands like TGFα also significantly correlated with advanced disease, suggesting that elevated levels of ligand and its receptor might create an autocrine signaling loop[68].
Deregulation of ErbB signaling: Enhanced activity of the receptors resulting from overexpression, coexpression of the receptor, and their ligands, as well as activating mutations, is the hallmark of many human carcinomas[24]. However, the most common is the overexpression of the receptor along with the expression of the respective ligands like TGFα, EGF, amphiregulin and HB-EGF leading to persistent autocrine stimulation. Another common occurrence is the activating mutation in the EGFR extracelluler domain; where the exons 2-/7 are deleted, leading to a persistently active receptor EGFRΔ[69-74], that is persistently active in the absence of a ligand[73,74]. This activating mutation is the hallmark of many tumors that overexpress EGFR. Activation of the EGFR stimulates tumor growth and progression, including the promotion of proliferation, angiogenesis, invasion, metastasis and inhibition of apoptosis[18,75,76]. The emergence of this mutation represents the most aggressive form of the tumor. ErbB receptors appear to have the potential to acquire novel survival signaling pathways when overexpressed and/or mutated within certain tumor cell types. ErbB survival signals can also prevent tumor cells from responding to chemotherapeutic agents[77-81], which often function by activating apoptotic pathways within targeted tumor cells[82,83]. Studies by Yu et al[78,79] have demonstrated that overexpression of ErbB-2 permits the MDA-MB-435 breast cancer cell line to withstand ten times the usual dose of the chemotherapeutic agent Taxol before undergoing apoptosis. Similarly, studies by Nagane et al[77], indicate that expression of a mutated version of ErbB-1 in human malignant gliomas results in tumor cell resistance to the apoptosis-inducing chemotherapeutic drug cisplatin. Enhanced tumorigenicity and escape from cisplatin-induced apoptosis have been correlated with mutant ErbB-1 induced upregulation of the anti-apoptotic Bcl-XL protein.
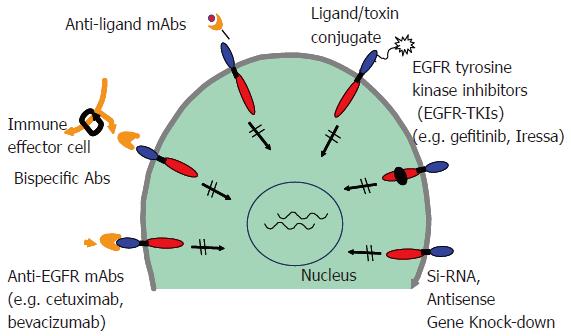
Over the years, different strategies have been developed that inhibit signaling by ErbB receptors. These agents with degrees of receptor specificity include monoclonal antibodies, tyrosine kinase inhibitors, immunotoxin conjugates, antisense oligonucleotides, and bispecific antibodies (Figure 3). Among the classes of agents targeting ErbB receptors, the monoclonal antibodies and tyrosine kinase inhibitors are furthest in development. In general, monoclonal antibodies against the extracellular domain target an individual ErbB receptor, whereas tyrosine kinase inhibitors that compete with the ATP binding site of the intracellular kinase domain[84-92]. The first ErbB-targeted compound approved for use in human malignancies was trastuzumab (Herceptin), a monoclonal antibody directed against ErbB-2. Subsequently, cetuximab (Erbitux), a monoclonal antibody directed against EGFR; and gefitinib (Iressa), an EGFR tyrosine kinase inhibitor were approved by the FDA for treatment of a number of epithelial cancers. Individually, they target a single ErbB receptor (Table 1). However, because members of the ErbB receptor family cooperate in signal transduction and malignant transformation, the efficacy of these agents has been limited. As mentioned earlier, the ErbB signaling is quite diverse due to different combinations of ligand binding and ErbB partners and the cross talk with other pathways. Preclinical studies have shown that cancer cells can escape the antiproliferative activity of an agent directed against one ErbB receptor by overexpressing ligand for another ErbB receptor. For these reasons there is an ever-increasing requirement for the development of therapies that can induce concurrent inhibition of two or more receptors/pathways. Using both in vitro and in vivo models, strategies that employ a dual ErbB approach seem to have a greater antitumor efficacy than agents targeting an individual ErbB receptor[93-99].
| Drug/agent | Type | Target | Company/institution | Stage of development |
| Trastuzumab (Herceptin) | Humanized mAb | ErbB-2 | Genentech/Roche | Approved for ErbB-2 over expressing breast cancer in 1998 |
| Cetuximab (Erbitux/IMC-225) | Human-mouse Chimeric mAb | EGFR | ImClone/Merck KGaA Bristol-Myers Squibb | Approved for colorectal cancer, Phase III trials ongoing for HNSCC and NSCLC in 2004 |
| Panitumumab (ABX-EGF) | Fully Human mAb | EGFR | Abgenix | Phase III trials for renal cancer, prostate cancer, pancreatic cancer, colorectal and NSCLC, esophageal cancer |
| Pertuzumab (Omnitarg/2C4) | Humanized mAb | ErbB-2 | Genentech | Phase II trials for ovarian cancer, breast cancer, prostate cancer and NSCLC |
| Matuzumab (EMD-72000) | Humanized mAb | EGFR | Merck KGaA | Phase II trials ongoing for gynaecological cancer, pancreatic cancer and esophageal cancer |
| Thera CIM (hR3) | Humanized mAb | EGFR | YM Biosciences/CIM | Phase II trials for HNSCC |
| HuMab-Mouse (MDX-447) | Humanized mAb | EGFR | Medarex/Merck KGaA | Preclinical trials ongoing. Phase II trials ongoing for HNSCC |
| Mab 806 | - | EGFR(del 2-7)/ EGFR vIII | Ludwig Institute | Preclinical trials ongoing. |
Different multi-target therapies can be categorized in the following three groups:
Combinations of agents that target individual ErbB receptors: As an ErbB-targeted approach, the combina-tion of a monoclonal antibody together with a small-molecule tyrosine kinase inhibitor uses two agents with different sites of action (Table 2). A phase II clinical study is currently underway to test the dual therapy with trastuzumab (mAb to ErbB-2) and EGFR tyrosine kinase inhibitors. The potential mechanisms of action of trastuzumab combined with an ErbB tyrosine kinase inhibitor include receptor down-regulation, signaling perturbation, angiogenesis inhibition, and antibody-dependent cell-mediated cytotoxicity[54,100,101].
| Drug/agent | Molecular properties | Target Selectivity | Clinical activity in cancer type | Company/Institution | Stage of development |
| Gefitinib (ZD 1839; Iressa) | Reversible TKI | EGFR inhibitor | NSCLC, HNSCC, colorectal cancer and breast cancer | AstraZeneca | Approved for NSCLC in 2003, ongoing Phase III Trials for other cancers |
| Erlotinib (OSI-774; Tarceva) | Reversible TKI | EGFR inhibitor | NSCLC, HNSCC, colorectal cancer and pancreatic cancer | Genentech/ OSI pharmaceuticals | Approved for NSCLC in 2005, ongoing Phase III Trials for other cancers |
| Canertinib (CI-1033)/(PD183805) | Irreversible TKI | EGFR/ErbB-2 inhibitor | NSCLC, HNSCC, Ovarian cancer, breast cancer | Pfizer | Phase II |
| Lapatinib (GW2016) | Reversible TKI | EGFR/ErbB-2 dual inhibitor | Breast cancer | GlaxoSmithkline | Phase III |
| EKB-569 | Irreversible TKI | EGFR inhibitor | Colorectal cancer, cancer, HNSCC, and NSCLC | Wyeth-Ayerst | Phase II |
| AEE788 | TKI | EGFR/ErbB-2/VEGFR | Anti-proliferative effects in tumor cell lines and animal models of cancer | Novartis | Phase I |
| EXEL 7647/EXEL 0999 | TKI | EGFR/ErbB-2/VEGFR | EXELIXIS | Phase I | |
| PKI-166 | Reversible TKI | EGFR/ErbB-2 | Thyroid, Renal, colorectal, HNSCC, and NSCLC | Novartis | Phase I |
| PD 168393 | Irreversible TKI | EGFR | - | Calbiochem | Preclinical |
| AG-1478 | Irreversible TKI | EGFR | - | Calbiochem | Preclinical |
| CGP-59326A | Reversible TKI | EGFR | - | Novartis/ | Preclinical |
| BIBX 1382 | TKI | EGFR | - | Boehringer/Ingelheim | Preclinical |
Single agents that target multiple ErbB receptors: They are either dual or pan-ErbB tyrosine kinase inhibitors (Table 2). Most tyrosine kinase inhibitors compete with the ATP binding site to inhibit phosphorylation. Among these agents, canertinib, targeting EGFR, ErbB-2, and ErbB-4, and lapatinib, targeting EGFR and ErbB-2, are the furthest in development. Lapitinib (GW2016) is a quinazoline derivative that functions as a reversible, dual ErbB tyrosine kinase inhibitor.
Agents that interfere with ErbB receptor interactions: Another approach to inhibiting multiple ErbB receptors is provided by pertuzumab (Omnitarg, 2C4), a monoclonal antibody against ErbB-2 that interferes with ErbB receptor interactions. Pertuzumab binds to a different epitope of the ErbB-2 extracellular domain than trastuzumab[102,103] and seems to differ from trastuzumab in its mechanism of action. Trastuzumab is only active in cells that overexpress ErbB-2, and it does not directly affect the ability of ErbB-2 to function as a coreceptor[104,105]. In contrast, pertuzumab is active in cells that do not overexpress ErbB-2, and it inhibits ligand-mediated signaling by preventing the recruitment of ErbB-2 into ligand/ErbB receptor complexes[105,106]. In this manner, pertuzumab provides a unique opportunity to study the contribution of individual heterodimers to the activation of specific signaling pathways.
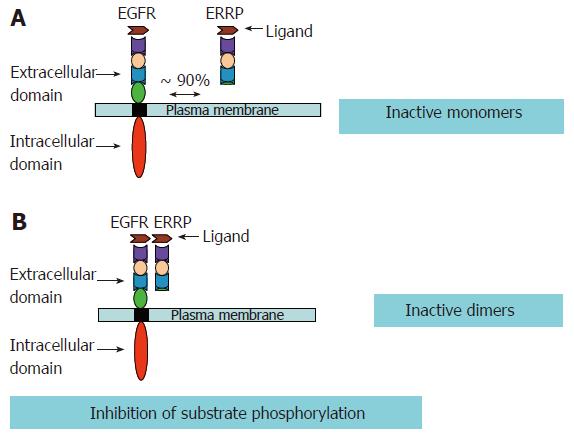
Several strategies have been developed to inhibit EGFR and other members of the ErbB family receptors. As mentioned above, usually more than one ErbB member may be involved in a given type of cancer. Thus, in such a scenario it becomes imperative that strategies are developed to target multiple members of the EGFR family. EGFR Related Protein (ERRP) recently isolated from the rat gastro-duodenal mucosa, was found to be a pan-ErbB inhibitor that targets multiple members of the EGFR family. ERRP, a 53-55 kDa protein, possesses three of the four extracellular domains of EGFR, which are responsible for the ligand binding, and subsequent homo/hetero dimerization of various ErbB members. The ERRP cDNA shows 85%-90% homology to the external domain of EGFR and 50%-60% homology to ErbB-2, ErbB-3 and ErbB-4[107]. Though the human counter part of the rat ERRP remains to be isolated, the rat ERRP shows approximately 85% homology to the extracellular domain of human EGFR. Nevertheless, immunohistological analyses in conjunction with anti-rat ERRP polyclonal antibodies revealed that ERRP expression changes in the gastrointestinal tissues (as discussed below) of the rat and human during carcinogenesis as well as aging. These data suggest the presence of an ERRP like molecule in humans.
Garrett et al[108] has reported that a truncated EGFR, lacking the extracellular domain IV of the receptor that binds EGF and TGF-α with higher affinity than the full-length extracellular domain of EGFR. ERRP, a naturally occurring molecule, lacking most of the extracellular domain IV, also binds TGF-α and is expected to be effective in preferentially binding/sequestering other ligands of ErbBs. In addition, recent biochemical studies utilizing EGFR mutant lacking exons 2-7 of the receptor extracellular domain demonstrated intermolecular inhibitory function of EGFR extracellular domains[109]. Such mutants dimerize with EGFR and cause phosphorylation of wild type EGFR in the absence of ligands[108]. In the light of these findings and the available experimental data, it is suggested that loss of such subdomains is associated with constitutive activation of EGFR, while the truncated EGFRs containing only the extracellular domains serve as repressors of EGFR functions. Ectopic expression of recombinant ERRP causes increased binding/sequestration of EGFR ligand(s) resulting in decreased availability of the ligand(s) for binding to and activation of EGFR with a subsequent attenuation of EGFR signaling pathways. A schematic representation of our hypothesis is depicted in Figure 4. This is further supported by experimental data. Marcinaik et al, have demonstrated that exposure of HCT-116 cells to recombinant ERRP and TGF-α results in the formation of heterodimers of EGFR and ERRP with molecular weight of about 220 kDa[110]. TGF-α also induces the formation of a 340 kDa homodimer of EGFR[110].
ERRP and carcinogenesis: In order to investigate the correlation between expression of ERRP protein and carcinogenesis, benign and neoplastic tissues from the pancreas, liver and gastric and colonic mucosa were examined. Expression of ERRP was found to be high in benign human colonic and gastric mucosa as well as in the liver and pancreas but low in the respective carcinomas of these tissues[110-118]. It was further observed, that in colorectal and pancreatic cancers, expression of ERRP decreases progressively with decrease in differentiation[112,113]. In the colon, ERRP expression became more attenuated in polyps with increasing grades of dysplasia. Expression of EGFR inversely related to ERRP in representative samples of normal and neoplastic colon[112]. In light of these observations it is speculated that the loss of ERRP may partly be responsible for induction of EGFR, and may play a causative role in the development of carcinogenesis.
To further evaluate the role of ERRP in the development of cancer, colonic mucosa from rats treated with the colonic carcinogen dimethylhydrazine (DMH) or vehicle (controls) was analyzed for ERRP and EGFR expression. ERRP expression was significantly lost in early stages of chemically induced colon cancer[113]. These observations suggest a potential role for ERRP in the development and progression of carcinogenesis. Supporting data for this inference was further obtained when EGFR and ERRP expression was analyzed in the gastric mucosa of rats during advancing age. In the rat model, it is generally accepted that aging is associated with increased proliferation of colonic mucosal cells that frequently involves the enhanced activity and expression of EGFRs. It has been postulated that age-associated enhanced EGFR activity is due in part, to loss of ERRP which acts as a negative modulator of EGFRs. Immunohistochemical studies revealed that ERRP expression decreased in the gastric mucosa with aging in contrast to increasing expression of EGFR[117].
ERRP as a potential pan-ErbB inhibitor: Our hypothesis that ERRP could be a potential therapeutic agent for epithelial cancers came from initial observation that transfection of ERRP cDNA into colon cancer cells inhibited proliferation in the matrix-dependent and -independent systems. This inhibition was associated with attenuation of tyrosine phosphorylation and tyrosine kinase activity of EGFR[107]. The similar phenomenon was also noted in prostate cancer cells (PC-3) following transfection with ERRP cDNA (unpublished data).
To further determine the therapeutic potential of ERRP, we generated and purified recombinant ERRP using the drosophila expression system (Invitrogen)[110]. The affinity-purified recombinant protein was utilized to investigate its effects on the growth of colon and other epithelial cancer cells in vitro and in vivo. ERRP was reported to inhibit proliferation of colon, and prostate cancer cell lines in a dose-dependent manner. These changes included the inhibition of EGFR signaling and attenuation of downstream signaling involving activation of Akt, mitogen activated protein kinase (MAPK) and nuclear factor (NF-κB)[110,118]. The similar effects were observed in other studies involving non-small cell lung cancer (NSCLC) cell lines, breast cancer and pancreatic cancer[119-121]. These epithelial cancer cells express varying levels of EGFR and other ErbB receptors. Thus, a pan-ErbB inhibitory role of ERRP was suggested. The results from efficacy trials using SCID mice have further shown tumor regression in some and arrested growth in other animals[110,122]. ERRP was effective at dose levels of 25 μg/kg and could be tolerated up to 100 μg/kg without producing signs of toxicity in the SCID mice. Although the withdrawal of ERRP administration leads to reappearance of the tumors, the growth rate was significantly reduced afterwards.
Immunohistochemical analysis of ERRP-treated tumors revealed that ERRP-induced inhibition of growth, accompanied by a marked stimulation in expression of active caspase-3 and reductions in phosphorylated (activated) forms of Akt and ERKs[122]. This suggests that ERRP inhibits tumor growth, in part by inducing apoptosis, which was further supported by in vitro experiments[122]. ERRP induced apoptosis follows arrest of the cells in the Go/G1 phase of the cell cycle[122]. In order to test the therapeutic value of ERRP, its immuno-reactivity in humans has been further investigated. In vitro studies in human peripheral blood lymphocytes have shown marginal activation of the immune response (unpublished data). In an attempt to understand the mechanisms underlying ERRP induced growth inhibition and apoptosis of the tumors, further in vitro studies have been conducted. Rishi et al observed that ERRP inhibits the processes of cell invasion and blood vessel formation by colon cancer cells. Further, ERRP also inhibited tubule formation by aortic endothelial cells and invasion by colon cancer cells through matrigel[123]. This finding suggests that ERRP inhibits different processes of invasion, metastasis and angiogenesis that are critical in the progression of carcinogenesis. Thus, ERRP, a novel pan-ErbB inhibitor, has a potential utility as a therapeutic for a wide variety of epithelial cancers.
Limited success of chemotherapy/adjuvant therapies in cancer treatment has necessitated the development of novel targeted therapies. A number of pharmacologic as well as biologic agents have been developed that target specific aspects of intracellular processes and interfere with development and progression of tumors. In light of the fact that the ErbB family of receptors plays an important role in epithelial cancers, a number of inhibitors that target these receptors have been developed. In particular, the monoclonal antibodies Cetuximab and Herceptin, as well as small molecule inhibitors gefitinib and tarceva have shown some promise in the treatment of cancer. However, these agents target EGFR or Her2 but not both. Since most cancers over-express multiple ErbBs, targeting a single receptor often leads to activation and signaling by other ErbBs resulting in development of resistance. In this regard, the agents/inhibitors that target multiple ErbBs are anticipated to display superior efficacy. In our pursuit to develop a pan-ErbB inhibitor, we have recently isolated and characterized ERRP. ERRP have been shown to inhibit proliferation and induce apoptosis of the prostate, colon, gastric, pancreatic, breast and lung cancer cells in in vitro models. ERRP attenuates the basal and ligand induced (TGF-α, HB-EGF and heregulin) activation of EGFR and Her-2 in a variety of epithelial cancers, suggesting a pan-ErbB inhibitory property. Recombinant ERRP also inhibits growth of colon and pancreatic cancer cell-derived xenografts in SCID mice. ERRP, is unlikely to initiate an immune response as it is an endogenous, secretory protein. ERRP thus is a potential therapeutic for a wide variety of epithelial cancers.
| 1. | Schottenfeld D. Gastrointestinal cancer: epidemiology. Gastrointestinal Oncology: Principles and Practice. Philadelphia: Lippincott, Williams & Wilkins 2002; 3-24. |
| 2. | Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999;49:33-64, 1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1285] [Cited by in RCA: 1257] [Article Influence: 46.6] [Reference Citation Analysis (1)] |
| 3. | Levin B, Brooks D, Smith RA, Stone A. Emerging technologies in screening for colorectal cancer: CT colonography, immunochemical fecal occult blood tests, and stool screening using molecular markers. CA Cancer J Clin. 2003;53:44-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Burch PA, Block M, Schroeder G, Kugler JW, Sargent DJ, Braich TA, Mailliard JA, Michalak JC, Hatfield AK, Wright K. Phase III evaluation of octreotide versus chemotherapy with 5-fluorouracil or 5-fluorouracil plus leucovorin in advanced exocrine pancreatic cancer: a North Central Cancer Treatment Group study. Clin Cancer Res. 2000;6:3486-3492. [PubMed] |
| 5. | Risch HA. Etiology of pancreatic cancer, with a hypothesis concerning the role of N-nitroso compounds and excess gastric acidity. J Natl Cancer Inst. 2003;95:948-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 121] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Schally AV, Szepeshazi K, Nagy A, Comaru-Schally AM, Halmos G. New approaches to therapy of cancers of the stomach, colon and pancreas based on peptide analogs. Cell Mol Life Sci. 2004;61:1042-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Gill S, Thomas RR, Goldberg RM. New targeted therapies in gastrointestinal cancers. Curr Treat Options Oncol. 2003;4:393-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Berlin J. New directions in the treatment of advanced colorectal cancer. Oncology (Williston Park). 2001;15:27-30. [PubMed] |
| 9. | Herbst RS, Shin DM. Monoclonal antibodies to target epidermal growth factor receptor-positive tumors: a new paradigm for cancer therapy. Cancer. 2002;94:1593-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 357] [Article Influence: 14.9] [Reference Citation Analysis (1)] |
| 10. | Mendelsohn J. The epidermal growth factor receptor as a target for cancer therapy. Endocr Relat Cancer. 2001;8:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 229] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 11. | Carpenter G. The EGF receptor: a nexus for trafficking and signaling. Bioessays. 2000;22:697-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Riese DJ 2nd, Stern DF. Specificity within the EGF family/ErbB receptor family signaling network. Bioessays. 1998;20:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 13. | Aroian RV, Sternberg PW. Multiple functions of let-23, a Caenorhabditis elegans receptor tyrosine kinase gene required for vulval induction. Genetics. 1991;128:251-267. [PubMed] |
| 14. | Price JV, Clifford RJ, Schüpbach T. The maternal ventralizing locus torpedo is allelic to faint little ball, an embryonic lethal, and encodes the Drosophila EGF receptor homolog. Cell. 1989;56:1085-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 216] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Schejter ED, Shilo BZ. The Drosophila EGF receptor homolog (DER) gene is allelic to faint little ball, a locus essential for embryonic development. Cell. 1989;56:1093-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 177] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Sibilia M, Wagner EF. Strain-dependent epithelial defects in mice lacking the EGF receptor. Science. 1995;269:234-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 724] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 17. | Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris RC. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995;269:230-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1070] [Cited by in RCA: 1051] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 18. | Wells A. EGF receptor. Int J Biochem Cell Biol. 1999;31:637-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 720] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 19. | Bogdan S, Klämbt C. Epidermal growth factor receptor signaling. Curr Biol. 2001;11:R292-R295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 163] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4920] [Cited by in RCA: 5198] [Article Influence: 207.9] [Reference Citation Analysis (1)] |
| 21. | Qu CK. Role of the SHP-2 tyrosine phosphatase in cytokine-induced signaling and cellular response. Biochim Biophys Acta. 2002;1592:297-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 104] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Carpenter G, Cohen S. Epidermal growth factor. J Biol Chem. 1990;265:7709-7712. [PubMed] |
| 23. | Cohen RB. Epidermal growth factor receptor as a therapeutic target in colorectal cancer. Clin Colorectal Cancer. 2003;2:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 101] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 24. | Yarden Y. The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur J Cancer. 2001;37 Suppl 4:S3-S8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1091] [Cited by in RCA: 1143] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 25. | Schaeffer L, Duclert N, Huchet-Dymanus M, Changeux JP. Implication of a multisubunit Ets-related transcription factor in synaptic expression of the nicotinic acetylcholine receptor. EMBO J. 1998;17:3078-3090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 123] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Daly RJ. Take your partners, please--signal diversification by the erbB family of receptor tyrosine kinases. Growth Factors. 1999;16:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Massagué J, Pandiella A. Membrane-anchored growth factors. Annu Rev Biochem. 1993;62:515-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 474] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 28. | Zhang D, Sliwkowski MX, Mark M, Frantz G, Akita R, Sun Y, Hillan K, Crowley C, Brush J, Godowski PJ. Neuregulin-3 (NRG3): a novel neural tissue-enriched protein that binds and activates ErbB4. Proc Natl Acad Sci USA. 1997;94:9562-9567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 283] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 29. | Harari D, Tzahar E, Romano J, Shelly M, Pierce JH, Andrews GC, Yarden Y. Neuregulin-4: a novel growth factor that acts through the ErbB-4 receptor tyrosine kinase. Oncogene. 1999;18:2681-2689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 208] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 30. | Tzahar E, Waterman H, Chen X, Levkowitz G, Karunagaran D, Lavi S, Ratzkin BJ, Yarden Y. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol. 1996;16:5276-5287. [PubMed] |
| 31. | Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997;16:1647-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1132] [Cited by in RCA: 1149] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 32. | Beerli RR, Graus-Porta D, Woods-Cook K, Chen X, Yarden Y, Hynes NE. Neu differentiation factor activation of ErbB-3 and ErbB-4 is cell specific and displays a differential requirement for ErbB-2. Mol Cell Biol. 1995;15:6496-6505. [PubMed] |
| 33. | Graus-Porta D, Beerli RR, Hynes NE. Single-chain antibody-mediated intracellular retention of ErbB-2 impairs Neu differentiation factor and epidermal growth factor signaling. Mol Cell Biol. 1995;15:1182-1191. [PubMed] |
| 34. | Earp HS, Dawson TL, Li X, Yu H. Heterodimerization and functional interaction between EGF receptor family members: a new signaling paradigm with implications for breast cancer research. Breast Cancer Res Treat. 1995;35:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 263] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 35. | Peles E, Levy RB, Or E, Ullrich A, Yarden Y. Oncogenic forms of the neu/HER2 tyrosine kinase are permanently coupled to phospholipase C gamma. EMBO J. 1991;10:2077-2086. [PubMed] |
| 36. | Batzer AG, Rotin D, Ureña JM, Skolnik EY, Schlessinger J. Hierarchy of binding sites for Grb2 and Shc on the epidermal growth factor receptor. Mol Cell Biol. 1994;14:5192-5201. [PubMed] |
| 37. | Fedi P, Pierce JH, di Fiore PP, Kraus MH. Efficient coupling with phosphatidylinositol 3-kinase, but not phospholipase C gamma or GTPase-activating protein, distinguishes ErbB-3 signaling from that of other ErbB/EGFR family members. Mol Cell Biol. 1994;14:492-500. [PubMed] |
| 38. | Muthuswamy SK, Muller WJ. Direct and specific interaction of c-Src with Neu is involved in signaling by the epidermal growth factor receptor. Oncogene. 1995;11:271-279. [PubMed] |
| 39. | Ricci A, Lanfrancone L, Chiari R, Belardo G, Pertica C, Natali PG, Pelicci PG, Segatto O. Analysis of protein-protein interactions involved in the activation of the Shc/Grb-2 pathway by the ErbB-2 kinase. Oncogene. 1995;11:1519-1529. [PubMed] |
| 40. | Di Fiore PP, Segatto O, Taylor WG, Aaronson SA, Pierce JH. EGF receptor and erbB-2 tyrosine kinase domains confer cell specificity for mitogenic signaling. Science. 1990;248:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 103] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159-3167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1802] [Cited by in RCA: 1861] [Article Influence: 71.6] [Reference Citation Analysis (1)] |
| 42. | Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res. 2003;284:31-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1083] [Cited by in RCA: 1137] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 43. | Dankort D, Jeyabalan N, Jones N, Dumont DJ, Muller WJ. Multiple ErbB-2/Neu Phosphorylation Sites Mediate Transformation through Distinct Effector Proteins. J Biol Chem. 2001;276:38921-38928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 44. | Marone R, Hess D, Dankort D, Muller WJ, Hynes NE, Badache A. Memo mediates ErbB2-driven cell motility. Nat Cell Biol. 2004;6:515-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 45. | Kim HH, Vijapurkar U, Hellyer NJ, Bravo D, Koland JG. Signal transduction by epidermal growth factor and heregulin via the kinase-deficient ErbB3 protein. Biochem J. 1998;334:189-195. [PubMed] |
| 46. | Prigent SA, Gullick WJ. Identification of c-erbB-3 binding sites for phosphatidylinositol 3'-kinase and SHC using an EGF receptor/c-erbB-3 chimera. EMBO J. 1994;13:2831-2841. [PubMed] |
| 47. | Casalini P, Iorio MV, Galmozzi E, Ménard S. Role of HER receptors family in development and differentiation. J Cell Physiol. 2004;200:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 167] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 48. | Gibson S, Tu S, Oyer R, Anderson SM, Johnson GL. Epidermal growth factor protects epithelial cells against Fas-induced apoptosis. Requirement for Akt activation. J Biol Chem. 1999;274:17612-17618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 193] [Article Influence: 7.1] [Reference Citation Analysis (5)] |
| 49. | Grandis JR, Drenning SD, Zeng Q, Watkins SC, Melhem MF, Endo S, Johnson DE, Huang L, He Y, Kim JD. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proc Natl Acad Sci USA. 2000;97:4227-4232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 486] [Article Influence: 18.7] [Reference Citation Analysis (13)] |
| 50. | Rubin Grandis J, Zeng Q, Drenning SD. Epidermal growth factor receptor--mediated stat3 signaling blocks apoptosis in head and neck cancer. Laryngoscope. 2000;110:868-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 51. | Jost M, Huggett TM, Kari C, Boise LH, Rodeck U. Epidermal growth factor receptor-dependent control of keratinocyte survival and Bcl-xL expression through a MEK-dependent pathway. J Biol Chem. 2001;276:6320-6326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 109] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 52. | Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37 Suppl 4:S9-S15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1424] [Cited by in RCA: 1496] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 53. | Herbst RS. Targeted therapy in non-small-cell lung cancer. Oncology (Williston Park). 2002;16:19-24. [PubMed] |
| 54. | Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. 2003;21:2787-2799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 944] [Cited by in RCA: 923] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 55. | Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8336] [Cited by in RCA: 8629] [Article Influence: 221.3] [Reference Citation Analysis (0)] |
| 56. | Hynes NE, Stern DF. The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim Biophys Acta. 1994;1198:165-184. [PubMed] |
| 57. | Shintani S, Funayama T, Yoshihama Y, Alcalde RE, Matsumura T. Prognostic significance of ERBB3 overexpression in oral squamous cell carcinoma. Cancer Lett. 1995;95:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Haugen DR, Akslen LA, Varhaug JE, Lillehaug JR. Expression of c-erbB-3 and c-erbB-4 proteins in papillary thyroid carcinomas. Cancer Res. 1996;56:1184-1188. [PubMed] |
| 59. | Kew TY, Bell JA, Pinder SE, Denley H, Srinivasan R, Gullick WJ, Nicholson RI, Blamey RW, Ellis IO. c-erbB-4 protein expression in human breast cancer. Br J Cancer. 2000;82:1163-1170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 60. | Kataoka H, Joh T, Kasugai K, Okayama N, Moriyama A, Asai K, Kato T. Expression of mRNA for heregulin and its receptor, ErbB-3 and ErbB-4, in human upper gastrointestinal mucosa. Life Sci. 1998;63:553-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 61. | Bacus SS, Zelnick CR, Plowman G, Yarden Y. Expression of the erbB-2 family of growth factor receptors and their ligands in breast cancers. Implication for tumor biology and clinical behavior. Am J Clin Pathol. 1994;102:S13-S24. [PubMed] |
| 62. | Knowlden JM, Gee JM, Seery LT, Farrow L, Gullick WJ, Ellis IO, Blamey RW, Robertson JF, Nicholson RI. c-erbB3 and c-erbB4 expression is a feature of the endocrine responsive phenotype in clinical breast cancer. Oncogene. 1998;17:1949-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 63. | Bacus SS, Chin D, Yarden Y, Zelnick CR, Stern DF. Type 1 receptor tyrosine kinases are differentially phosphorylated in mammary carcinoma and differentially associated with steroid receptors. Am J Pathol. 1996;148:549-558. [PubMed] |
| 64. | Lyne JC, Melhem MF, Finley GG, Wen D, Liu N, Deng DH, Salup R. Tissue expression of neu differentiation factor/heregulin and its receptor complex in prostate cancer and its biologic effects on prostate cancer cells in vitro. Cancer J Sci Am. 1997;3:21-30. [PubMed] |
| 65. | Srinivasan R, Poulsom R, Hurst HC, Gullick WJ. Expression of the c-erbB-4/HER4 protein and mRNA in normal human fetal and adult tissues and in a survey of nine solid tumour types. J Pathol. 1998;185:236-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 66. | Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF 3rd, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci USA. 2003;100:8933-8938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 771] [Article Influence: 33.5] [Reference Citation Analysis (1)] |
| 67. | Gilbertson RJ, Perry RH, Kelly PJ, Pearson AD, Lunec J. Prognostic significance of HER2 and HER4 coexpression in childhood medulloblastoma. Cancer Res. 1997;57:3272-3280. [PubMed] |
| 68. | Saeki T, Salomon DS, Johnson GR, Gullick WJ, Mandai K, Yamagami K, Moriwaki S, Tanada M, Takashima S, Tahara E. Association of epidermal growth factor-related peptides and type I receptor tyrosine kinase receptors with prognosis of human colorectal carcinomas. Jpn J Clin Oncol. 1995;25:240-249. [PubMed] |
| 69. | Alroy I, Yarden Y. The ErbB signaling network in embryogenesis and oncogenesis: signal diversification through combinatorial ligand-receptor interactions. FEBS Lett. 1997;410:83-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 518] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 70. | Yaish P, Gazit A, Gilon C, Levitzki A. Blocking of EGF-dependent cell proliferation by EGF receptor kinase inhibitors. Science. 1988;242:933-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 431] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 71. | Levitzki A. Protein tyrosine kinase inhibitors as novel therapeutic agents. Pharmacol Ther. 1999;82:231-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 151] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 72. | Baselga J. Why the epidermal growth factor receptor The rationale for cancer therapy. Oncologist. 2002;7 Suppl 4:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 348] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 73. | Huang HS, Nagane M, Klingbeil CK, Lin H, Nishikawa R, Ji XD, Huang CM, Gill GN, Wiley HS, Cavenee WK. The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J Biol Chem. 1997;272:2927-2935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 439] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 74. | Wikstrand CJ, Reist CJ, Archer GE, Zalutsky MR, Bigner DD. The class III variant of the epidermal growth factor receptor (EGFRvIII): characterization and utilization as an immunotherapeutic target. J Neurovirol. 1998;4:148-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 116] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 75. | Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1911] [Cited by in RCA: 1929] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 76. | Woodburn JR. The epidermal growth factor receptor and its inhibition in cancer therapy. Pharmacol Ther. 1999;82:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 595] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 77. | Nagane M, Levitzki A, Gazit A, Cavenee WK, Huang HJ. Drug resistance of human glioblastoma cells conferred by a tumor-specific mutant epidermal growth factor receptor through modulation of Bcl-XL and caspase-3-like proteases. Proc Natl Acad Sci USA. 1998;95:5724-5729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 255] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 78. | Yu D, Jing T, Liu B, Yao J, Tan M, McDonnell TJ, Hung MC. Overexpression of ErbB2 blocks Taxol-induced apoptosis by upregulation of p21Cip1, which inhibits p34Cdc2 kinase. Mol Cell. 1998;2:581-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 252] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 79. | Yu D, Liu B, Jing T, Sun D, Price JE, Singletary SE, Ibrahim N, Hortobagyi GN, Hung MC. Overexpression of both p185c-erbB2 and p170mdr-1 renders breast cancer cells highly resistant to taxol. Oncogene. 1998;16:2087-2094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 85] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 80. | Liu B, Fang M, Lu Y, Mendelsohn J, Fan Z. Fibroblast growth factor and insulin-like growth factor differentially modulate the apoptosis and G1 arrest induced by anti-epidermal growth factor receptor monoclonal antibody. Oncogene. 2001;20:1913-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 81. | Liu B, Fang M, Lu Y, Lu Y, Mills GB, Fan Z. Involvement of JNK-mediated pathway in EGF-mediated protection against paclitaxel-induced apoptosis in SiHa human cervical cancer cells. Br J Cancer. 2001;85:303-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 82. | Mesner PW Jr, Budihardjo II, Kaufmann SH. Chemotherapy-induced apoptosis. Adv Pharmacol. 1997;41:461-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 106] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 83. | Kaufmann SH, Earnshaw WC. Induction of apoptosis by cancer chemotherapy. Exp Cell Res. 2000;256:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 893] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 84. | Wakeling AE, Guy SP, Woodburn JR, Ashton SE, Curry BJ, Barker AJ, Gibson KH. ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 2002;62:5749-5754. [PubMed] |
| 85. | Moyer JD, Barbacci EG, Iwata KK, Arnold L, Boman B, Cunningham A, DiOrio C, Doty J, Morin MJ, Moyer MP. Induction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res. 1997;57:4838-4848. [PubMed] |
| 86. | Barbacci EG, Pustilnik LR, Rossi AM, Emerson E, Miller PE, Boscoe BP, Cox ED, Iwata KK, Jani JP, Provoncha K. The biological and biochemical effects of CP-654577, a selective erbB2 kinase inhibitor, on human breast cancer cells. Cancer Res. 2003;63:4450-4459. [PubMed] |
| 87. | Wissner A, Brawner Floyd MB, Rabindran SK, Nilakantan R, Greenberger LM, Shen R, Wang YF, Tsou HR. Syntheses and EGFR and HER-2 kinase inhibitory activities of 4-anilinoquinoline-3-carbonitriles: analogues of three important 4-anilinoquinazolines currently undergoing clinical evaluation as therapeutic antitumor agents. Bioorg Med Chem Lett. 2002;12:2893-2897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 88. | Jani JP, Barbacci G, Bhattacharya S. CP-724714, a novel erbB2 receptor tyrosine kinase inhibitor for cancer therapy. Proc AACR. 2004;45:4637. |
| 89. | Naito K, Matsutani E, Tamura T. TAK-165, a selective inhibitor of HER2 tyrosine kinase. I. Nature of tyrosine kinase inhibition and selective antitumor activity in vivo and in vitro. Proc AACR. 2002;43:3897. |
| 90. | Allen LF, Eiseman IA, Fry DW, Lenehan PF. CI-1033, an irreversible pan-erbB receptor inhibitor and its potential application for the treatment of breast cancer. Semin Oncol. 2003;30:65-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 91. | Rusnak DW, Lackey K, Affleck K, Wood ER, Alligood KJ, Rhodes N, Keith BR, Murray DM, Knight WB, Mullin RJ. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther. 2001;1:85-94. [PubMed] |
| 92. | Traxler P, Bold G, Buchdunger E, Caravatti G, Furet P, Manley P, O'Reilly T, Wood J, Zimmermann J. Tyrosine kinase inhibitors: from rational design to clinical trials. Med Res Rev. 2001;21:499-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 227] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 93. | Moulder SL, Yakes FM, Muthuswamy SK, Bianco R, Simpson JF, Arteaga CL. Epidermal growth factor receptor (HER1) tyrosine kinase inhibitor ZD1839 (Iressa) inhibits HER2/neu (erbB2)-overexpressing breast cancer cells in vitro and in vivo. Cancer Res. 2001;61:8887-8895. [PubMed] |
| 94. | Moasser MM, Basso A, Averbuch SD, Rosen N. The tyrosine kinase inhibitor ZD1839 ("Iressa") inhibits HER2-driven signaling and suppresses the growth of HER2-overexpressing tumor cells. Cancer Res. 2001;61:7184-7188. [PubMed] |
| 95. | Normanno N, Campiglio M, De LA, Somenzi G, Maiello M, Ciardiello F, Gianni L, Salomon DS, Menard S. Cooperative inhibitory effect of ZD1839 (Iressa) in combination with trastuzumab (Herceptin) on human breast cancer cell growth. Ann Oncol. 2002;13:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 186] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 96. | Finn RS, Wilson CA, Chen J. Biologic effects of CP-724,714, a selective HER-2/neu kinase inhibitor, on human breast cancer cells with variable expression of EGFR and HER-2. Proc AAC. 2004;45:4556. |
| 97. | Ye D, Mendelsohn J, Fan Z. Augmentation of a humanized anti-HER2 mAb 4D5 induced growth inhibition by a human-mouse chimeric anti-EGF receptor mAb C225. Oncogene. 1999;18:731-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 96] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 98. | Ritter CA, Bianco R, Dugger T. HER2-overexpressing human breast cancer cells selected for Herceptin (trastuzumab) resistance in vivo retain HER2 gene amplification and overexpress HER1/EGF receptor ligands. Proc AACR. 2003;44:R4869. |
| 99. | Konecny G, Finn R, Venkatesan N. The novel dual kinase inhibitor GW572016 is particularly active in HER2-positive and trastuzumab- conditioned breast cancer cells. Breast Cancer Res Treat. 2003;82:S171. |
| 100. | Sliwkowski MX, Lofgren JA, Lewis GD, Hotaling TE, Fendly BM, Fox JA. Nonclinical studies addressing the mechanism of action of trastuzumab (Herceptin). Semin Oncol. 1999;26:60-70. [PubMed] |
| 101. | Citri A, Alroy I, Lavi S, Rubin C, Xu W, Grammatikakis N, Patterson C, Neckers L, Fry DW, Yarden Y. Drug-induced ubiquitylation and degradation of ErbB receptor tyrosine kinases: implications for cancer therapy. EMBO J. 2002;21:2407-2417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 176] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 102. | Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW Jr, Leahy DJ. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421:756-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1185] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 103. | Fendly BM, Winget M, Hudziak RM, Lipari MT, Napier MA, Ullrich A. Characterization of murine monoclonal antibodies reactive to either the human epidermal growth factor receptor or HER2/neu gene product. Cancer Res. 1990;50:1550-1558. [PubMed] |
| 104. | Albanell J, Codony J, Rovira A, Mellado B, Gascón P. Mechanism of action of anti-HER2 monoclonal antibodies: scientific update on trastuzumab and 2C4. Adv Exp Med Biol. 2003;532:253-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 145] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 105. | Agus DB, Akita RW, Fox WD, Lewis GD, Higgins B, Pisacane PI, Lofgren JA, Tindell C, Evans DP, Maiese K. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 666] [Cited by in RCA: 672] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 106. | Mendoza N, Phillips GL, Silva J, Schwall R, Wickramasinghe D. Inhibition of ligand-mediated HER2 activation in androgen-independent prostate cancer. Cancer Res. 2002;62:5485-5488. [PubMed] |
| 107. | Yu Y, Rishi AK, Turner JR, Liu D, Black ED, Moshier JA, Majumdar AP. Cloning of a novel EGFR-related peptide: a putative negative regulator of EGFR. Am J Physiol Cell Physiol. 2001;280:C1083-C1089. [PubMed] |
| 108. | Garrett TP, McKern NM, Lou M, Elleman TC, Adams TE, Lovrecz GO, Zhu HJ, Walker F, Frenkel MJ, Hoyne PA. Crystal structure of a truncated epidermal growth factor receptor extracellular domain bound to transforming growth factor alpha. Cell. 2002;110:763-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 584] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 109. | Zhu HJ, Iaria J, Orchard S, Walker F, Burgess AW. Epidermal growth factor receptor: association of extracellular domain negatively regulates intracellular kinase activation in the absence of ligand. Growth Factors. 2003;21:15-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 110. | Marciniak DJ, Moragoda L, Mohammad RM, Yu Y, Nagothu KK, Aboukameel A, Sarkar FH, Adsay VN, Rishi AK, Majumdar AP. Epidermal growth factor receptor-related protein: a potential therapeutic agent for colorectal cancer. Gastroenterology. 2003;124:1337-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 111. | Majumdar AP. Therapeutic potential of EGFR-related protein, a universal EGFR family antagonist. Future Oncol. 2005;1:235-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 112. | Feng J, Adsay NV, Kruger M, Ellis KL, Nagothu K, Majumdar AP, Sarkar FH. Expression of ERRP in normal and neoplastic pancreata and its relationship to clinicopathologic parameters in pancreatic adenocarcinoma. Pancreas. 2002;25:342-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 113. | Schmelz EM, Levi E, Du J, Xu H, Majumdar AP. Age-related loss of EGF-receptor related protein (ERRP) in the aging colon is a potential risk factor for colon cancer. Mech Ageing Dev. 2004;125:917-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 114. | Jaszewski R, Levi E, Sochacki P, Frank J, Kucuk O, Axelrod BN, Majumdar AP. Expression of epidermal growth factor-receptor related protein (ERRP) in human colorectal carcinogenesis. Cancer Lett. 2004;213:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 115. | Moon WS, Chang KJ, Majumdar AP, Tarnawski AS. Reduced expression of epidermal growth factor receptor-related protein in hepatocellular carcinoma: implications for cancer growth. Digestion. 2004;69:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 116. | Moon WS, Tarnawski AS, Chai J, Yang JT, Majumdar AP. Reduced expression of epidermal growth factor receptor related protein in gastric cancer. Gut. 2005;54:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 117. | Majumdar AP, Du J, Hatfield JS, Levi E, Adsay V, Schmelz EM, Nagothu KK, Jaszewski R, Kucuk O, Sarkar FH. Expression of EGF-receptor related protein (ERRP) decreases in gastric mucosa during aging and carcinogenesis. Dig Dis Sci. 2003;48:856-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 118. | Marciniak DJ, Rishi AK, Sarkar FH, Majumdar AP. Epidermal growth factor receptor-related peptide inhibits growth of PC-3 prostate cancer cells. Mol Cancer Ther. 2004;3:1615-1621. [PubMed] |
| 119. | Xu H, Yu Y, Marciniak D, Rishi AK, Sarkar FH, Kucuk O, Majumdar AP. Epidermal growth factor receptor (EGFR)-related protein inhibits multiple members of the EGFR family in colon and breast cancer cells. Mol Cancer Ther. 2005;4:435-442. [PubMed] |
| 120. | Zhang Y, Banerjee S, Wang Z, Xu H, Zhang L, Mohammad R, Aboukameel A, Adsay NV, Che M, Abbruzzese JL. Antitumor activity of epidermal growth factor receptor-related protein is mediated by inactivation of ErbB receptors and nuclear factor-kappaB in pancreatic cancer. Cancer Res. 2006;66:1025-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 121. | Zhang Y, Banerjee S, Wang ZW, Marciniak DJ, Majumdar AP, Sarkar FH. Epidermal growth factor receptor-related protein inhibits cell growth and induces apoptosis of BxPC3 pancreatic cancer cells. Cancer Res. 2005;65:3877-3882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 122. | Levi E, Mohammad R, Kodali U, Marciniak D, Reddy S, Aboukameel A, Sarkar FH, Kucuk O, Rishi AK, Majumdar AP. EGF-receptor related protein causes cell cycle arrest and induces apoptosis of colon cancer cells in vitro and in vivo. Anticancer Res. 2004;24:2885-2891. [PubMed] |
| 123. | Rishi AK, Parikh R, Wali A, Durko L, Zhang L, Yu Y, Majumdar AP. EGF receptor-related protein (ERRP) inhibits invasion of colon cancer cells and tubule formation by endothelial cells in vitro. Anticancer Res. 2006;26:1029-1037. [PubMed] |
S- Editor Liu Y L- Editor Rampone B E- Editor Bi L