Published online Dec 14, 2006. doi: 10.3748/wjg.v12.i46.7428
Revised: August 5, 2006
Accepted: August 11, 2006
Published online: December 14, 2006
Cell proliferation is an important process in life for growth of normal and cancer cells. The signal transduction pathways activated during this process are strictly regulated. This editorial focuses on the role of nicotine, a mitogen, in the induction of signaling pathways resulting in proliferation of pancreatic tumor cells and compares these events with those in normal acinar cells isolated from the rat pancreas. The data shows striking similarities between these two cellular systems. In addition, the editorial reviews very recent literature of the contribution of MAPK signaling in cell lines associated with human diseases. A prospective cellular model of nicotine induced activation of MAPK cascade is presented.
- Citation: Chowdhury P, Udupa KB. Nicotine as a mitogenic stimulus for pancreatic acinar cell proliferation. World J Gastroenterol 2006; 12(46): 7428-7432
- URL: https://www.wjgnet.com/1007-9327/full/v12/i46/7428.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i46.7428
Cell proliferation is a strictly regulated event. The rate of cell division depends upon instructions by which the cell responds to mitogenic or other stimuli in order to enter the cell cycle substantiated by cyclin dependent kinases (CDKs) which are activated by cyclins. In a normal resting cell the intracellular signaling proteins and genes, activated by extracellular growth factors, are quiescent. However, when stimulated by growth factors, the cells undergo proliferation. In cancer cells, intracellular signaling proteins and oncogenes activated by the growth factors remain constitutively active[1]. In response to growth factors or other mitogenic stimuli, three major types of signal transduction pathways are activated in a cell: mitogen activated protein kinase (MAPK) pathways, protein kinase C (PKC) pathways and JAK/STAT pathways. Activation of each of these pathways requires a phosphorylation step that regulates cell proliferation and differentiation[1].
Understanding of the complex molecular mechanisms involved in the regulation of cell proliferation in normal and cancer cells will provide us with tools to intervene in the regulation and control of the cell cycle in the presence of a mitogen. Qualitatively speaking, the biochemistry of growth of tumor and normal cells appear similar[2]. The fundamental difference most probably lies in a relaxation of regulation of cell growth[3,4]. Here we will discuss the role of nicotine in cell proliferation in reference to AR42J cells (a rat pancreatic tumor cell line) and look further into extended observations from our recent findings using primary cells derived from the normal rat pancreas when exposed to nicotine in culture.
Nicotine is a major component of cigarette smoke and is a known risk factor for the development of numerous diseases[5-9]. The role of nicotine/smoking as a risk factor for the induction of pancreatic inflammation and pancreatic cancer has been reported recently[10,11]. The mechanism by which nicotine induces such pathologies is as yet unknown. Understanding of the proliferative potential of nicotine in primary and tumor cells of the pancreas will allow us to develop measures that will ultimately lead to intervention, prevention and treatment.
AR42J is a stable, rat pancreatic tumor cell line derived from the hyperplastic pancreatic nodules of male rats following the administration of azaserine[12]. Because of their unique properties of stability, secretory capacity, and growth potential[13], these cells have been extensively used as an in vitro model for studying exocytotic secretory processes and activation of signal transduction pathways[14-16]. In response to regulatory peptides, AR42J cells can be stimulated to secrete enzymes and induce proliferation[13,17,18].
MAPK enzymes play critical roles in the regulation of cell proliferation, differentiation, and apoptosis, and are comprised of a ubiquitous family of tyrosine/threonine kinases that include the extracellular signal-regulated kinases (ERK1/2), c-jun NH2-terminal kinases (JNK1/2), and p38 MAPK. Cytokines and mitogens trigger signaling cascades that lead to the activation of MAPK, as reported in studies with different cell lines[19-24].
Activation of ERK has been primarily implicated in cell proliferation and survival, whereas activation of JNK and p38 are associated with growth arrest and apoptosis[25-27]. Induction of MAPK’s leads to the phosphorylation and activation of a variety of proteins, including a number of transcription factors involved in regulating the expression of genes controlling cellular proliferation[28-30]. ERK1/2 is found in most tumors and is involved in gastric carcinogenesis[31]. Differential activation of MAPK by cholecystokinin (CCK) and bombesin has been reported in AR42J cells[17].
Nicotine is known to activate several MAPK signaling pathways in a variety of tissue and cell types[32-37]. It also behaves like a growth factor promoting survival of human lung cancer cells[38]. Studies show that nicotine can increase the cell numbers of certain cancer cell lines[39-41]. This suggests that nicotine exposure can lead to the disruption of the dynamic balance between cell death and proliferation, which is required for normal functioning of cells.
In pulmonary neuroendocrine cells, nicotine binds to nicotinic receptors, resulting in the phosphorylation of ERK and stimulation of DNA synthesis[32,42]. Furthermore, nicotine induces Ca2+ influx and stimulates the Ras/ERK cascade that promotes cell survival in neuronal cells[43]. Thus, ERK1/2 is one of the possible signaling pathways involved in nicotine-induced cell proliferation. Studies by Bose et al[44] on the mitogenic effect of nicotine on AR42J cells show that nicotine activates ERK1/2 in AR42J cells and induces proliferation without affecting basal and stimulated enzyme secretion. These data suggest that MAPK signaling by nicotine in AR42J cells is independent of the secretory response.
Primary cells are normally derived from intact rat pancreas. The functional status of the primary acinar cells exposed to nicotine under basal and stimulated conditions has been reported[45,46]. However, the ability of nicotine to activate MAPK signaling pathways and subsequent proliferation has not yet been reported. It has been shown that p42 MAPK is fully activated at 5 min by cholecystokinin (CCK) in freshly isolated pancreatic acini, and JNK is activated maximally at 30 min and remains significantly elevated at 60 min[47,14]. Unpublished studies from our laboratory show that exposure of primary cells to nicotine in short term culture activates ERK1/2 MAPK with very little or no effect on JNK and p38 MAPK. The results on primary cells from this study and the studies cited above thus demonstrate that some parallelism in the time-dependent activation of ERK by CCK and nicotine exists, although these two secretagogues are completely different from one another in terms of their biological actions on the pancreas[15,14,48]. Further, cell proliferation studies conducted and monitored with three independent methods (MTT assay, BrdU assay and flow cytometry) confirmed the proliferative ability of primary cells in the presence of nicotine. This response is reversed in the presence of the ERK1/2 inhibitor UO126, suggesting that the proliferation induced by nicotine in primary cells is MAPK-dependent. Activation of these signals and application of their inhibitors in the presence of nicotine had no effect on the stimulated enzyme secretion by these cells (unpublished observations). The proliferation of primary cells by nicotine thus appears to be independent of the stimulus-secretion coupling response of amylase secretion.
MAPKs are known to exert several complex functions, such as regulation of cellular growth, proliferation, and differentiation[29,49,50]. It has also been shown that activation of ERK1/2 MAPK is associated with cell proliferative signals whereas activations of c-jun NH2-terminal kinases 1/2 (JNK1/2) and p38 MAPKs are associated with stress-response signaling[51,52]. Zhao et al[53] have shown that in the human T lymphoma cell line Molt-4, ERK and p-38 mitogen activated protein kinase (MAPK) signaling are induced in response to hepatitis C virus E2 envelope protein resulting in alterations in cell behavior. In human squamous cell carcinomas (SCC) of the larynx, the potential derangement of MAPK pathways which showed decreased activity of ERK1/2 p44/42 reflecting alterations in tumor suppressing activity, has been reported[54]. Application of low power laser irradiation (LPLI) has been shown to promote cellular proliferation of human dental pulp derived fibroblast-like cells (dental pulp cells) inducing the activation of ERK 1/2 with no induction pf p38 MAPK or c-Jun N-terminal kinase (JNK) phosphorylation[55]. Hepatocyte growth factor (HGF) also enhanced proliferation and differentiation of dental pulp cells by partial activation of the ERK/MAPK pathway[56].
Recently Li et al have shown that anti-apoptotic human phosphatidylethanolamine-binding protein (hPEBP4) silencing, promotes tumor necrosis factor related apoptosis-inducing ligand (TRAIL)-induced apoptosis of human ovarian cancer cells by activating ERK and JNK pathways[57]. Mutant huntingtin, a protein derived from Huntington disease (HD) affects signaling at upstream points activating ERK and JNK, suggesting that pharmacological intervention of MAPK pathways may be an appropriate approach to HD therapy[58]. ERK 1 expression has been shown to be an early marker of cervical carcinogenesis[59]. In human glomerulopathies, activation of ERK pathways has been correlated with cell proliferation, histologic lesions, and renal dysfunction[60]. In human neutrophils, Rac/Cdc-dependent activation of MAPK/ERK is a critical event in the immediate phagocytic response of PMNs to microbial challenge[61]. IL-1 beta stimulated human airway smooth muscle cells demonstrate activated p38 MAPK, JNK kinase and p42/p44 ERK suggesting their role in the inflammatory process in asthma[62]. In the human myeloma cell line SKO-007, activation of ERK in the Ras/MAPK signaling pathway has been shown to play important differences in their responsiveness to IFN-alpha[63]. Signaling through SAPK/MAK pathways is shown to be a typical feature of chronic synovitis in rheumatoid arthritis, but not in degenerative joint disease. SAPK/MAPK signaling is found at distinct sites in the synovial tissue and is induced by proinflammatory cytokines[64].
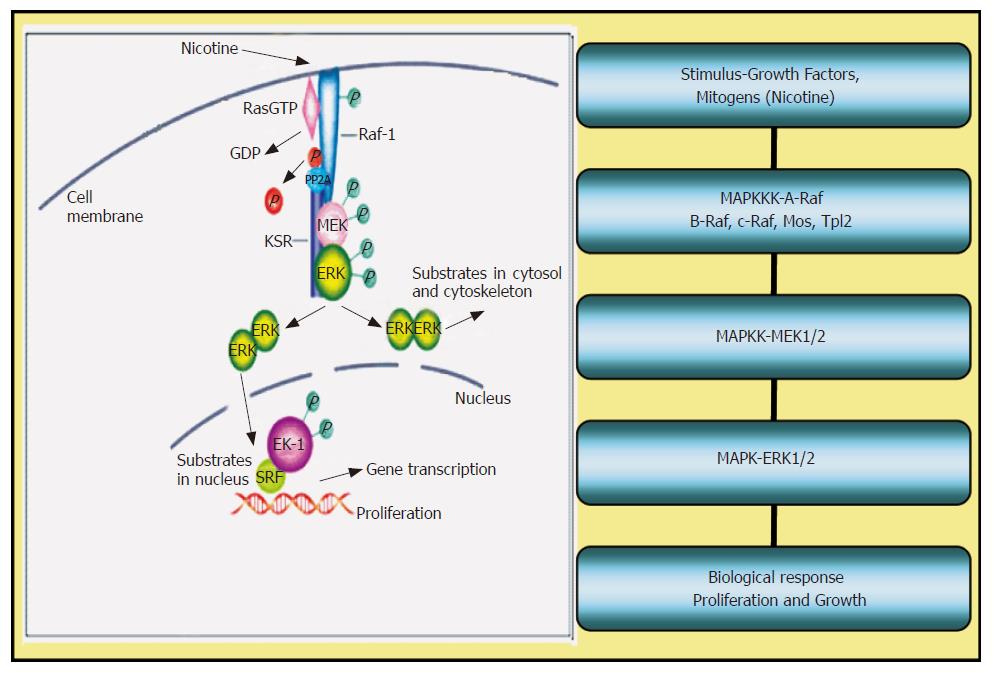
The possible signaling pathways leading to cell proliferation by nicotine are shown in Figure 1 below. The schematic shows that nicotine enters the cell either by diffusion or via a calcium regulated pathway as demonstrated earlier[65]. Entry of nicotine induces the activation of Ras-Raf-MEK-ERK pathways inducing phosphorylation of the MAP kinase cascade. Substrates of ERK in the cytosol include tyrosine kinase receptors among others. Substrates of ERK in the nucleus include transcription factors such as ELK-1 and others. The endpoint of ERK phosphorylation leads to the assembly of transcription factors which stimulate the production of proteins causing cells to proliferate and grow.
The parallelism observed in nicotine-induced cell proliferation studies conducted in a mutant pancreatic cell line and freshly isolated pancreatic acinar cells suggest the possibility that this stable mutant line can be used for extensive evaluation of signal transduction pathways mediating oncogenesis. The data gathered from these studies can be extended to assess the mechanisms of development of pancreatic diseases induced in animal models exposed to chronic/sub-chronic exposure to nicotine or cigarette smoking.
| 1. | Molecular Biology of the Cell. Cell Proliferation. Alberts B, Bray D, Johnson A, Lewis J, Raff M, Roberts K, Walter P, eds. London: Garland Publishing INC 1998; . |
| 2. | Weber G. Biochemical strategy of cancer cells and the design of chemotherapy: G. H. A. Clowes Memorial Lecture. Cancer Res. 1983;43:3466-3492. [PubMed] |
| 3. | Fingert HJ, Campisi J, Pardee AB. Molecular Biology and Biochemistry of Cancer. Gynecologic Oncology. New York: Macmillan 1991; 30. |
| 4. | Gibbs JB, Oliff A, Kohl NE. Farnesyltransferase inhibitors: Ras research yields a potential cancer therapeutic. Cell. 1994;77:175-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 388] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 5. | National Center for Chronic Disease Prevention and Health Promotion. Health Consequences of Smoking: a Report of the Surgeon General. Washington D. C: National Institutes of Health 2004; . |
| 6. | Zhang H, Cai B. The impact of tobacco on lung health in China. Respirology. 2003;8:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 124] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Slattery ML, Edwards S, Curtin K, Schaffer D, Neuhausen S. Associations between smoking, passive smoking, GSTM-1, NAT2, and rectal cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:882-889. [PubMed] |
| 8. | Ulrich CM, Bigler J, Whitton JA, Bostick R, Fosdick L, Potter JD. Epoxide hydrolase Tyr113His polymorphism is associated with elevated risk of colorectal polyps in the presence of smoking and high meat intake. Cancer Epidemiol Biomarkers Prev. 2001;10:875-882. [PubMed] |
| 9. | Lowenfels AB, Maisonneuve P, Lankisch PG. Chronic pancreatitis and other risk factors for pancreatic cancer. Gastroenterol Clin North Am. 1999;28:673-685, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Malfertheiner P, Schütte K. Smoking--a trigger for chronic inflammation and cancer development in the pancreas. Am J Gastroenterol. 2006;101:160-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Wittel UA, Pandey KK, Andrianifahanana M, Johansson SL, Cullen DM, Akhter MP, Brand RE, Prokopczyk B, Batra SK. Chronic pancreatic inflammation induced by environmental tobacco smoke inhalation in rats. Am J Gastroenterol. 2006;101:148-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Longnecker DS, Lilja HS, French J, Kuhlmann E, Noll W. Transplantation of azaserine-induced carcinomas of pancreas in rats. Cancer Lett. 1979;7:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Christophe J. Pancreatic tumoral cell line AR42J: an amphicrine model. Am J Physiol. 1994;266:G963-G971. [PubMed] |
| 14. | Duan RD, Williams JA. Cholecystokinin rapidly activates mitogen-activated protein kinase in rat pancreatic acini. Am J Physiol. 1994;267:G401-G408. [PubMed] |
| 15. | Duan RD, Zheng CF, Guan KL, Williams JA. Activation of MAP kinase kinase (MEK) and Ras by cholecystokinin in rat pancreatic acini. Am J Physiol. 1995;268:G1060-G1065. [PubMed] |
| 16. | Williams JA. Intracellular signaling mechanisms activated by cholecystokinin-regulating synthesis and secretion of digestive enzymes in pancreatic acinar cells. Annu Rev Physiol. 2001;63:77-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 176] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 17. | Kiehne K, Herzig KH, Fölsch UR. Differential activation of p42ERK2 and p125FAK by cholecystokinin and bombesin in the secretion and proliferation of the pancreatic amphicrine cell line AR42J. Pancreatology. 2002;2:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Thommesen L, Nørsett K, Sandvik AK, Hofsli E, Laegreid A. Regulation of inducible cAMP early repressor expression by gastrin and cholecystokinin in the pancreatic cell line AR42J. J Biol Chem. 2000;275:4244-4250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Bose C, Bhuvaneswaran C, Udupa KB. Altered mitogen-activated protein kinase signal transduction in human skin fibroblasts during in vitro aging: differential expression of low-density lipoprotein receptor. J Gerontol A Biol Sci Med Sci. 2004;59:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Clarke N, Arenzana N, Hai T, Minden A, Prywes R. Epidermal growth factor induction of the c-jun promoter by a Rac pathway. Mol Cell Biol. 1998;18:1065-1073. [PubMed] |
| 21. | Kumar A, Middleton A, Chambers TC, Mehta KD. Differential roles of extracellular signal-regulated kinase-1/2 and p38(MAPK) in interleukin-1beta- and tumor necrosis factor-alpha-induced low density lipoprotein receptor expression in HepG2 cells. J Biol Chem. 1998;273:15742-15748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | McDermott EP, O'Neill LA. Ras participates in the activation of p38 MAPK by interleukin-1 by associating with IRAK, IRAK2, TRAF6, and TAK-1. J Biol Chem. 2002;277:7808-7815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Minden A, Lin A, McMahon M, Lange-Carter C, Dérijard B, Davis RJ, Johnson GL, Karin M. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science. 1994;266:1719-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 843] [Cited by in RCA: 882] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 24. | Westwick JK, Weitzel C, Minden A, Karin M, Brenner DA. Tumor necrosis factor alpha stimulates AP-1 activity through prolonged activation of the c-Jun kinase. J Biol Chem. 1994;269:26396-26401. [PubMed] |
| 25. | Cobb MH. MAP kinase pathways. Prog Biophys Mol Biol. 1999;71:479-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 637] [Cited by in RCA: 635] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 26. | Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1191] [Cited by in RCA: 1250] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 27. | Paul A, Wilson S, Belham CM, Robinson CJ, Scott PH, Gould GW, Plevin R. Stress-activated protein kinases: activation, regulation and function. Cell Signal. 1997;9:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 253] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 28. | Bose C, Bhuvaneswaran C, Udupa KB. Age-related alteration in hepatic acyl-CoA: cholesterol acyltransferase and its relation to LDL receptor and MAPK. Mech Ageing Dev. 2005;126:740-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Davis RJ. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993;268:14553-14556. [PubMed] |
| 30. | Whitmarsh AJ, Shore P, Sharrocks AD, Davis RJ. Integration of MAP kinase signal transduction pathways at the serum response element. Science. 1995;269:403-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 729] [Cited by in RCA: 762] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 31. | Shin VY, Wu WK, Ye YN, So WH, Koo MW, Liu ES, Luo JC, Cho CH. Nicotine promotes gastric tumor growth and neovascularization by activating extracellular signal-regulated kinase and cyclooxygenase-2. Carcinogenesis. 2004;25:2487-2495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 32. | Heusch WL, Maneckjee R. Signalling pathways involved in nicotine regulation of apoptosis of human lung cancer cells. Carcinogenesis. 1998;19:551-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 153] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Nakayama H, Numakawa T, Ikeuchi T, Hatanaka H. Nicotine-induced phosphorylation of extracellular signal-regulated protein kinase and CREB in PC12h cells. J Neurochem. 2001;79:489-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 111] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 34. | Nakayama H, Numakawa T, Ikeuchi T. Nicotine-induced phosphorylation of Akt through epidermal growth factor receptor and Src in PC12h cells. J Neurochem. 2002;83:1372-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Schuller HM, Porter B, Riechert A, Walker K, Schmoyer R. Neuroendocrine lung carcinogenesis in hamsters is inhibited by green tea or theophylline while the development of adenocarcinomas is promoted: implications for chemoprevention in smokers. Lung Cancer. 2004;45:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Tang K, Wu H, Mahata SK, O'Connor DT. A crucial role for the mitogen-activated protein kinase pathway in nicotinic cholinergic signaling to secretory protein transcription in pheochromocytoma cells. Mol Pharmacol. 1998;54:59-69. [PubMed] |
| 37. | Wang J, Chen YB, Zhu XN, Chen RZ. Activation of p42/44 mitogen-activated protein kinase pathway in long-term potentiation induced by nicotine in hippocampal CA1 region in rats. Acta Pharmacol Sin. 2001;22:685-690. [PubMed] |
| 38. | Jin Z, Gao F, Flagg T, Deng X. Nicotine induces multi-site phosphorylation of Bad in association with suppression of apoptosis. J Biol Chem. 2004;279:23837-23844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 104] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 39. | Cattaneo MG, Codignola A, Vicentini LM, Clementi F, Sher E. Nicotine stimulates a serotonergic autocrine loop in human small-cell lung carcinoma. Cancer Res. 1993;53:5566-5568. [PubMed] |
| 40. | Quik M, Chan J, Patrick J. alpha-Bungarotoxin blocks the nicotinic receptor mediated increase in cell number in a neuroendocrine cell line. Brain Res. 1994;655:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | Schuller HM. Carbon dioxide potentiates the mitogenic effects of nicotine and its carcinogenic derivative, NNK, in normal and neoplastic neuroendocrine lung cells via stimulation of autocrine and protein kinase C-dependent mitogenic pathways. Neurotoxicology. 1994;15:877-886. [PubMed] |
| 42. | Aguayo SM. Pulmonary neuroendocrine cells in tobacco-related lung disorders. Anat Rec. 1993;236:122-127; discussion 127-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Itano N, Atsumi F, Sawai T, Yamada Y, Miyaishi O, Senga T, Hamaguchi M, Kimata K. Abnormal accumulation of hyaluronan matrix diminishes contact inhibition of cell growth and promotes cell migration. Proc Natl Acad Sci USA. 2002;99:3609-3614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 241] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 44. | Bose C, Zhang H, Udupa KB, Chowdhury P. Activation of p-ERK1/2 by nicotine in pancreatic tumor cell line AR42J: effects on proliferation and secretion. Am J Physiol Gastrointest Liver Physiol. 2005;289:G926-G934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 45. | Chowdhury P, Hosotani R, Chang L, Rayford PL. Metabolic and pathologic effects of nicotine on gastrointestinal tract and pancreas of rats. Pancreas. 1990;5:222-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 46. | Chowdhury P, Hosotani R, Rayford PL. Inhibition of CCK or carbachol-stimulated amylase release by nicotine. Life Sci. 1989;45:2163-2168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 47. | Dabrowski A, Grady T, Logsdon CD, Williams JA. Jun kinases are rapidly activated by cholecystokinin in rat pancreas both in vitro and in vivo. J Biol Chem. 1996;271:5686-5690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 48. | Doi R, Chowdhury P, Rayford PL. Agonist-regulated alteration of the affinity of pancreatic muscarinic cholinergic receptors. J Biol Chem. 1993;268:22436-22443. [PubMed] |
| 49. | Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3632] [Cited by in RCA: 3693] [Article Influence: 119.1] [Reference Citation Analysis (0)] |
| 50. | Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143-180. [PubMed] |
| 51. | Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4166] [Cited by in RCA: 4316] [Article Influence: 139.2] [Reference Citation Analysis (0)] |
| 52. | Harper SJ, LoGrasso P. Signalling for survival and death in neurones: the role of stress-activated kinases, JNK and p38. Cell Signal. 2001;13:299-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 234] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 53. | Zhao LJ, Zhang XL, Zhao P, Cao J, Cao MM, Zhu SY, Liu HQ, Qi ZT. Up-regulation of ERK and p38 MAPK signaling pathways by hepatitis C virus E2 envelope protein in human T lymphoma cell line. J Leukoc Biol. 2006;80:424-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 54. | Garavello W, Nicolini G, Aguzzi A, Maggioni D, Leone BE, Viganò P, Gaini RM, Tredici G. Selective reduction of extracellular signal-regulated protein kinase (ERK) phosphorylation in squamous cell carcinoma of the larynx. Oncol Rep. 2006;16:479-484. [PubMed] |
| 55. | Miyata H, Genma T, Ohshima M, Yamaguchi Y, Hayashi M, Takeichi O, Ogiso B, Otsuka K. Mitogen-activated protein kinase/extracellular signal-regulated protein kinase activation of cultured human dental pulp cells by low-power gallium-aluminium-arsenic laser irradiation. Int Endod J. 2006;39:238-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 56. | Ye L, Peng L, Tan H, Zhou X. HGF enhanced proliferation and differentiation of dental pulp cells. J Endod. 2006;32:736-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | Li P, Wang X, Li N, Kong H, Guo Z, Liu S, Cao X. Anti-apoptotic hPEBP4 silencing promotes TRAIL-induced apoptosis of human ovarian cancer cells by activating ERK and JNK pathways. Int J Mol Med. 2006;18:505-510. [PubMed] |
| 58. | Apostol BL, Illes K, Pallos J, Bodai L, Wu J, Strand A, Schweitzer ES, Olson JM, Kazantsev A, Marsh JL. Mutant huntingtin alters MAPK signaling pathways in PC12 and striatal cells: ERK1/2 protects against mutant huntingtin-associated toxicity. Hum Mol Genet. 2006;15:273-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 59. | Branca M, Ciotti M, Santini D, Bonito LD, Benedetto A, Giorgi C, Paba P, Favalli C, Costa S, Agarossi A. Activation of the ERK/MAP kinase pathway in cervical intraepithelial neoplasia is related to grade of the lesion but not to high-risk human papillomavirus, virus clearance, or prognosis in cervical cancer. Am J Clin Pathol. 2004;122:902-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Masaki T, Stambe C, Hill PA, Dowling J, Atkins RC, Nikolic-Paterson DJ. Activation of the extracellular-signal regulated protein kinase pathway in human glomerulopathies. J Am Soc Nephrol. 2004;15:1835-1843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Zhong B, Jiang K, Gilvary DL, Epling-Burnette PK, Ritchey C, Liu J, Jackson RJ, Hong-Geller E, Wei S. Human neutrophils utilize a Rac/Cdc42-dependent MAPK pathway to direct intracellular granule mobilization toward ingested microbial pathogens. Blood. 2003;101:3240-3248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 62. | Wuyts WA, Vanaudenaerde BM, Dupont LJ, Demedts MG, Verleden GM. Involvement of p38 MAPK, JNK, p42/p44 ERK and NF-kappaB in IL-1beta-induced chemokine release in human airway smooth muscle cells. Respir Med. 2003;97:811-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 63. | Song L, Li Y, Shen B. Protein kinase ERK contributes to differential responsiveness of human myeloma cell lines to IFNalpha. Cancer Cell Int. 2002;2:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 64. | Schett G, Tohidast-Akrad M, Smolen JS, Schmid BJ, Steiner CW, Bitzan P, Zenz P, Redlich K, Xu Q, Steiner G. Activation, differential localization, and regulation of the stress-activated protein kinases, extracellular signal-regulated kinase, c-JUN N-terminal kinase, and p38 mitogen-activated protein kinase, in synovial tissue and cells in rheumatoid arthritis. Arthritis Rheum. 2000;43:2501-2512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 65. | Chowdhury P, MacLeod S, Udupa KB, Rayford PL. Pathophysiological effects of nicotine on the pancreas: an update. Exp Biol Med (Maywood). 2002;227:445-454. [PubMed] |
S- Editor Liu Y L- Editor Lalor PF E- Editor Bai SH